Abstract
Using data collected from 9,823 participants in the 2007–2008 and 2009–2010 cycles of the National Health and Nutrition Examination Survey, we formally investigated potentially modifiable factors linking low socioeconomic status (SES) to chronic kidney disease (CKD) for their presence and magnitude of mediation. SES was defined using the poverty income ratio. The main outcome was CKD, defined as estimated glomerular filtration rate <60 mL/minute/1.73 m2 (using the Chronic Kidney Disease Epidemiology Collaboration equation) and/or urinary albumin:creatinine ratio ≥30 mg/g. In mediation analyses, we tested the contributions of health-related behaviors (smoking, alcohol intake, diet, physical activity, and sedentary time), comorbid conditions (diabetes, hypertension, obesity, abdominal obesity, and hypercholesterolemia), and access to health care (health insurance and routine health-care visits) to this association. Except for sedentary time and diet, all examined health-related behaviors, comorbid conditions, and factors related to health-care access mediated the low SES–CKD association and contributed 20%, 32%, and 11%, respectively, to this association. In race/ethnicity-specific analyses, identified mediators tended to explain more of the association between low SES and CKD in non-Hispanic blacks than in other racial/ethnic groups. In conclusion, potentially modifiable factors like health-related behaviors, comorbid conditions, and health-care access contribute substantially to the association between low SES and CKD in the United States, especially among non-Hispanic blacks.
Keywords: chronic kidney disease, mediation, socioeconomic status
Chronic kidney disease (CKD) is a major public health problem. CKD is associated with a number of adverse health outcomes, including end-stage renal disease, cardiovascular mortality, and all-cause mortality (1), and its burden is increasing. In the United States, the prevalence of CKD is estimated to be 15% (2), and lifetime risk of developing CKD is nearly 60% (3). However, the burden of CKD is not equally distributed among populations. Substantial inequality is observed in incidence and prevalence of CKD across socioeconomic (4–6) and racial/ethnic (7–9) groups.
Socioeconomic status (SES) is a major contributor to inequalities in CKD prevalence, and its effects are potentially modifiable. The US national blueprint for public health goals as per Healthy People 2020 (http://www.healthypeople.gov/) explicitly aims for the elimination of socioeconomic health disparities related to kidney disease in the United States by 2020 (10). To reach this aim, the identification of factors linking SES to CKD is pivotal. Adverse health-related behaviors, comorbid conditions, and limited access to health care have been suggested as potential mediators that link low SES to CKD (11). However, to our knowledge, no previous study has formally tested for mediation or estimated the extent to which mediators contribute to socioeconomic disparities in CKD. Consequently, evidence is largely lacking on mediators actually linking SES to CKD and on the extent to which correction for these mediators could mitigate socioeconomic disparities in CKD.
Therefore, we sought to 1) identify mediators (health-related behaviors, comorbid conditions, and health-care access) linking SES and CKD in a US population and 2) determine the extent to which identified mediators contribute to the association between low SES and CKD in the United States.
METHODS
Study design and population
We analyzed National Health and Nutrition Examination Survey (NHANES) data from the years 2007–2008 and 2009–2010. NHANES surveys a cross-sectional, multistage, stratified, clustered probability sample of the noninstitutionalized US civilian population and is conducted by the National Center for Health Statistics (12). Data on participants aged 20 years or older with measured serum creatinine levels were included (n = 10,822). We excluded participants with missing information on income (n = 966) or missing urinary albumin and creatinine measurements (n = 133), leaving 9,823 participants for the final analysis.
SES measurement
Income was used as the primary SES measure. While other SES measures, including education and occupation, capture individual-based dimensions of social position, household income is more indicative of standard of living and access to goods and services. Moreover, income has also been shown to have a stronger association with CKD than education in the United States (4). Information on income was obtained via questionnaire and was recorded as the poverty income ratio (PIR). PIR is calculated by dividing household income by the US federal poverty threshold specific to that family size and year. The PIR is adjusted for family size, composition, and age of the members of a household and is updated annually for inflation (12). For analytical purposes (to maintain a sufficient number of participants in each category), the PIR was divided into tertiles. The lowest (≤1.36), middle (1.37–3.29), and highest (3.30–5.00) PIR tertiles were designated low-, medium-, and high-SES groups, respectively.
Health-related behaviors
Among health-related behaviors, smoking, alcohol intake, diet, physical activity, and sedentary time are generally more prevalent in low-SES groups and are associated with health-related outcomes. Although alcohol intake and diet may not always be related to SES (13), studies in the United States generally have shown an association between alcohol intake/diet and SES (14, 15). We therefore considered them also as potential mediators.
Smoking status was self-reported, and information on smoking was collected during the household interview of adults aged 20 years or older. Participants were classified as current smokers if they reported having smoked at least 100 cigarettes in their lifetime and reported smoking every day or on some days of the week at the time of the interview. All others were defined as nonsmokers (16). Alcohol consumption was assessed by asking participants to quantify the number of alcoholic drinks consumed in the previous 12 months. We categorized alcohol intake (monthly number of any type of alcoholic drink) into 3 categories: 1) none to low (<1 per month), 2) medium (1–19 per month), and 3) high (≥20 per month) (17).
Dietary behavior was characterized as unhealthy if participants reported consuming fruits or vegetables fewer than 5 times per week (18) or if they reported that fruits or vegetables were never or rarely available at home. Since information on actual consumption of fruits and vegetables was not available in NHANES 2007–2008, we used availability of fruits and vegetables at home, which has been shown to be correlated with actual consumption, to assess dietary behavior in this population (19).
Physical activity was measured from self-reported information on recreational physical activity. Participants were classified as physically active if they reported engaging in moderate-to-vigorous recreational physical activity at least 3–5 times per week. Other participants were classified as nonactive (12).
Sedentary time was recorded as the number of hours spent sitting or reclining at work, at home, or at school each day (excluding time spent sleeping) and was categorized as low (≤1 hour/day), medium (2–3 hours/day), or high (≥4 hours/day) (20).
Comorbid conditions
Among comorbid conditions, diabetes, hypertension, obesity, abdominal obesity, and hypercholesterolemia have been shown to be socioeconomically patterned and are associated with poor health. Therefore, we examined them as potentially mediating comorbid conditions in the relationship between low SES and CKD.
