ACTG A5230 was a pilot study of lopinavir/ritonavir monotherapy as second-line HIV treatment. Over 104 weeks, 60% remained on monotherapy with viral load <400 copies/mL. For subjects with viral loads >400 copies/mL, emtricitabine/tenofovir was added, and most suppressed to <400 copies/mL.
Keywords: second-line antiretroviral therapy, protease inhibitor monotherapy, intensification, ACTG 5230
Abstract
Background. The AIDS Clinical Trials Group (ACTG) A5230 study evaluated lopinavir/ritonavir (LPV/r) monotherapy following virologic failure (VF) on first-line human immunodeficiency virus (HIV) regimens in Africa and Asia.
Methods. Eligible subjects had received first-line regimens for at least 6 months and had plasma HIV-1 RNA levels 1000–200 000 copies/mL. All subjects received LPV/r 400/100 mg twice daily. VF was defined as failure to suppress to <400 copies/mL by week 24, or confirmed rebound to >400 copies/mL at or after week 16 following confirmed suppression. Subjects with VF added emtricitabine 200 mg/tenofovir 300 mg (FTC/TDF) once daily. The probability of continued HIV-1 RNA <400 copies/mL on LPV/r monotherapy through week 104 was estimated with a 95% confidence interval (CI); predictors of treatment success were evaluated with Cox proportional hazards models.
Results. One hundred twenty-three subjects were enrolled. Four subjects died and 2 discontinued prematurely; 117 of 123 (95%) completed 104 weeks. Through week 104, 49 subjects met the primary endpoint; 47 had VF, and 2 intensified treatment without VF. Of the 47 subjects with VF, 41 (33%) intensified treatment, and 39 of 41 subsequently achieved levels <400 copies/mL. The probability of continued suppression <400 copies/mL over 104 weeks on LPV/r monotherapy was 60% (95% CI, 50%–68%); 80%–85% maintained levels <400 copies/mL with FTC/TDF intensification as needed. Ultrasensitive assays on specimens with HIV-1 RNA level <400 copies/mL at weeks 24, 48, and 104 revealed that 61%, 62%, and 65% were suppressed to <40 copies/mL, respectively.
Conclusions. LPV/r monotherapy after first-line VF with FTC/TDF intensification when needed provides durable suppression of HIV-1 RNA over 104 weeks.
Clinical Trials Registration. NCT00357552.
Nearly 14 000 000 persons are receiving antiretroviral therapy (ART), with the majority living in resource-limited settings [1]. The 2013 World Health Organization (WHO) guidelines recommend first-line treatment with nonnucleoside reverse transcriptase inhibitor (NNRTI)–containing combinations [2]. Approximately 15% of persons receiving first-line ART may fail within 1–2 years [3]. Limited monitoring of ART in resource-limited settings means that virologic failure (VF) is often recognized late, after drug resistance mutations have already accumulated [4–6]. The WHO guidelines for second-line ART recommend the use of a boosted protease inhibitor (PI) in combination with 2 nucleoside reverse transcriptase inhibitors (NRTIs) [2]. It is unclear if the inclusion of NRTIs as a component of second-line ART provides additional virologic suppression, or simply adds potential cost and toxicity.
The primary results of the AIDS Clinical Trials Group (ACTG) A5230 study demonstrated short-term virologic activity of lopinavir/ritonavir (LPV/r) monotherapy as second-line therapy in resource-limited settings, with an 87% estimated probability of remaining on LPV/r monotherapy without VF at week 24 and no evidence of accumulated LPV-related resistance mutations [7]. ACTG A5230 was designed to continue for 104 weeks to provide observations on the long-term activity and safety of the strategy of LPV/r monotherapy with intensification as needed.
METHODS
Study Design
ACTG A5230 was a 104-week open-label, multicenter pilot study of LPV/r monotherapy in subjects failing a first-line regimen containing an NNRTI and 2 NRTIs (ClinicalTrials.gov identifier NCT00357552). The study was conducted at 5 AIDS Clinical Trials Group (ACTG) Clinical Research Sites, including 3 sites in Africa (Lilongwe, Malawi; Moshi, Tanzania; and Johannesburg, South Africa) and 2 sites in Asia (Chiang Mai, Thailand; and Chennai, India).
