Abstract
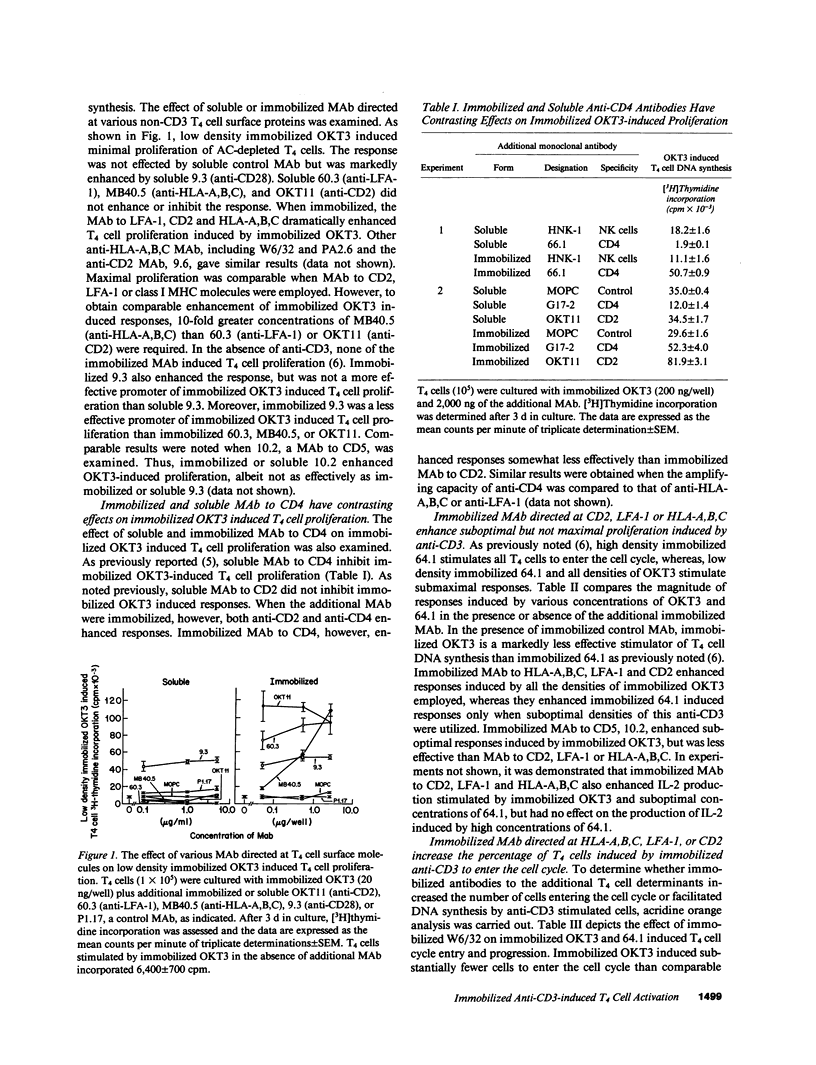
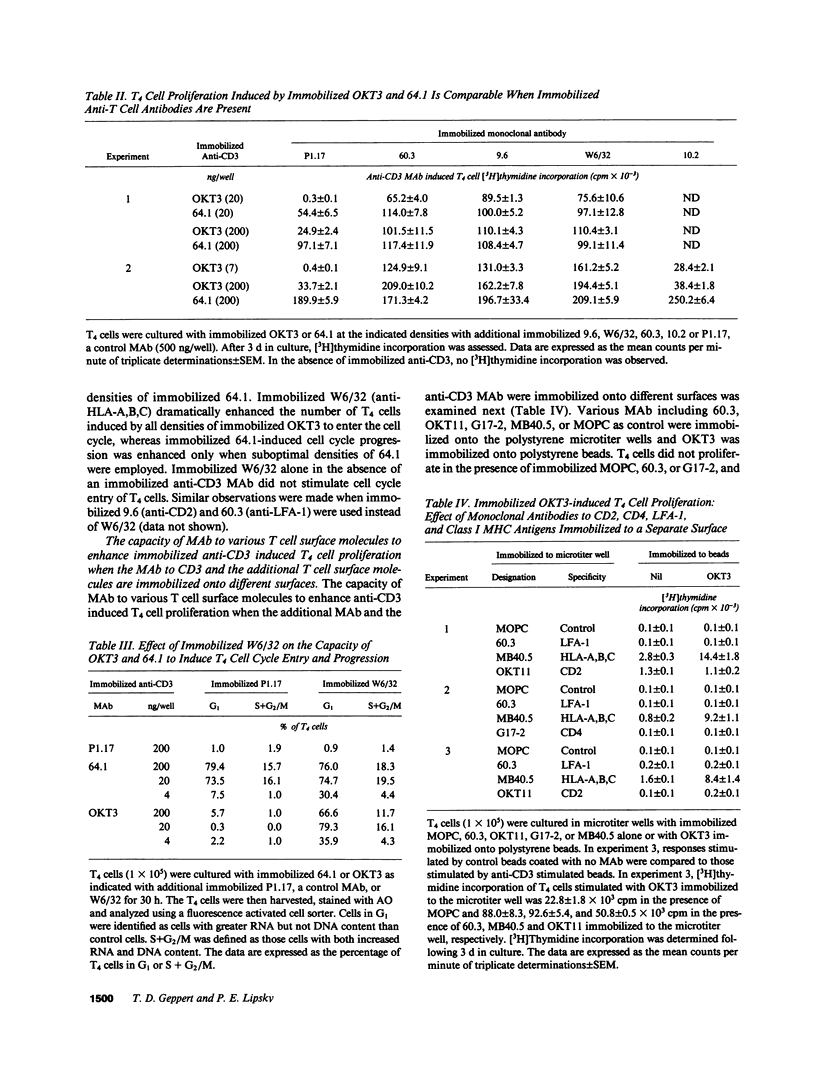
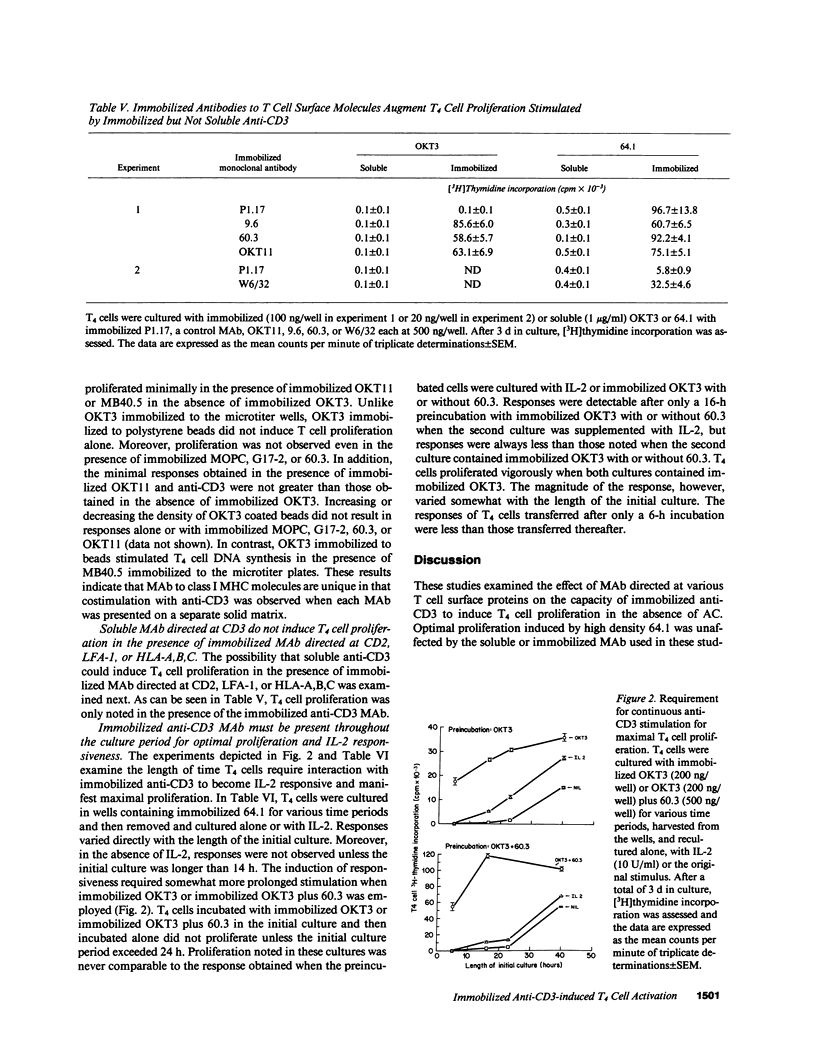
The effect of soluble or immobilized MAb directed at various additional surface proteins on the proliferation of highly purified T4 cells induced by two immobilized MAb to CD3, OKT3 and 64.1, was examined. High density 64.1 stimulated nearly all T4 cells to enter and progress through the cell cycle. Maximal T4 cell proliferation required stimulation with immobilized 64.1 throughout the length of the incubation and was not effected by any of the additional soluble or immobilized MAb employed. In contrast, low density immobilized 64.1 and all densities of immobilized OKT3 employed stimulated a minority of the cells to enter the cell cycle and proliferate. Immobilized MAb directed at CD2, class I major histocompatibility complex (MHC) encoded gene products or CD11a (LFA-1) dramatically enhanced the response, whereas soluble MAb directed at these determinants did not. Both immobilized and soluble MAb directed at CD5 and CD28 (Tp44) enhanced responses, but they were less effective than immobilized MAb to CD2, LFA-1 or HLA-A,B,C. Soluble anti-CD4 MAb inhibited responses somewhat, whereas immobilized anti-CD4 enhanced responses. Costimulation was observed when MAb to CD3 and class I MHC molecules but not CD2, LFA-1 or CD4 were immobilized to separate surfaces. The data suggest that when anti-CD3 stimulation is suboptimal, responses can be enhanced by MAb to CD5 or CD28 (Tp44) or by immobilized MAb to CD4, CD2, CD11a (LFA-1), or class I MHC encoded gene products. Although crosslinking of CD4, CD2, or CD11a with CD3 may be necessary for costimulation, immobilized MAb to CD3 and class I MHC molecules appear to deliver independent signals that result in enhanced T4 cell activation and proliferation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Anderson P., Blue M. L., Morimoto C., Schlossman S. F. Cross-linking of T3 (CD3) with T4 (CD4) enhances the proliferation of resting T lymphocytes. J Immunol. 1987 Aug 1;139(3):678–682. [PubMed] [Google Scholar]