Diabetes was defined as a nonfasting glucose level ≥200 mg/dL, the use of oral antidiabetic drugs or insulin, or self-reported diabetes mellitus (21). Blood pressure was measured in accordance with the recommendations of the American Heart Association (22), and hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg, self-reported presence of hypertension, or use of antihypertensive medication (23). Body mass index was calculated as weight (kg) divided by the square of height (m2). Participants were classified as obese if body mass index was ≥30 (24). Abdominal obesity was defined as waist circumference ≥88 cm in women and ≥102 cm in men (25). Hypercholesterolemia was defined as a serum total cholesterol concentration ≥5.2 mmol/L (≥200 mg/dL) or reported current use of cholesterol-lowering medication (26).
Access to health care
Access to health care was assessed in terms of 1) health insurance coverage and 2) self-reported use of health-care services (27). Compared with high-SES groups, people in low-SES groups are more likely to have no health insurance and to experience barriers in the use of health-care services. Because health insurance and routine health-care visits are known determinants of health (28), both were examined as potential meditators of the SES-CKD association.
For health insurance, participants were classified as either having or not having health insurance. Self-reported use of health-care services was assessed from the number of routine health-care visits made in the past year and was categorized as no visits, 1–3 visits, or ≥4 visits (29).
CKD measurement
Serum creatinine measurements for NHANES 2007–2008 were performed by Collaborative Laboratory Services LLC (Hartford, Connecticut). In 2007, creatinine was measured using the Synchron LX20 chemistry analyzer (Beckman Coulter, Inc., Fullerton, California) (30). On the basis of a recommendation by the National Center for Health Statistics, NHANES 2007 serum creatinine values were reduced by 0.08 mg/dL to convert them to standardized creatinine values (31). From 2008 onwards, serum creatinine measurements were performed using the UniCel DxC 800 Synchron Clinical System (Beckman Coulter, Inc.) and were standardized according to National Kidney Disease Education Program guidelines (32). Estimated glomerular filtration rate (eGFR) was determined using the creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation (33). Urinary albumin and creatinine levels were measured using enzymatic methods in spot urine samples collected during the examination. Urinary albumin:creatinine ratio (measured in mg/g) was calculated as urine albumin concentration divided by urine creatinine concentration. CKD was defined as eGFR <60 mL/minute/1.73 m2 and/or urinary albumin:creatinine ratio ≥30 mg/g (34).
Statistical analysis
Mediation analysis was performed according to the method advised by Lange et al. (35) for multiple mediators, which allows decomposition of the total effect of an exposure into natural direct and indirect effects regardless of the underlying statistical model. This approach is flexible to potential biases that exist in traditional mediation analysis resulting from exposure-mediator interaction and from confounding in exposure-outcome, exposure-mediator, and mediator-outcome relationships (35). Point estimates for the natural direct effect and the natural indirect effects related to the mediators are obtained by means of a weighted regression of the outcome on the exposure, the confounders, and additional counterfactual variables. Additional counterfactual variables are the exposure variables generated in an extended set of the original data in which exposure takes its opposite (“counterfactual”) value. An extended data set is constructed by repeating each observation in the original data set xk times, where x refers to the number of levels of exposure and k refers to the number of mediators (e.g., in the case of a binary exposure and 2 mediators, each observation is repeated 22 = 4 times). The weights are determined by
with P deriving from the logistic regression (binomial in the case of binary variables and multinomial in the case of multicategory variables) of the mediator (M) on the exposure (A) and the confounders (C). i refers to row i in the extended data set. A weighted regression model yields odds ratios for the levels of exposure that serve as estimates for the natural direct effect and indirect effects. The product of the odds ratios yields the odds ratio for the total effect (i.e., direct and indirect jointly). Standard errors and confidence intervals are determined by bootstrap methods.
In each domain of mediators, the status “potential mediator” for an individual variable was established on the basis of directed acyclic graphs after consensus among all authors (Figure 1). In the case of multiple mediators within a domain, the assumption that the mediators are independent of each other is tested by adding mediators one by one in the mediator models and subsequently testing the significance of their association. Any exposure-mediator interaction is adjusted for in the analysis. Given that the various domains of mediators may be causally linked, such as behavioral mediators causing comorbidity mediators, we examined each domain of mediators separately (35). We first explored mediation from the domain of health-related behaviors in the SES-CKD association. Similarly, direct and indirect effects were obtained for comorbid conditions and factors related to health-care access. While assessing mediation, only age, sex, and race were considered as confounders and were included as covariates in the final models. For multicategory variables, we present results for the contrast between the highest and lowest categories.
Figure 1.
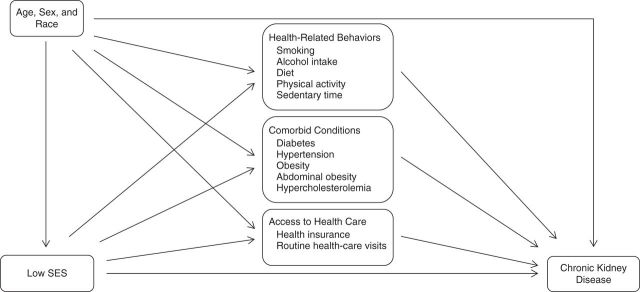
Pathways assumed for the domains of mediators between low socioeconomic status (SES) and chronic kidney disease.
In descriptions of the mediation analyses, we use the term “effect” rather than “association” to be consistent with terminology used in mediation analysis within the counterfactual framework, although we realize that the analyses were cross-sectional and no conclusions can be drawn with regard to the direction of causality.
Previous studies observed racial differences in the association between SES and CKD (6, 36); therefore, we also assessed whether findings differed by race/ethnicity. Considering that urinary albumin:creatinine ratio may be increased temporarily, we performed a sensitivity analyses for low eGFR alone (eGFR <60 mL/minute/1.73 m2).
To achieve representativeness for the US population, we used the recommended 4-year sampling weights for NHANES 2007–2010, by calculating the mean of the weights for NHANES 2007–2008 and the weights for NHANES 2009–2010 (12). Sampling weights and mediation weights were multiplied, and their multiplication was incorporated into the analyses. A P value less than 0.05 was considered to indicate statistical significance. All analyses were conducted using STATA software, version 12.0 (StataCorp LP, College Station, Texas).