Subjects
Individuals 18 years or older with documented human immunodeficiency virus type 1 (HIV-1) infection who had received a first-line regimen containing an NNRTI and 2 NRTIs for at least 6 months continuously with documented plasma HIV-1 RNA levels between 1000 and 200 000 copies/mL were eligible for the study. Laboratory entry criteria are described in a previous publication [7]. Pregnant or breastfeeding women and persons with prior PI use, a serious medical condition within the previous 14 days, active substance abuse, or detectable hepatitis B surface antigen were excluded. All subjects provided written informed consent, and the study was approved by ethics and appropriate institutional review boards in each country.
Study Evaluations
Study visits for clinical assessments, self-reported adherence, and pill counts occurred at weeks 2, 4, 8, 12, 16, 20, and 24, followed by every 8 weeks to week 56, and then every 12 weeks to week 104. Serum chemistries, liver enzymes, and hematology values were measured at weeks 4, 8, 12, 24, 32, 48, 56, and every 12 weeks thereafter; fasting lipid panels were measured at study entry and at weeks 12, 24, 48, 68, and 104. Plasma HIV-1 RNA levels were evaluated at baseline and all study visits from week 12 onward; CD4 cell counts were measured at study entry and weeks 16, 24, 48, 68, and 104. All laboratories at clinical sites participated in defined international external quality assurance programs.
In a post hoc analysis, all week 24, 48, and 104 samples with HIV-1 RNA <400 copies/mL were evaluated with the Abbott m2000 RealTime assay (Abbott Laboratories, Abbott Park, Illinois) with a lower limit of detection 40 copies/mL. HIV-1 sequencing was performed in batch for all subjects at screening and at time of VF. Sequencing was performed with an ABI Prism 3100-Avant Genetic Analyzer (Applied Biosystems). HIV-1 subtype and drug resistance were determined from protease (PR) and reverse transcriptase (RT) sequences, and analyzed using the Stanford version 6.1 algorithm [8, 9].
Study Interventions
All subjects received fixed-dose combination LPV/r 400 mg/100 mg twice daily. If subjects developed VF, fixed-dose combination emtricitabine 200 mg/tenofovir 300 mg (FTC/TDF) daily was added to LPV/r.
Primary Endpoint
The primary endpoint was time to discontinuation of LPV/r monotherapy or VF where virologic failure was defined as (1) failure to suppress plasma HIV-1 RNA level to <400 copies/mL by week 24, or (2) confirmed rebound of plasma HIV-1 RNA level to >400 copies/mL after confirmed suppression to <400 copies/mL. With some exceptions per protocol, discontinuation of LPV/r monotherapy was considered as a primary endpoint; exceptions included discontinuation for rifampin-based tuberculosis treatment, addition of antiretroviral drugs due to pregnancy, and death with HIV-1 RNA level <400 copies/mL.
Sample Size
The target sample size of 120 subjects was chosen to provide at least 90% power to show that LPV/r monotherapy could provide at least a 65% success rate over 24 weeks. The study was continued for 104 weeks to observe the long-term activity and safety of the strategy of LPV/r monotherapy with intensification as needed.
Statistical Analysis
The probabilities of remaining on LPV/r monotherapy without VF at weeks 24 and 104 weeks were estimated via methods of Kaplan–Meier; estimation of confidence intervals (CIs) used Greenwood variance. The proportion of subjects with plasma HIV-1 RNA levels <400 copies/mL over time and <400 copies/mL on LPV/r monotherapy were calculated with exact confidence intervals assuming missing data were ignorable. Cox proportional hazards regression was used to assess factors associated with the hazard of failure. Risk factors of interest included prior use of efavirenz (EFV) vs nevirapine (NVP), prior therapy duration (>3 years, 1–3 years, and <1 year), sex, baseline CD4 cell count (≤200 vs >200 cells/µL), baseline plasma HIV-1 RNA level (<10 000 vs ≥10 000 copies/mL), and preexisting NRTI resistance mutations (defined as K65R or thymidine analogue mutations [TAMs] vs no NRTI resistance); these were defined a priori. Proportional hazards were assessed by visual examination of the score process.
RESULTS
Baseline Characteristics
One hundred twenty-three subjects entered the study; their baseline characteristics are described in Table 1. The majority (57%) of subjects were female. Median plasma HIV-1 RNA level was 4.34 log10 copies/mL; 22 subjects (18%) had HIV-1 RNA levels >100 000 copies/mL. Median CD4 count at study entry was 164 cells/µL; 70 subjects (57%) had counts <200 cells/µL. The most common first-line ART regimen was a combination of NVP, lamivudine (3TC), and stavudine (n = 63 [51%]); the majority of subjects had received ART for >3 years (n = 70 [57%]).