- Bank I., Chess L. Perturbation of the T4 molecule transmits a negative signal to T cells. J Exp Med. 1985 Oct 1;162(4):1294–1303. doi: 10.1084/jem.162.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty P. G., Ledbetter J. A., Martin P. J., Price T. H., Hansen J. A. Definition of a common leukocyte cell-surface antigen (Lp95-150) associated with diverse cell-mediated immune functions. J Immunol. 1983 Dec;131(6):2913–2918. [PubMed] [Google Scholar]
- Biddison W. E., Rao P. E., Talle M. A., Goldstein G., Shaw S. Possible involvement of the OKT4 molecule in T cell recognition of class II HLA antigens. Evidence from studies of cytotoxic T lymphocytes specific for SB antigens. J Exp Med. 1982 Oct 1;156(4):1065–1076. doi: 10.1084/jem.156.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P. Monomorphic anti-HLA-A,B,C monoclonal antibodies detecting molecular subunits and combinatorial determinants. J Immunol. 1982 Jan;128(1):129–135. [PubMed] [Google Scholar]
- Ceuppens J. L., Bloemmen F. J., Van Wauwe J. P. T cell unresponsiveness to the mitogenic activity of OKT3 antibody results from a deficiency of monocyte Fc gamma receptors for murine IgG2a and inability to cross-link the T3-Ti complex. J Immunol. 1985 Dec;135(6):3882–3886. [PubMed] [Google Scholar]
- Chang T. W., Kung P. C., Gingras S. P., Goldstein G. Does OKT3 monoclonal antibody react with an antigen-recognition structure on human T cells? Proc Natl Acad Sci U S A. 1981 Mar;78(3):1805–1808. doi: 10.1073/pnas.78.3.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouaib S., Welte K., Dupont B. Differential effect of anti-beta 2-microglobulin on IL 2 production and IL 2 receptor expression in the primary mixed lymphocyte culture reaction. J Immunol. 1985 Feb;134(2):940–948. [PubMed] [Google Scholar]
- Clement L. T., Tilden A. B., Dunlap N. E. Analysis of the monocyte Fc receptors and antibody-mediated cellular interactions required for the induction of T cell proliferation by anti-T3 antibodies. J Immunol. 1985 Jul;135(1):165–171. [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T., Melamed M. R. Lymphocyte stimulation: a rapid multiparameter analysis. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2881–2884. doi: 10.1073/pnas.73.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmrich F., Kanz L., Eichmann K. Cross-linking of the T cell receptor complex with the subset-specific differentiation antigen stimulates interleukin 2 receptor expression in human CD4 and CD8 T cells. Eur J Immunol. 1987 Apr;17(4):529–534. doi: 10.1002/eji.1830170415. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Strittmatter U., Eichmann K. Synergism in the activation of human CD8 T cells by cross-linking the T-cell receptor complex with the CD8 differentiation antigen. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8298–8302. doi: 10.1073/pnas.83.21.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert T. D., Lipsky P. E. Accessory cell independent proliferation of human T4 cells stimulated by immobilized monoclonal antibodies to CD3. J Immunol. 1987 Mar 15;138(6):1660–1666. [PubMed] [Google Scholar]
- Geppert T. D., Lipsky P. E. Accessory cell-T cell interactions involved in anti-CD3-induced T4 and T8 cell proliferation: analysis with monoclonal antibodies. J Immunol. 1986 Nov 15;137(10):3065–3073. [PubMed] [Google Scholar]
- Geppert T. D., Lipsky P. E. Dissection of defective antigen presentation by interferon-gamma-treated fibroblasts. J Immunol. 1987 Jan 15;138(2):385–392. [PubMed] [Google Scholar]
- Huet S., Wakasugi H., Sterkers G., Gilmour J., Tursz T., Boumsell L., Bernard A. T cell activation via CD2 [T, gp50]: the role of accessory cells in activating resting T cells via CD2. J Immunol. 1986 Sep 1;137(5):1420–1428. [PubMed] [Google Scholar]
- Kamoun M., Martin P. J., Hansen J. A., Brown M. A., Siadak A. W., Nowinski R. C. Identification of a human T lymphocyte surface protein associated with the E-rosette receptor. J Exp Med. 1981 Jan 1;153(1):207–212. doi: 10.1084/jem.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Martin P. J., Spooner C. E., Wofsy D., Tsu T. T., Beatty P. G., Gladstone P. Antibodies to Tp67 and Tp44 augment and sustain proliferative responses of activated T cells. J Immunol. 1985 Oct;135(4):2331–2336. [PubMed] [Google Scholar]