RESULTS
Baseline characteristics
Baseline characteristics of the study population by tertile of SES are shown in Table 1. Participants in the low-SES tertile were younger and less likely to be male. Non-Hispanic whites comprised the majority of participants in the high-SES tertile, while non-Hispanic blacks and Mexican Americans comprised a greater proportion of participants in the low-SES tertile. The proportions of participants with adverse health-related behaviors, such as current smoking, high alcohol intake, no leisure-time physical activity, high sedentary time, and unhealthy diet, were higher in participants with low SES than in participants with high SES. The same was true for all comorbid conditions (i.e., diabetes, hypertension, obesity, abdominal obesity, and hypercholesterolemia) and for having no health insurance and no routine health-care visits in the past year. Associations of health-related behaviors, comorbid conditions, and factors related to health-care access with SES are shown for the overall population and for each racial/ethnic group in Web Table 1 (available at http://aje.oxfordjournals.org/).
Table 1.
Baseline Characteristics of the Study Population According to Socioeconomic Status, National Health and Nutrition Examination Survey, 2007–2008 and 2009–2010a
| Characteristic | Total (n = 9,823) |
Socioeconomic Statusb |
P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low (n = 3,289) |
Medium (n = 3,262) |
High (n = 3,272) |
|||||||
| % | Median (IQR) | % | Median (IQR) | % | Median (IQR) | % | Median (IQR) | ||
| Age, years | 49 (35–64) | 44 (31–61) | 51 (36–68) | 51 (38–63) | <0.001 | ||||
| Male sex | 49.0 | 44.5 | 49.9 | 52.7 | <0.001 | ||||
| Race/ethnicity | <0.001 | ||||||||
| Non-Hispanic white | 50.1 | 39.9 | 46.7 | 63.5 | |||||
| Non-Hispanic black | 18.0 | 17.9 | 20.2 | 15.9 | |||||
| Mexican-American | 17.2 | 24.2 | 18.8 | 8.5 | |||||
| Otherc | 14.7 | 17.9 | 14.3 | 12.1 | |||||
| Less than high school education | 35.1 | 55.1 | 34.2 | 10.8 | <0.001 | ||||
| Current smokingd | 22.1 | 32.9 | 19.1 | 14.3 | <0.001 | ||||
| High alcohol intakee | 20.4 | 26.3 | 21.1 | 14.7 | <0.001 | ||||
| Physical inactivityf | 53.4 | 67.3 | 57.8 | 41.2 | <0.001 | ||||
| Unhealthy dietg | 76.7 | 82.5 | 79.3 | 72.4 | <0.001 | ||||
| Sedentary time ≥4 hours/day | 32 | 41 | 33 | 24 | <0.001 | ||||
| Obesityh | 40.6 | 46.9 | 41.5 | 35.8 | <0.001 | ||||
| Abdominal obesityi | 51 | 58.7 | 51.9 | 40.5 | <0.001 | ||||
| Hypertension | 21.2 | 23.3 | 23.1 | 18.4 | <0.001 | ||||
| Diabetes mellitus | 14.6 | 17.4 | 15.1 | 11.5 | <0.001 | ||||
| Hypercholesterolemia, ≥6.2 mmol/L | 14.3 | 14.4 | 14.8 | 13.6 | 0.04 | ||||
| Health insurance | 23.5 | 39.3 | 23.9 | 7.3 | <0.001 | ||||
| No health-care visits in last year | 15.9 | 21.5 | 16.2 | 9.2 | <0.001 | ||||
| Serum creatinine level, mg/dL | 0.84 (0.72–1.01) | 0.82 (0.69–0.96) | 0.83 (0.72–1.02) | 0.88 (0.74–1.02) | 0.006 | ||||
| eGFR, mL/minute/1.73 m2 | 95.7 (78.4–111.1) | 92.6 (77.1–106.4) | 95.3 (75.5–111.6) | 101.5 (83.9–115.9 | <0.001 | ||||
| Low eGFR, <60 mL/minute/1.73 m2 | 9.2 | 8.7 | 7.7 | <0.001 | |||||
| Albumin:creatinine ratio, mg/g | 7.0 (4.5–13.6) | 7.7 (4.7–6.1) | 7.2 (4.5–14.4) | 6.3 (4.2–10.9) | <0.001 | ||||
| Moderate albuminuria, ≥30 mg/g | 12.1 | 13.1 | 9.2 | <0.001 | |||||
| Chronic kidney disease | 18.4 | 17.5 | 14.8 | <0.001 | |||||
Abbreviations: eGFR, estimated glomerular filtration rate; IQR, interquartile range.
a There was no significant difference in age and sex between the participants who were excluded because of missing income information and the final study participants.
b Socioeconomic status was defined as tertile of poverty income ratio (≤1.36 (low), 1.37–3.29 (middle), or 3.30–5.00 (high)). The poverty income ratio is calculated by dividing household income by the US federal poverty threshold.
c “Other” included other Hispanics as well as persons of other ethnicities, including multiracial individuals.
d Having smoked ≥100 cigarettes in one's lifetime and smoking every day or on some days of the week at the time of the interview.
e Having consumed ≥20 alcoholic drinks per month in the previous 12 months.
f Engaging in moderate-to-vigorous recreational physical activity less than 3 times per week.
g Consuming fruits or vegetables fewer than 5 times per week or reporting that fruits or vegetables were never or rarely available at home.
h Body mass index (weight (kg)/height (m)2) ≥30.
i Waist circumference ≥88 cm in women and ≥102 cm in men.