Table 1.
Baseline Characteristics of 123 Study Subjects
| Characteristic | No. |
|---|---|
| Age, y, median (min–max) | 39 (22–60) |
| Sex, male | 53 (43%) |
| Site | |
| Malawi | 40 (33%) |
| Tanzania | 35 (28%) |
| Thailand | 24 (20%) |
| South Africa | 22 (18%) |
| India | 12 (10%) |
| Plasma HIV-1 RNA level, median | 4.34 log10 copies/mL |
| ≥100 000 copies/mL | 22 (18%) |
| CD4 count, median | 164 cells/µL |
| <200 cells/µL | 70 (57%) |
| HIV subtypes | |
| C | 75 |
| AE | 21 |
| A1 | 8 |
| D | 7 |
| R10 | 1 |
| U | 1 |
| Not available | 10 |
| Total continuous NNRTI exposure before entry | |
| >3 years | 66 (54%) |
| 1–3 y | 45 (37%) |
| <1 y | 12 (10%) |
| NNRTI component of prior regimen | |
| EFV | 29 (24%) |
| NVP | 94 (76%) |
| Resistance | |
| ≥1 NNRTI mutation | 120 (98%) |
| ≥1 NRTI mutation | 117 (95%) |
| >1 PI mutation | 2 (1.6%) |
| Thymidine analogue mutationa | |
| TAM1 only | 14 (13%) |
| TAM2 only | 27 (25%) |
| TAM1 + TAM2 | 15 (14%) |
Abbreviations: EFV, efavirenz; HIV, human immunodeficiency virus; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; TAM, thymidine analogue mutation.
a TAMs (M41L, D67N, K70R, L210W, T215Y/F, K219Q/E) were further categorized to TAM1 (M41L, L210W, T215Y) and TAM2 (D67N, K70R, T215F, K219Q/E).
Baseline Resistance and Subtyping
At baseline, all of the 123 subjects had samples available for sequencing; 114 (93%) were successfully sequenced. Nearly all preenrollment samples had mutations associated with drug resistance. Most subjects were found to have mutations associated with reduced susceptibility to 3TC (n = 108 [95%]), EFV (n = 112 [98%]), and NVP (n = 112 [98%]). TAMs were noted in 56 of 108 subjects (52%) with full RT sequencing available: 14 (13%) TAM1, 27 (25%) TAM2, and 15 (14%) with mutations consistent with both TAM1 and TAM2 pathways. Two subjects had low-level, or potentially low-level, resistance to LPV/r linked to L10F and L90M, and V32I and M64L mutation pairs; isolated A71T, L10I, and L10V mutations were observed for 17 participants. Overall, 112 of 114 (98%) demonstrated full susceptibility to LPV/r. Based on genotyping of the PR and partial RT, HIV-1 subtype C was found to be most prevalent (66%), followed by subtypes AE (19%), A1 (7%), and D (6%).
Subject Disposition
Of 123 subjects who entered the study, 117 completed 104 weeks of study follow-up. Four subjects died of conditions unrelated to study treatment (2 with HIV-1 RNA levels <400 copies/mL), and 2 subjects discontinued prematurely. All 117 subjects completing the study remained on study treatment (LPV/r monotherapy or LPV/r with FTC/TDF) through 104 weeks.
Virologic Responses
At weeks 24 and 104, respectively, 107 and 74 subjects remained on LPV/r monotherapy without VF. The estimated probability of remaining on LPV/r monotherapy without VF at 24 weeks was 87% (95% CI, 80%–92%) [7], and 60% at week 104 (95% CI, 50%–68%). By week 104, 49 subjects had met the primary endpoint criteria; 47 had reached VF criteria, and 2 had intensified LPV/r monotherapy without VF. The cumulative probability of VF or discontinuation of LPV/r monotherapy by 104 weeks was 40% (95% CI, 32%–50%). Median HIV-1 RNA level at VF was 1434 (1st–3rd quartile, 696–5495) copies/mL.