- Marrack P., Endres R., Shimonkevitz R., Zlotnik A., Dialynas D., Fitch F., Kappler J. The major histocompatibility complex-restricted antigen receptor on T cells. II. Role of the L3T4 product. J Exp Med. 1983 Oct 1;158(4):1077–1091. doi: 10.1084/jem.158.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. J., Hansen J. A., Nowinski R. C., Brown M. A. A new human T-cell differentiation antigen: unexpected expression on chronic lymphocytic leukemia cells. Immunogenetics. 1980;11(5):429–439. doi: 10.1007/BF01567812. [DOI] [PubMed] [Google Scholar]
- Martin P. J., Longton G., Ledbetter J. A., Newman W., Braun M. P., Beatty P. G., Hansen J. A. Identification and functional characterization of two distinct epitopes on the human T cell surface protein Tp50. J Immunol. 1983 Jul;131(1):180–185. [PubMed] [Google Scholar]
- Mentzer S. J., Gromkowski S. H., Krensky A. M., Burakoff S. J., Martz E. LFA-1 membrane molecule in the regulation of homotypic adhesions of human B lymphocytes. J Immunol. 1985 Jul;135(1):9–11. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Mishra G. C., Berton M. T., Oliver K. G., Krammer P. H., Uhr J. W., Vitetta E. S. A monoclonal anti-mouse LFA-1 alpha antibody mimics the biological effects of B cell stimulatory factor-1 (BSF-1). J Immunol. 1986 Sep 1;137(5):1590–1598. [PubMed] [Google Scholar]
- Moreno J., Lipsky P. E. Functional heterogeneity of human antigen-presenting cells: presentation of soluble antigen but not self-Ia by monocytes. J Clin Immunol. 1986 Jan;6(1):9–20. doi: 10.1007/BF00915359. [DOI] [PubMed] [Google Scholar]
- Parham P., Bodmer W. F. Monoclonal antibody to a human histocompatibility alloantigen, HLA-A2. Nature. 1978 Nov 23;276(5686):397–399. doi: 10.1038/276397a0. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Springer T. A. The requirement for lymphocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986 May 1;163(5):1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless T., Traganos F., Darzynkiewicz Z., Melamed M. R. Flow cytofluorimetry: discrimination between single cells and cell aggregates by direct size measurements. Acta Cytol. 1975 Nov-Dec;19(6):577–581. [PubMed] [Google Scholar]