SES, CKD, and mediating factors
The relationship between low SES and CKD was statistically significant after adjustment for age, sex, and race (P < 0.001). Table 2 shows that changing SES from the highest level to the lowest level increased the odds of CKD (odds ratio = 1.64, 95% confidence interval: 1.42, 1.89). While assessing health-related behaviors, the natural direct and indirect effects operating via smoking, high alcohol intake, and physical activity pathways were statistically significant. The indirect effects operating via sedentary time and diet were not statistically significant. Smoking, alcohol intake, and physical inactivity contributed 8%, 7%, and 4%, respectively, to the total effect of low SES on CKD. Twenty percent of the total effect of SES on CKD was explained by health-related behaviors. Regarding comorbid conditions, the direct as well as indirect effects operating via diabetes, hypertension, obesity, abdominal obesity, and hypercholesterolemia were significant. Diabetes and hypertension explained 13% and 7% of the association between low SES and CKD, respectively; obesity and abdominal obesity explained 4% each; and hypercholesterolemia explained 3%. Comorbid conditions together explained 32% of the association between low SES and CKD. Among factors related to health-care access, the indirect effects operating via the health insurance pathway and health-care visits were significant and contributed to the association between low SES and CKD by 6% and 5%, respectively. Factors related to health-care access together contributed 11% to the SES-CKD association.
Table 2.
Natural Direct and Indirect Effects (Odds Ratio Scale) of Socioeconomic Status on Chronic Kidney Disease Operating via Health-Related Behaviors, Comorbid Conditions, and Access to Health Care, National Health and Nutrition Examination Survey, 2007–2008 and 2009–2010a
| Potential Mediator | Odds Ratio | 95% CI | % Mediated | 95% CI |
|---|---|---|---|---|
| Health-related behaviors | ||||
| Direct effect | 1.49b | 1.24, 1.74 | 80b | 69, 95 |
| Indirect effect of: | ||||
| Smoking | 1.04b | 1.01, 1.07 | 8b | 3, 11 |
| Alcohol intakec | 1.04b | 1.01, 1.08 | 7b | 2, 12 |
| Physical activity | 1.02b | 1.00, 1.05 | 4b | 1, 7 |
| Sedentary timec | 1.01 | 0.98, 1.04 | 3 | −5, 9 |
| Dietc | 0.99 | 0.95, 1.03 | 1 | −6, 11 |
| All togethere | 1.10b | 1.04, 1.17 | 20b | 15, 26 |
| Total effectf | 1.64b | 1.42, 1.89 | ||
| Comorbid conditions | ||||
| Direct effect | 1.41b | 1.22, 1.62 | 68 | 57, 83 |
| Indirect effect of: | ||||
| Diabetes | 1.07b | 1.03, 1.11 | 13b | 8, 21 |
| Hypertension | 1.04b | 1.03, 1.07 | 7b | 4, 9 |
| Obesity | 1.02b | 1.00, 1.05 | 4b | 1, 7 |
| Abdominal obesity | 1.02b | 1.00, 1.04 | 4b | 1, 8 |
| Hypercholesterolemia | 1.01b | 1.00, 1.03 | 3b | 1, 6 |
| All together | 1.16b | 1.11, 1.21 | 32b | 25, 40 |
| Total effect | 1.64b | 1.42, 1.89 | ||
| Access to health care | ||||
| Direct effect | 1.56b | 1.35, 1.77 | 89b | 80, 99 |
| Indirect effect of: | ||||
| Health insurance | 1.03b | 1.00, 1.05 | 6b | 1, 11 |
| Health-care visits | 1.02b | 1.00, 1.04 | 5b | 1, 10 |
| Both together | 1.05b | 1.02, 1.08 | 11b | 4, 18 |
| Total effect | 1.64b | 1.42, 1.89 |
Abbreviations: CI, confidence interval; SES, socioeconomic status.
a Results are shown for high SES versus low SES, with SES defined as tertile of poverty income ratio (≤1.36 (low), 1.37–3.29 (middle), or 3.30–5.00 (high)). The poverty income ratio is calculated by dividing household income by the US federal poverty threshold.
b P < 0.05.
c Mediation via high alcohol intake, high sedentary time, or unhealthy diet (see Table 1).
e Indirect effect when all of the above examined mediators were added together in the model.
f Direct effect × indirect effects.
Conditional on age, sex, and SES, each of the mediators was independent of the other mediators within each domain of mediators (P < 0.05). Similarly, none of the mediators of interest interacted with SES regarding its association with CKD (P < 0.05).
The association of SES with CKD differed significantly by racial/ethnic group (P < 0.001 for interaction). Therefore, analyses stratified by racial/ethnic group were performed. Indirect effects and percentages of mediation for various potential mediators of the low SES–CKD association are shown by racial/ethnic group in Figures 2–5. Identified health-related behaviors together contributed 29%, 33%, 17%, and 13% to the association between SES and CKD in non-Hispanic whites, non-Hispanic blacks, Mexican Americans, and other racial/ethnic groups, respectively, and comorbid conditions together contributed 28%, 38%, 21%, and 24%, respectively. The percentage of mediation from factors related to health-care access was 8% in non-Hispanic whites, 10% in non-Hispanic blacks, 9% in Mexican Americans, and 5% in other racial/ethnic groups.
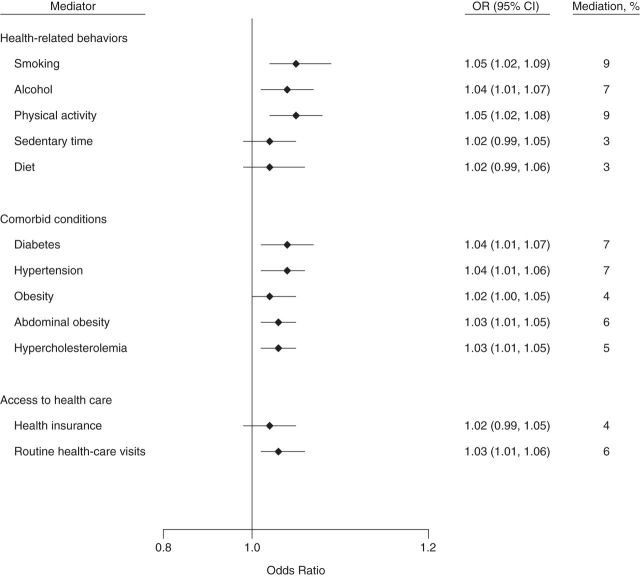
Figure 3.