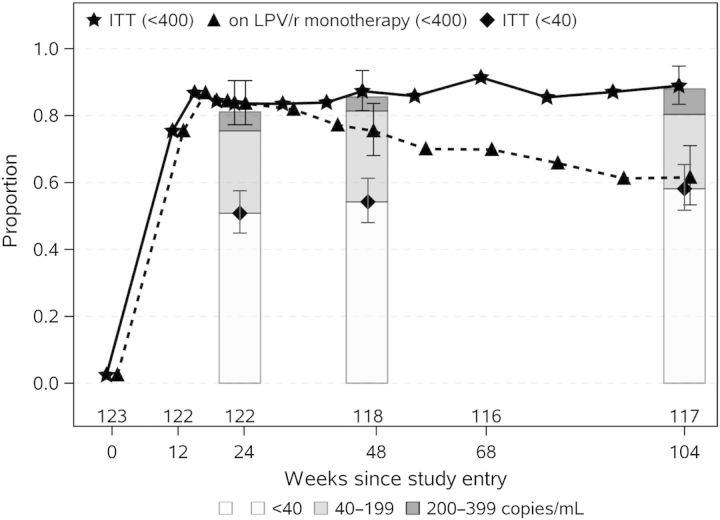
Among the 47 subjects with VF, 41 subjects intensified LPV/r monotherapy with TDF/FTC. Following intensification, 39 of 41 (95%) achieved an HIV-1 RNA level <400 copies/mL. From week 12 onward, >80% of subjects had HIV-1 RNA levels <400 copies/mL (intent to treat [ITT], regardless of intensification); among these, 61%, 62%, and 65% were further suppressed to <40 copies/mL at weeks 24, 48, and 104 respectively (Figure 1). The proportion of subjects with HIV-1 RNA levels <400 copies/mL and on LPV/r monotherapy peaked at 87% at week 16, falling to 83% at week 24 and 75% at week 48; at 2 years, 62% of participants remained on LPV/r monotherapy with HIV-1 RNA levels <400 copies/mL.
Figure 1.
The solid line and stars give the proportion of subjects with human immunodeficiency virus type 1 (HIV-1) RNA level <400 copies/mL over time regardless of prior intensification and thus show the ability of the strategy of lopinavir/ritonavir (LPV/r) monotherapy with intensification as needed to maintain HIV-1 RNA suppression; the dashed line and triangles give the proportion of subjects with HIV-1 RNA level <400 copies/mL and on LPV/r monotherapy and thus give the ability of LPV/r monotherapy to maintain HIV-1 RNA suppression. Stars and triangles are staggered at each week to avoid overlap. Bar charts and point estimates at weeks 24, 48, and 104 give the results of retesting of samples with HIV-1 RNA level <400 copies/mL with the Abbott Real-Time assay with a lower quantification limit of 40 copies/mL; shaded sections of each bar show the proportion of subjects with HIV-1 RNA level <40 copies/mL (white), 40–199 copies/mL (light gray), and 200–399 copies/mL (dark gray). Error bars shown for weeks 24, 48, and 104 estimates give 95% confidence intervals. Abbreviation: ITT, intent to treat.
Resistance at Virologic Failure
PR sequence was obtained from 43 of 47 subjects at time of VF. Nineteen subjects (43%) had PR mutations associated with resistance to LPV/r. Of these, 15 had single mutations not predicted to confer reduced susceptibility to LPV/r by themselves (L10I, L10V, L33F, M46I, I54V, A71T, and V82A). Three subjects had 2 PR resistance mutations; 2 subjects had 1 mutation present at baseline and added a second mutation, and 1 subject developed 2 PR resistance mutations. Of note, 1 subject had evidence of high-level resistance to LPV/r linked to 6 mutations at VF (L10F, L33F, M46I, I54V, V82A, and L90M). This individual was 1 of the 2 subjects with low-level (or potentially low-level) resistance to LPV/r apparent at study entry, and was not observed to achieve HIV-1 RNA <400 copies/mL following intensification. The other subject with evidence of low-level (or potentially low-level) resistance to LPV/r at screening maintained HIV-1 RNA <400 copies/mL on LPV/r monotherapy throughout the study.
Many NRTI- and NNRTI-associated resistance mutations detected at screening were not apparent at VF: 5 of 9 with TAMs, 20 of 27 with M184V, and 5 of 5 with K65R (among subjects with sequence available at screening and at VF). Similar observations were made in subjects with NNRTI resistance mutation at baseline (8/19 with K101E/Q or K103N, 14/18 with Y181C, and 14/21 with other NNRTI mutations). In analyzing the impact of loss of resistance mutations on response to intensification with FTC/TDF, there were 39 subjects with sequences at baseline and at VF who received intensification. Two subjects failed to suppress their HIV-1 RNA level to <400 copies/mL after intensification: 1 subject with no resistance mutations at baseline or VF, and the 1 subject who had persistent TAM1, M184V, and NNRTI mutations and added 6 new PI mutations. All of the 19 subjects who lost NRTI mutations were able to suppress their HIV-1 RNA levels with the addition of FTC/TDF. Six subjects had persistent NRTI mutations, and nonetheless did suppress levels of HIV-1 RNA with intensification.