- Sprent J., Schaefer M. Properties of purified T cell subsets. I. In vitro responses to class I vs. class II H-2 alloantigens. J Exp Med. 1985 Dec 1;162(6):2068–2088. doi: 10.1084/jem.162.6.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterkers G., Henin Y., Kalil J., Bagot M., Levy J. P. Influence of HLA class I- and class II-specific monoclonal antibodies on DR-restricted lymphoproliferative responses. I. Unseparated populations of effector cells. J Immunol. 1983 Dec;131(6):2735–2740. [PubMed] [Google Scholar]
- Thiele D. L., Kurosaka M., Lipsky P. E. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J Immunol. 1983 Nov;131(5):2282–2290. [PubMed] [Google Scholar]
- Turco M. C., De Felice M., Corbo L., Morrone G., Mertelsmann R., Ferrone S., Venuta S. Regulatory role of a monomorphic determinant of HLA Class I antigens in T cell proliferation. J Immunol. 1985 Oct;135(4):2268–2273. [PubMed] [Google Scholar]
- Van Wauwe J. P., De Mey J. R., Goossens J. G. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980 Jun;124(6):2708–2713. [PubMed] [Google Scholar]
- Verbi W., Greaves M. F., Schneider C., Koubek K., Janossy G., Stein H., Kung P., Goldstein G. Monoclonal antibodies OKT 11 and OKT 11A have pan-T reactivity and block sheep erythrocyte "receptors". Eur J Immunol. 1982 Jan;12(1):81–86. doi: 10.1002/eji.1830120115. [DOI] [PubMed] [Google Scholar]
- Vollger L. W., Tuck D. T., Springer T. A., Haynes B. F., Singer K. H. Thymocyte binding to human thymic epithelial cells is inhibited by monoclonal antibodies to CD-2 and LFA-3 antigens. J Immunol. 1987 Jan 15;138(2):358–363. [PubMed] [Google Scholar]
- Walker C., Bettens F., Pichler W. J. Activation of T cells by cross-linking an anti-CD3 antibody with a second anti-T cell antibody: mechanism and subset-specific activation. Eur J Immunol. 1987 Jun;17(6):873–880. doi: 10.1002/eji.1830170622. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. Y., Chouaib S., Dupont B. A common pathway for T lymphocyte activation involving both the CD3-Ti complex and CD2 sheep erythrocyte receptor determinants. J Immunol. 1986 Aug 15;137(4):1097–1100. [PubMed] [Google Scholar]