Indirect effects of and percentage of mediation from potential mediators of the socioeconomic status–chronic kidney disease association among non-Hispanic blacks in the National Health and Nutrition Examination Survey, 2007–2008 and 2009–2010. Direct and indirect effects (on the odds ratio scale): for health-related behaviors, odds ratio (OR) = 1.47 (95% confidence interval (CI): 1.05, 1.96) and OR = 1.19 (95% CI: 1.14, 1.25), respectively; for comorbid conditions, OR = 1.43 (95% CI: 1.02, 1.83) and OR = 1.23 (95% CI: 1.17, 1.30), respectively; and for access to health care, OR = 1.66 (95% CI: 1.20, 2.12) and OR = 1.06 (95% CI: 1.02, 1.10), respectively. Confidence intervals that include 1 indicate no statistical significance.
Figure 4.
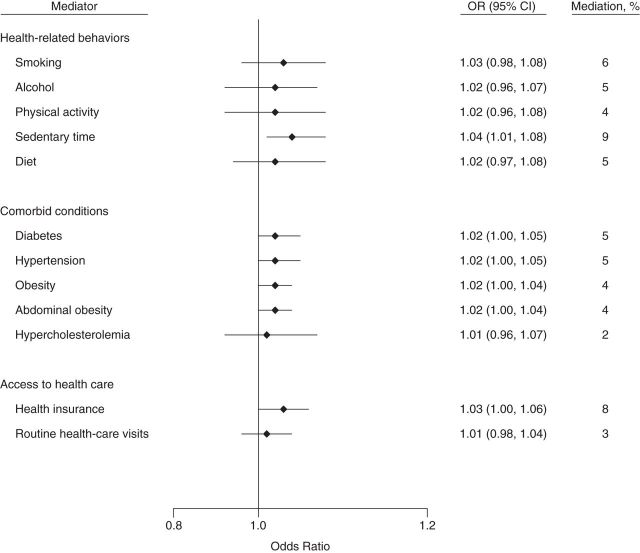
Indirect effects of and percentage of mediation from potential mediators of the socioeconomic status–chronic kidney disease association among Mexican Americans in the National Health and Nutrition Examination Survey, 2007–2008 and 2009–2010. Direct and indirect effects (on the odds ratio scale): for health-related behaviors, odds ratio (OR) = 1.37 (95% confidence interval (CI): 1.03, 1.96) and OR = 1.06 (95% CI: 1.02, 1.10), respectively; for comorbid conditions; OR = 1.35 (95% CI: 1.01, 1.67) and OR = 1.08 (95% CI: 1.04, 1.12), respectively; and for access to health care, OR = 1.42 (95% CI: 1.04, 1.89) and OR = 1.03 (95% CI: 1.00, 1.06), respectively. Confidence intervals that include 1 indicate no statistical significance.
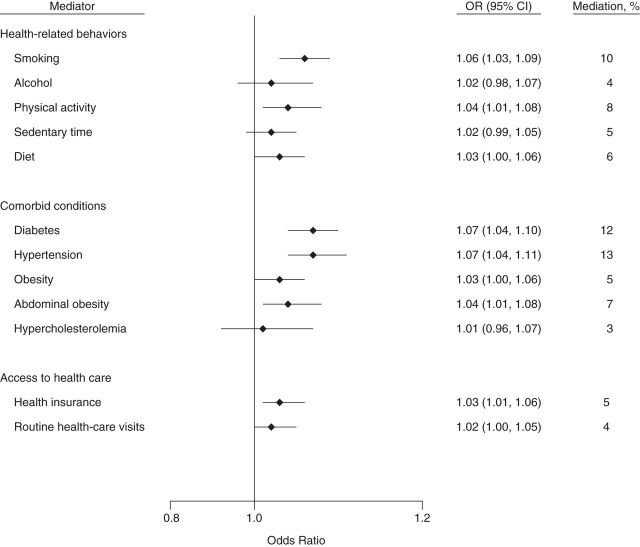
Figure 2.
Indirect effects of and percentage of mediation from potential mediators of the socioeconomic status–chronic kidney disease association among non-Hispanic whites in the National Health and Nutrition Examination Survey, 2007–2008 and 2009–2010. Direct and indirect effects (on the odds ratio scale): for health-related behaviors, odds ratio (OR) = 1.44 (95% confidence interval (CI): 1.15, 1.75) and OR = 1.15 (95% CI: 1.10, 1.20), respectively; for comorbid conditions, OR = 1.46 (95% CI: 1.11, 1.80) and OR = 1.13 (95% CI: 1.08, 1.19), respectively; and for access to health care, OR = 1.56 (95% CI: 1.29, 1.93) and OR = 1.04 (95% CI: 1.01, 1.06), respectively. Confidence intervals that include 1 indicate no statistical significance.
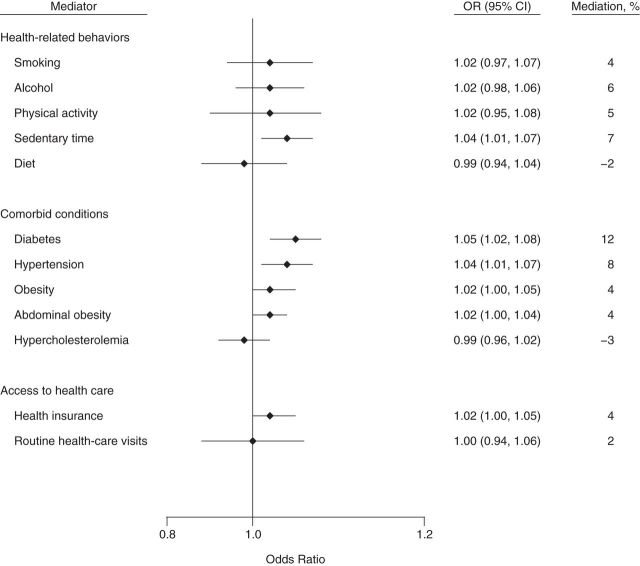
Figure 5.
Indirect effects of and percentage of mediation from potential mediators of the socioeconomic status–chronic kidney disease association among other racial/ethnic groups in the National Health and Nutrition Examination Survey, 2007–2008 and 2009–2010. Direct and indirect effects (on the odds ratio scale): for health-related behaviors, odds ratio (OR) = 1.45 (95% confidence interval (CI): 1.05, 1.90) and OR = 1.06 (95% CI: 1.02, 1.10), respectively; for comorbid conditions, OR = 1.39 (95% CI: 1.00, 1.79) and OR = 1.10 (95% CI: 1.05, 1.15), respectively; and for access to health care, OR = 1.51 (95% CI: 1.08, 1.95) and OR = 1.02 (95% CI: 1.00, 1.05), respectively. Confidence intervals that include 1 indicate no statistical significance.