Predictors of Virologic Failure
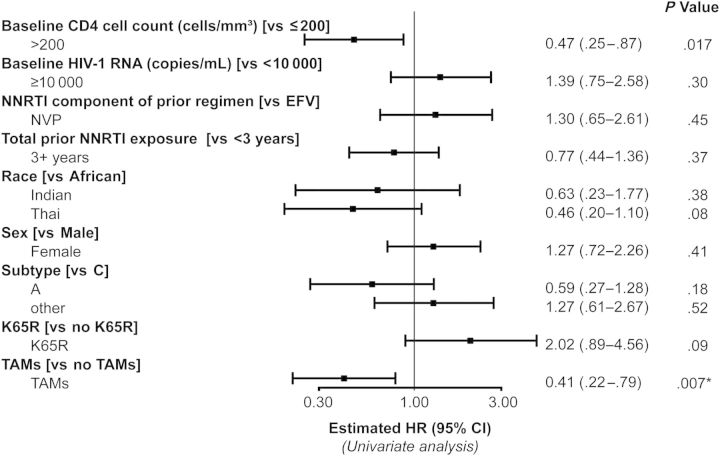
Baseline CD4 count >200 cells/µL was associated with an estimated 53% lower hazard of reaching a primary endpoint (hazard ratio, 0.47 [95% CI, .25–.87]). An association between the presence of NRTI resistance mutations at screening and of hazard of VF was also apparent, although proportional hazards assessment suggested attenuation of this effect over time. Given almost complete confounding between the presence of TAMs (associated with a lower hazard of failure) and presence of K65R (associated with a higher risk of failure), it was not possible to untangle these findings. Some evidence of a lower failure in Thai vs African participants was noted (hazard ratio, 0.46 [95% CI, .20–1.10]; P = .08). No other associations were identified (Figure 2), and no further violations of the proportional hazards assumption were apparent.
Figure 2.
Due to almost complete overlap in the populations with thymidine analogue mutations (TAMs) present and the K65R mutation absent, fully adjusted analyses were not possible. Multivariable analyses that adjusted for TAMs revealed little change in the unadjusted estimates presented. Multivariable analyses that adjusted for K65R revealed additional confounding with nonnucleoside reverse transcriptase inhibitor (NNRTI) use prior to study entry. *P < .05. Abbreviations: CI, confidence interval; EFV, efavirenz; HIV-1, human immunodeficiency virus type 1; HR, hazard ratio.
Immunologic and Clinical Responses
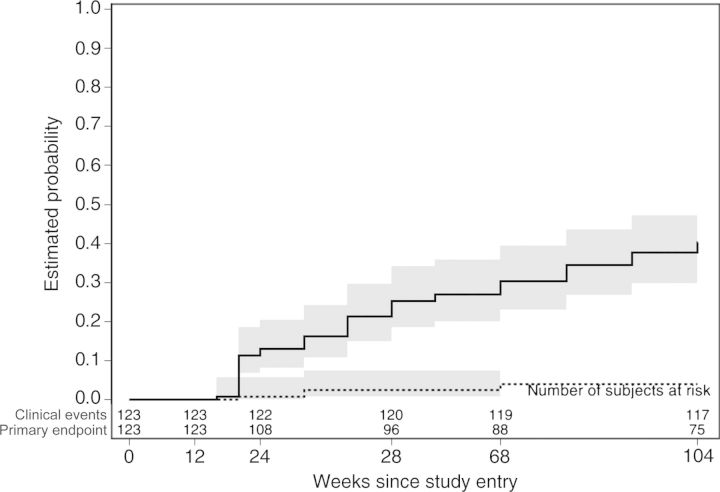
CD4 cell counts increased by a median of 213 (1st–3rd quartile, 111–326) cells/µL over 104 weeks. Four deaths occurred due to invasive cervical carcinoma, myocardial infarction, bacterial pneumonia, and probable cerebral malaria, respectively; all deaths were judged by site investigators as unrelated to study medications. HIV-1 disease progression (defined as meeting WHO stage 4 criteria) or death was rare (Figure 3).
Figure 3.