Results of sensitivity analyses for low eGFR alone (eGFR <60 mL/minute/1.73 m2) were essentially similar to the overall results (Web Table 2).
DISCUSSION
In this study, we examined a nationally representative sample of the US population to identify mediators of the association between low SES and CKD. We found that all examined health-related behaviors, comorbid conditions, and factors related to health-care access mediated the SES-CKD association, except for sedentary time and diet. Identified mediators contributed substantially to this association. Analyses stratified by racial/ethnic group revealed differential mediation across groups. The identified mediators tended to explain more of the SES-CKD association in non-Hispanic blacks than in other racial/ethnic groups.
Earlier studies quantified the role of modifiable risk factors in the associations of low SES with diabetes mellitus (37, 38) and overall mortality (39) and examined the contribution of obesity and the metabolic syndrome to reduced kidney function among low-SES individuals (40). In these studies, mediators explained 12%–94% of the socioeconomic disparities. In our study, the extent of mediation from smoking and obesity (factors also examined in previous studies) falls within the range observed in previous studies (38–41). It should be noted, however, that this wide range suggests heterogeneity of this association across study populations and outcomes (41).
Using the mediation analysis approach of Lange et al. (35), we were able to account for 2 of the main sources of potential bias which might occur because of any exposure-mediator interaction and mediator-outcome confounding. This approach is based upon the assumption of “nonintertwined” pathways—that is, that none of the mediators has a causal association with another. Because health-related behaviors are likely to be causally related to comorbid conditions (e.g., smoking causing hypertension (42)), we examined health-related behaviors, comorbid conditions, and factors related to limited health-care access separately for their mediating role in the SES-CKD association. Mediating factors within each domain of mediators might also be causally linked (e.g., smokers may more often drink alcohol and obesity might be causally linked to diabetes). Moreover, each of these mediating factors may form a distinct pathway between SES and CKD (43). To identify distinct causal pathways and to test the mediating role of each mediator, we examined all mediators within a domain simultaneously. Statistical testing indicated that each of the mediators within a domain was not causally related to other mediators. Although this does not guarantee that the causal pathways are indeed nonintertwined, it indicates that mediators within a domain were at least partially independent of each other. Therefore, the possibility of a causal link between mediators is unlikely to have resulted in any meaningful bias in identifying mediators in our study. In analysis of the overall population, the majority of the examined factors mediated the association between low SES and CKD and contributed substantially to this association, but sedentary time and diet did not appear to be mediators. In racial/ethnic group–specific analysis, though, mediation from sedentary time was significant in Mexican Americans and “other” racial/ethnic groups, and mediation from diet was significant in non-Hispanic blacks.
We found that the identified mediators, comorbid conditions in particular, explained the relatively higher proportion of the association between low SES and CKD in non-Hispanic blacks than in other racial/ethnic groups. Non-Hispanic blacks have steeper socioeconomic gradients in some of the identified mediators (e.g., physical inactivity and poor diet (41), diabetes (44), hypertension (45, 46), and limited access to health care (47)) than other racial/ethnic groups.
In separate analyses by racial/ethnic group, not all of the examined factors mediated the SES-CKD association. Sedentary time, diet, and health insurance were not mediators in non-Hispanic whites, and alcohol intake, sedentary time, and hypercholesterolemia were not mediators in non-Hispanic blacks. In Mexican Americans, only sedentary time, diabetes, hypertension, obesity, and health insurance appeared as mediators. The differential mediation across racial/ethnic groups might be a chance finding, but it may also be that these factors have differential influences across racial/ethnic groups. For instance, a lack of health insurance has been shown to more often be temporary in non-Hispanic whites than in other ethnic groups, which can weaken its mediating power (48). Similarly, in Mexican Americans, a weak or nonexistent relationship between low SES and health-related behaviors (e.g., smoking and alcohol intake) has been reported (14).
Some limitations of our study warrant consideration. First, the cross-sectional nature of the NHANES data did not allow longitudinal assessment of mediators which might be better suited to explaining the SES-CKD association, particularly for mediators that change over time, such as health-related behaviors. In addition, reverse causation might be possible in some scenarios—for example, CKD could contribute to hypertension and severe CKD might influence income. In our study, however, fewer than 5% of the CKD patients had severe CKD (eGFR <30 mL/minute/1.73 m2), the stage of CKD at which patients may experience inability to work. Therefore, this scenario is unlikely to have affected our findings. In the future, however, investigators should explore the role of potential mediators in longitudinal studies as well. Second, we did not assess mediation by psychological factors, such as depression, which have also been suggested to link SES with adverse health conditions, including CKD (49). However, the effect of psychosocial factors on CKD may be mediated by health-related behaviors. For example, distress and/or anxiety associated with socioeconomic disadvantage leads to more smoking, higher alcohol intake, and lower physical activity (50, 51). Therefore, testing for mediation by health-related behaviors may have largely accounted for the mediation from psychological factors. Third, in NHANES 2007–2008, information on healthy diet was recorded as availability of fruits and vegetables at home rather than their actual consumption (as in NHANES 2009–2010). This may have influenced the measured extent of mediation by healthy diet in the association between low SES and CKD. However, sensitivity analyses did not show a significant difference in attenuation for the 2 measures of healthy diet in the NHANES surveys (data not shown), suggesting that this did not affect our findings. Finally, we measured physical activity from recreational physical activity only. Recreational physical activity is a commonly used measure of physical activity, though it might not be able to capture level of physical activity from other sources (e.g., physical activities associated with work and transport).
This study had 3 major strengths. First, to our knowledge, it was the first study to formally test mediation of the SES-CKD association by health-related behaviors, comorbid conditions, and health-care access and to estimate the extent to which these factors contributed to that association in a large, nationally representative sample of the US adult population. Second, we formally investigated mediation in the SES-CKD relationship using a recommended statistical method (35) which is amenable to potential biases that exist in the traditional mediation analysis proposed by Baron and Kenny (52–54). Third, unlike previous studies (6, 37, 39), we added a degree of precision to the extent of mediation by providing a confidence interval around the percentage of attenuation, which is often expressed simply as a percentage.