Failure of lopinavir/ritonavir (LPV/r) monotherapy was defined as confirmed virologic failure (failure to suppress plasma human immunodeficiency virus type 1 [HIV-1] RNA level to <400 copies/mL by week 24, or confirmed rebound of plasma HIV-1 RNA level to >400 copies/mL after confirmed suppression to <400 copies/mL) or discontinuation of LPV/r monotherapy. The solid line represents the study's primary endpoint, defined as virologic failure or LPV/r intensification. The dotted line represents a clinical endpoint, defined as the first occurrence of disease progression (World Health Organization stage 4 event) or death. Shaded areas denote 95% confidence intervals.
Adherence
Adherence levels assessed by self-report were generally high throughout the study; at week 104, 88 (76%) of subjects reported never missing a dose of study medication. Similar patterns of adherence were observed across subjects with and without evidence of VF.
Safety and Tolerability
Fifty subjects (41%) experienced grade 3 or 4 adverse laboratory events on study. The most common laboratory abnormalities included lipid elevations (3 subjects with grade 3 fasting triglycerides, 7 subjects with grade 4 fasting triglycerides, 8 subjects with grade 3 fasting low-density lipoprotein cholesterol, and 11 subjects with grade 3 fasting total cholesterol), elevations in fasting blood sugar (3 subjects with grade 3 and 5 subjects with grade 4), and abnormalities in serum phosphorus (13 subjects with grade 3 hypophosphatemia and 1 with grade 4) (Table 2).
Table 2.
Adverse Events During the Study
| Sign/Symptom/ Laboratory Event | Grade |
No. of Subjects | |
|---|---|---|---|
| 3 | 4 | ||
| Any grade 3 or 4 event | 30 (24%) | 20 (16%) | 50 (41%) |
| Phosphorus | 13 | 1 | 14 (11%) |
| Fasting total cholesterol | 11 | 0 | 11 (9%) |
| Fasting total triglycerides | 3 | 7 | 10 (8%) |
| Fasting blood sugar | 3 | 5 | 8 (7%) |
| Fasting LDL | 8 | 0 | 8 (7%) |
| Absolute neutrophil count | 5 | 2 | 7 (6%) |
| Nonfasting glucose | 4 | 2 | 6 (5%) |
| Hemoglobin | 2 | 2 | 4 (3%) |
| Total bilirubin | 3 | 1 | 4 (3%) |
| Diarrhea/loose stools | 3 | 1 | 4 (3%) |
| Cachexia/wasting/weight loss | 4 | 0 | 4 (3%) |
| SGOT | 1 | 2 | 3 (2.4%) |
| SGPT | 1 | 2 | 3 (2.4%) |
| Ache/pain/discomfort | 2 | 1 | 3 (2.4%) |
Table gives number of subjects with at least 1 report of each given event during study follow-up (on lopinavir/ritonavir monotherapy or following intensification). Grade represents the highest reported grade during all follow-up for the subject. Events were graded by site staff according to the Division of AIDS grading scale. Only events reported in ≥3 subjects are shown; total (first) row includes all events.
Abbreviations: LDL, low-density lipoprotein; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase.
DISCUSSION
The week 104 results of ACTG 5230 extend observations on the strategy of LPV/r monotherapy with intensification as needed for second-line ART in resource-limited settings [7]. After 104 weeks, there was a 60% probability of remaining on LPV/r monotherapy with HIV-1 RNA levels <400 copies/mL. When intensification as needed with FTC/TDF was included, >80% of subjects maintained HIV-1 RNA suppression <400 copies/mL over 2 years. Ultrasensitive assays performed on archived specimens with HIV-1 RNA levels <400 copies/mL revealed that 61%–65% had suppression of HIV-1 RNA to <40 copies/mL. The study was conducted at 5 different sites, with important subtype diversity representative of the global HIV epidemic. The strengths of the study include the low loss to follow-up rate among subjects, and the durability of responses over 104 weeks. These results are promising, and suggest that such a strategy may offer a potent and simplified approach to the management of second-line ART in resource-limited settings.
Other studies of second-line ART in resource-limited settings have demonstrated success in achieving virologic suppression with use of LPV/r plus NRTIs in resource-limited settings [10–12]. A recent study done in Thailand compared LPV/r monotherapy with LPV/r plus 2 NTRIs, and showed that monotherapy was inferior in suppressing HIV-1 RNA levels to <50 copies/mL [11]. The Europe Africa Research Network for Evaluation of Secondline Therapy (EARNEST) trial compared LPV/r monotherapy with LPV/r plus 2 NRTIs, and LPV/r with raltegravir [12]. The authors observed lower rates of virologic suppression in the LPV/r monotherapy arm. However, in ACTG 5230, subjects with VF received intensified treatment with FTC/TDF, an important difference from the Thai and EARNEST studies.