Our study may have important public health and policy implications. By understanding the mediating factors that explain the relationship between SES and CKD, it becomes possible to design interventions targeting modifiable health risk behaviors, existing comorbid conditions, and barriers to health care in order to reduce SES inequalities in CKD prevalence. The effect of these interventions may be most beneficial for non-Hispanic blacks.
In conclusion, our study provides further evidence of a link between socioeconomic inequality and CKD and shows that adverse health-related behaviors, existing comorbid conditions, and limited access to health care are pathways through which social adversity may lead to CKD. Identified mediators contribute substantially to the association between low SES and CKD in the United States, especially among non-Hispanic blacks, and may be suitable targets for interventions aimed at reducing disparities in CKD.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Community and Occupational Medicine, Department of Health Sciences, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands (Priya Vart, Sijmen A. Reijneveld, Ute Bültmann); Division of Nephrology, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands (Ron T. Gansevoort); Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Medical Institutions, Baltimore, Maryland (Deidra C. Crews); and Division of Nephrology, Department of Medicine, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Deidra C. Crews).
No specific funding was received for this work. P.V., R.T.G., S.A.R., and U.B. received funding from their respective institutions. D.C.C. was supported by grant K23DK097184 from the National Institute of Diabetes and Digestive and Kidney Diseases and by the Gilbert S. Omenn Anniversary Fellowship of the Institute of Medicine. The National Health and Nutrition Examination Survey is funded by the US Department of Health and Human Services.
The funding bodies played no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15(5):1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 2.Collins AJ, Foley RN, Chavers B, et al. United States Renal Data System 2011 Annual Data Report: atlas of chronic kidney disease and end-stage renal disease in the United States. Am J Kidney Dis. 2012;59(1 suppl 1):e1–e420. doi: 10.1053/j.ajkd.2011.11.015. A7. [DOI] [PubMed] [Google Scholar]
- 3.Grams ME, Chow EK, Segev DL, et al. Lifetime incidence of CKD stages 3–5 in the United States. Am J Kidney Dis. 2013;62(2):245–252. doi: 10.1053/j.ajkd.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vart P, Gansevoort RT, Coresh J, et al. Socioeconomic measures and CKD in the United States and the Netherlands. Clin J Am Soc Nephrol. 2013;8(10):1685–1693. doi: 10.2215/CJN.12521212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merkin SS, Diez Roux AV, Coresh J, et al. Individual and neighborhood socioeconomic status and progressive chronic kidney disease in an elderly population: the Cardiovascular Health Study. Soc Sci Med. 2007;65(4):809–821. doi: 10.1016/j.socscimed.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Crews DC, McClellan WM, Shoham DA, et al. Low income and albuminuria among REGARDS (Reasons for Geographic and Racial Differences in Stroke) study participants. Am J Kidney Dis. 2012;60(5):779–786. doi: 10.1053/j.ajkd.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruce MA, Beech BM, Crook ED, et al. Association of socioeconomic status and CKD among African Americans: the Jackson Heart Study. Am J Kidney Dis. 2010;55(6):1001–1008. doi: 10.1053/j.ajkd.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norris K, Nissenson AR. Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol. 2008;19(7):1261–1270. doi: 10.1681/ASN.2008030276. [DOI] [PubMed] [Google Scholar]
- 9.Tarver-Carr ME, Powe NR, Eberhardt MS, et al. Excess risk of chronic kidney disease among African-American versus white subjects in the United States: a population-based study of potential explanatory factors. J Am Soc Nephrol. 2002;13(9):2363–2370. doi: 10.1097/01.asn.0000026493.18542.6a. [DOI] [PubMed] [Google Scholar]
- 10.Agency for Healthcare Research and Quality, US Department of Health and Human Services. 2010 National Healthcare Quality and Disparities Reports. Rockville, MD: Agency for Healthcare Research and Quality; 2011. [Google Scholar]
- 11.Patzer RE, McClellan WM. Influence of race, ethnicity and socioeconomic status on kidney disease. Nat Rev Nephrol. 2012;8(9):533–541. doi: 10.1038/nrneph.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention, US Department of Health and Human Services. National Health and Nutrition Examination Survey (NHANES). Analytic and reporting guidelines. http://www.cdc.gov/nchs/nhanes/analytic_guidelines.htm. Published September 2013. Updated January 16, 2014. Accessed April 15, 2014.