Despite extensive baseline resistance to NRTI, 39 of 41 subjects reached HIV-1 RNA levels <400 copies/mL following intensification, and 80%–85% maintained levels <400 copies/mL over 104 weeks. These responses to intensification were better than expected. Analysis of resistance mutations at baseline and at VF revealed that among most subjects, NRTI and NNRTI resistance mutations were not detectable upon the reemergence of virus replication, a possible explanation for the success of intensification. However, 6 subjects retained NRTI resistance mutations, and yet still were suppressed upon intensification. Fifty-seven percent of the baseline samples were susceptible to TDF [13], suggesting that TDF contributed significantly to the success of intensification. Even among subjects with TDF resistance mutations at baseline (5 with K65R and 2 with Q151M), these mutations were not detected at VF. This finding of retained NRTI activity in the presence of preexisting NRTI resistance after first-line failure is consistent with findings from the second-line trial, EARNEST, and observational datasets [10, 12, 14].
Of the baseline factors examined as associated with failure of LPV/r monotherapy, only low CD4 counts (<200 cells/µL) and preexisting NRTI mutations were identified as significant predictors for the risk of failure. The findings with respect to preexisting NRTI mutations were mixed, with the presence of TAMs at study entry associated with a lower risk and the presence of K65R associated with a higher risk. Given the confounding between TAMs and K65R, no conclusions can be offered regarding their predictive value.
The PR resistance findings at VF do demonstrate an evolving pattern of resistance. Forty-three percent of subjects experiencing VF had PR resistance mutations, similar to the EARNEST study results and higher than reported in previous trials of monotherapy. However, most of these subjects had single mutations not predicted to confer clinical resistance, and only 4 subjects had multiple mutations. Recently, Rabi and colleagues described a multistep mechanism for PI activity associated with viral entry and reverse transcription in which phenotypic PI resistance could be demonstrated in vitro, mediated by changes in the cytoplasmic tail of gp41 [15]. Comparing envelope genes from baseline to VF of the ACTG 5230 envelope may offer insight into the genetic changes associated with VF of LPV/r.
Among subjects with HIV-1 RNA levels <400 copies/mL in ACTG 5230 over time, 61%–65% had levels <40 copies/mL and 35%–39% had levels 40–399 copies/mL. These results are similar to the observations of other trials evaluating LPV/r monotherapy [10–12]. This low-level viremia raises concern about the evolution of drug resistance mutations. The ACTG A5230 results suggest that in the absence of selective drug pressure, drug resistance mutations may diminish, and that the PR resistance consequences of using a strategy of intensification with an HIV-1 RNA threshold of >400 copies/mL are limited.
The durability of virologic suppression in ACTG A5230 over 104 weeks does provide reassurance regarding outcomes associated with low-level viremia. Adverse events included metabolic and phosphorous abnormalities, similar to previous trial results with LPV/r and FTC/TDF [10].
This approach offers the possibility of lowered drug cost by avoiding the inclusion of NRTI, the potential for improved safety at lower cost by reducing the risk of long term NRTI toxicities, and an option for patients in whom NRTIs are contraindicated. Conversely, HIV-1 RNA level monitoring during LPV/r monotherapy to identify persons in need of intensification incurs additional costs. If the strategic approach of LPV/r monotherapy with intensification as needed is evaluated further, cost-effectiveness must be considered.
ACTG A5230 does have important limitations. It is a single-arm, open-label pilot study with a relatively small number of subjects, and therefore it is premature to utilize this strategy in clinical practice. The success of this strategy is contingent on the availability of viral load monitoring, which is frequently not available in resource-limited settings. Alternative boosted PI strategies may represent more attractive options, such as those with once daily dosing or with newer pharmacologic enhancers (cobicistat).
In summary, second-line ART in resource-limited settings with LPV/r monotherapy and FTC/TDF intensification when needed provided durable suppression of HIV-1 RNA level over 104 weeks. LPV/r monotherapy with the option for intensification should be compared to currently recommended 3-drug second-line regimens in fully powered, randomized clinical trials and the cost-effectiveness of the strategy carefully examined.