- 13.van Oers JA, Bongers IM, van de Goor LA, et al. Alcohol consumption, alcohol-related problems, problem drinking, and socioeconomic status. Alcohol Alcohol. 1999;34(1):78–88. doi: 10.1093/alcalc/34.1.78. [DOI] [PubMed] [Google Scholar]
- 14.Lantz PM, House JS, Lepkowski JM, et al. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA. 1998;279(21):1703–1708. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- 15.Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87(5):1107–1117. doi: 10.1093/ajcn/87.5.1107. [DOI] [PubMed] [Google Scholar]
- 16.Clair C, Bitton A, Meigs JB, et al. Relationships of cotinine and self-reported cigarette smoking with hemoglobin A1c in the U.S.: results from the National Health and Nutrition Examination Survey, 1999–2008. Diabetes Care. 2011;34(10):2250–2255. doi: 10.2337/dc11-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freiberg MS, Cabral HJ, Heeren TC, et al. Alcohol consumption and the prevalence of the metabolic syndrome in the US.: a cross-sectional analysis of data from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2954–2959. doi: 10.2337/diacare.27.12.2954. [DOI] [PubMed] [Google Scholar]
- 18.Andaya AA, Arredondo EM, Alcaraz JE, et al. The association between family meals, TV viewing during meals, and fruit, vegetables, soda, and chips intake among Latino children. J Nutr Educ Behav. 2011;43(5):308–315. doi: 10.1016/j.jneb.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neumark-Sztainer D, Wall M, Perry C, et al. Correlates of fruit and vegetable intake among adolescents: findings from Project EAT. Prev Med. 2003;37(3):198–208. doi: 10.1016/s0091-7435(03)00114-2. [DOI] [PubMed] [Google Scholar]
- 20.Sisson SB, Camhi SM, Church TS, et al. Leisure time sedentary behavior, occupational/domestic physical activity, and metabolic syndrome in U.S. men and women. Metab Syndr Relat Disord. 2009;7(6):529–536. doi: 10.1089/met.2009.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Resnick HE, Foster GL, Bardsley J, et al. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999−2002: the National Health and Nutrition Examination Survey. Diabetes Care. 2006;29(3):531–537. doi: 10.2337/diacare.29.03.06.dc05-1254. [DOI] [PubMed] [Google Scholar]
- 22.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals. Part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 23.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. Geneva, Switzerland: World Health Organization; 1995. (WHO Technical Report Series no. 854) [PubMed] [Google Scholar]
- 25.Yusuf S, Anand S. Body-mass index, abdominal adiposity, and cardiovascular risk. Lancet. 2011;378(9787):226–227. doi: 10.1016/S0140-6736(11)61120-3. [DOI] [PubMed] [Google Scholar]
- 26.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 27.Ostchega Y, Hughes JP, Wright JD, et al. Are demographic characteristics, health care access and utilization, and comorbid conditions associated with hypertension among US adults? Am J Hypertens. 2008;21(2):159–165. doi: 10.1038/ajh.2007.32. [DOI] [PubMed] [Google Scholar]
- 28.Ward MM. Access to care and the incidence of end-stage renal disease due to diabetes. Diabetes Care. 2009;32(6):1032–1036. doi: 10.2337/dc09-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Bullard KM, Gregg EW, et al. Access to health care and control of ABCs of diabetes. Diabetes Care. 2012;35(7):1566–1571. doi: 10.2337/dc12-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myers GL, Miller WG, Coresh J, et al. National Kidney Disease Education Program Laboratory Working Group. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52(1):5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 31.National Center for Health Statistics, Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Laboratory Manual. Hyattsville, MD: National Center for Health Statistics; 2013. [Google Scholar]
- 32.Skurup A, Kristensen T, Wennecke G National Kidney Disease Education Program Laboratory Working Group. New creatinine sensor for point-of-care testing of creatinine meets the National Kidney Disease Education Program guidelines. Clin Chem Lab Med. 2008;46(1):3–8. doi: 10.1515/CCLM.2008.004. [DOI] [PubMed] [Google Scholar]
- 33.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lange T, Rasmussen M, Thygesen LC. Assessing natural direct and indirect effects through multiple pathways. Am J Epidemiol. 2014;179(4):513–518. doi: 10.1093/aje/kwt270. [DOI] [PubMed] [Google Scholar]
- 36.Crews DC, Charles RF, Evans MK, et al. Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis. 2010;55(6):992–1000. doi: 10.1053/j.ajkd.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams ED, Tapp RJ, Magliano DJ, et al. Health behaviours, socioeconomic status and diabetes incidence: the Australian Diabetes Obesity and Lifestyle Study (AusDiab) Diabetologia. 2010;53(12):2538–2545. doi: 10.1007/s00125-010-1888-4. [DOI] [PubMed] [Google Scholar]
- 38.Stringhini S, Tabak AG, Akbaraly TN, et al. Contribution of modifiable risk factors to social inequalities in type 2 diabetes: prospective Whitehall II cohort study. BMJ. 2012;345:e5452. doi: 10.1136/bmj.e5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stringhini S, Sabia S, Shipley M, et al. Association of socioeconomic position with health behaviors and mortality. JAMA. 2010;303(12):1159–1166. doi: 10.1001/jama.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Qaoud TM, Nitsch D, Wells J, et al. Socioeconomic status and reduced kidney function in the Whitehall II Study: role of obesity and metabolic syndrome. Am J Kidney Dis. 2011;58(3):389–397. doi: 10.1053/j.ajkd.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: One size does not fit all. JAMA. 2005;294(22):2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 42.Orth SR, Hallan SI. Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients—absence of evidence or evidence of absence? Clin J Am Soc Nephrol. 2008;3(1):226–236. doi: 10.2215/CJN.03740907. [DOI] [PubMed] [Google Scholar]
- 43.Haroun MK, Jaar BG, Hoffman SC, et al. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol. 2003;14(11):2934–2941. doi: 10.1097/01.asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 44.Brancati FL, Whelton PK, Kuller LH. Diabetes mellitus, race, and socioeconomic status: a population-based study. Ann Epidemiol. 1996;6(1):67–73. doi: 10.1016/1047-2797(95)00095-x. [DOI] [PubMed] [Google Scholar]
- 45.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 46.Boykin S, Diez-Roux AV, Carnethon M, et al. Racial/ethnic heterogeneity in the socioeconomic patterning of CVD risk factors in the United States: the Multi-Ethnic Study of Atherosclerosis. J Health Care Poor Underserved. 2011;22(1):111–127. doi: 10.1353/hpu.2011.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris MI. Racial and ethnic differences in health care access and health outcomes for adults with type 2 diabetes. Diabetes Care. 2001;24(3):454–459. doi: 10.2337/diacare.24.3.454. [DOI] [PubMed] [Google Scholar]
- 48.Harris MI. Racial and ethnic differences in health insurance coverage for adults with diabetes. Diabetes Care. 1999;22(10):1679–1682. doi: 10.2337/diacare.22.10.1679. [DOI] [PubMed] [Google Scholar]
- 49.Kop WJ, Seliger SL, Fink JC, et al. Longitudinal association of depressive symptoms with rapid kidney function decline and adverse clinical renal disease outcomes. Clin J Am Soc Nephrol. 2011;6(4):834–844. doi: 10.2215/CJN.03840510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schrijvers CT, Stronks K, van de Mheen HD, et al. Explaining educational differences in mortality: the role of behavioral and material factors. Am J Public Health. 1999;89(4):535–540. doi: 10.2105/ajph.89.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arbour-Nicitopoulos KP, Faulkner GE, Irving HM. Multiple health-risk behaviour and psychological distress in adolescence. J Can Acad Child Adolesc Psychiatry. 2012;21(3):171–178. [PMC free article] [PubMed] [Google Scholar]
- 52.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 53.VanderWeele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172(12):1339–1348. doi: 10.1093/aje/kwq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol. 2013;42(5):1511–1519. doi: 10.1093/ije/dyt127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.