Notes
Acknowledgments. We thank the study participants and the Clinical Research Site investigators and their staff for support of this study, (Drs Mina Hosseinipour, Agnes Moses, and Albert Mwafongo, Kamuzu Central Hospital, Lilongwe, Malawi; Drs John Crump, Ann Buchanan and Elizabeth Reddy, Kilimanjaro Christian Medical Centre, Moshi, Tanzania; Dr Prudence Ive, University of Witwatersrand, Johannesburg, South Africa; Dr N. Kumarasamy, Dr S. Poongulali, and Beulah Faith, CART Clinical Research Site, YRG CARE Medical Centre, Chennai, India; and Dr Khuanchai Supparatpinyo, Chiang Mai University, Chiang Mai, Thailand). We also thank all members of the ACTG A5230 Protocol Team.
Financial support. This clinical trial was supported by the ACTG, National Institutes of Health (grant numbers U01 AI068634, U01 AI069484, U01 AI068636, U01AI067854, and U01 AI069432). Drug supplies were provided by AbbVie and Gilead Sciences, Inc. Viral failure samples were processed in the Mellors Laboratory, Pittsburgh, Pennsylvania. Additional funding for laboratory tests was provided by AbbVie.
Potential conflicts of interest. M. R. N. is an employee of, and holds stock in, Abbvie. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Global update on HIV treatment 2013: results, impact and opportunities. WHO report in partnership with UNICEF and UNAIDS. Accessed 19 March 2014.
- 2.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, 2013. Available at: www.who.int/hiv/pub/guidelines/arv2013/. Accessed 20 March 2014. [PubMed]
- 3.Shet A, De Costa A, Kumarasamy N, et al. HIVIND Study Team. Effect of mobile telephone reminders on treatment outcome in HIV: evidence from a randomised controlled trial in India. BMJ 2014; 349:g5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumarasamy N, Madhavan V, Venkatesh K, et al. High frequency of clinically significant mutations following first-line generic HAART failure: implications for second line options in resource-limited settings. Clin Infect Dis 2009; 49:306–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosseinipour MC, van Oosterhout JJ, Weigel R, et al. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS 2009; 23:1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sungkanuparph S, Manosuthi W, Kiertiburanakul S, et al. Options for a second-line antiretroviral regimen for HIV type 1–infected patients whose initial regimen of a fixed-dose combination of stavudine, lamivudine, and nevirapine fails. Clin Infect Dis 2007; 44:447–52. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett JA, Ribaudo HJ, Wallis CL, et al. Lopinavir/ritonavir monotherapy after virologic failure of first-line antiretroviral therapy in resource-limited settings. AIDS 2012; 26:1345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee S-Y, Gonzales M, Kantor R, Betts B, Ravela J, Shafer R. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res 2003; 31:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shafer R. Rationale and uses of a public HIV drug-resistance database. J Infect Dis 2006; 194:S51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd MA, Kumarasamy N, Moore CL, et al. Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomized, open-label, non-inferiority study. Lancet 2013; 381:2091–9. [DOI] [PubMed] [Google Scholar]
- 11.Bunupuradah T, Chetchotisakd P, Ananworanich J, et al. A randomized comparison of second-line lopinavir/ritonavir monotherapy versus tenofovir/lamivudine/lopinavir/ritonavir in patients failing NNRTI regimens: the HIV STAR study. Antivir Ther 2012; 17:1351–61. [DOI] [PubMed] [Google Scholar]
- 12.Paton N, Kityo C, Hoppe A, et al. A pragmatic randomised controlled strategy trial of three second-line treatment options for use in public health rollout programme settings: the Europe-Africa Research Network for Evaluation of Second-line Therapy (EARNEST) Trial [abstract WELBB02]. In: 7th International Conference on HIV Pathogenesis, Treatment and Prevention (IAS 2013) Kuala Lumpur, Malaysia: International AIDS Society, 2013. [Google Scholar]
- 13.Wallis CL, Aga E, Ribaudo H, et al. ; A5230 team. Drug susceptibility and resistance mutations after first-line failure in resource limited settings. Clin Infect Dis 2014; 59:706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sigaloff K, Hamers R, Wallis C, et al. Second-line antiretroviral treatment successfully resuppresses drug-resistant HIV-1 after first-line failure: prospective cohort in sub-Saharan Africa. J Infect Dis 2012; 205:1739–44. [DOI] [PubMed] [Google Scholar]
- 15.Rabi SA, Laird G, Durand C, et al. Multi-step inhibition explains HIV-1 protease inhibitor pharmacodynamics and resistance. J Clin Invest 2013; 123:3848–60. [DOI] [PMC free article] [PubMed] [Google Scholar]