Abstract
Introduction:
Acute cigarette smoking may relieve withdrawal and negative affect due to tobacco abstinence to a greater extent in women versus men. Yet, the relative contribution of the cigarette’s nicotine content to this sex difference is not clear.
Methods:
Non-quitting dependent adult smokers (N = 44; 21 males, 23 females) participated in 2 virtually identical sessions, each after abstaining overnight (CO < 10 ppm) and differing only in the nicotine content of the designated cigarette. While blind to brand markings, they consumed a total of 24 puffs in controlled fashion for 2hr in each session, either from a nicotine (Quest 1, 0.6mg) or denicotinized (Quest 3, 0.05mg) cigarette. Withdrawal symptoms were obtained before and after smoking, and negative affect was assessed after each period of cigarette exposure consisting of 6 puffs every 25min.
Results:
Men and women did not differ in baseline withdrawal and negative affect due to overnight abstinence, but reductions in each symptom were significantly influenced by the interaction of sex × nicotine/denicotinized cigarette (both p < .05). In men, but not in women, each symptom was generally decreased more by the nicotine versus denicotinized cigarette, and the nicotine cigarette reduced each to a greater degree in men versus women.
Conclusions:
Sex differences in relief of abstinence-induced withdrawal and negative affect due to the nicotine content in cigarettes are consistent with prior research indicating that nicotine per se, compared to non-nicotine smoke stimuli, is less rewarding in women versus men.
Introduction
Smoking behavior acutely increases during negative mood experiences.1–3 This observation clearly may help explain why negative affect (e.g. sad mood, anxiety) is a critical symptom of tobacco withdrawal that is strongly predictive of smoking persistence4 and of smoking relapse among those trying to quit.5–7 Similarly, smoking acutely relieves negative affect that arises due to tobacco deprivation (i.e. withdrawal) but not most negative affect that is caused by “stressors” or other environmental challenges.8
Smokers often vary in their degree of relief from withdrawal-induced negative affect by smoking and in the influences of accompanying conditions.9 Yet, relatively few controlled studies have specifically assessed individual differences in affective responses to acute smoking behavior. Some responses to smoking during negative mood situations, including tobacco deprivation, may be greater in women than men. In one study, women reported greater increase in negative affect due to overnight abstinence and also greater subsequent relief of that negative affect by resuming smoking, compared to men.10 Indirect support for the notion that women are more responsive to smoking-related relief from negative affect also comes from tobacco industry documents that summarize industry research on smoking preferences in men and women (reported by 11). For example, British-American Tobacco (BAT) documents indicated “the role of smoking relates to emotions and sensory pleasure for women, and habit and reward for men”.11
Because a quit attempt requires refraining from smoking when experiencing negative affect due to abstinence, greater subjective relief from smoking in women could also help explain their often greater difficulty when quitting.12–16 Supporting this notion, women may be more likely to view smoking as a way to manage their mood17,18, and negative affect after quitting may predict relapse more strongly in women than men.19,20 In other research, compared to men, women admit to less ability to manage the perceived risks of attempting to quit smoking, which is predictive of poorer cessation outcome.21 Perhaps similarly, a history of depression more strongly predicts poor cessation outcome in women relative to men22.
Moreover, largely unexamined is sex differences in the relative contributions of pharmacological and nonpharmacological (e.g. smoke stimuli other than nicotine) factors to relief of negative affect from acute smoking behavior, although men and women generally do not appear to differ in nicotine pharmacokinetics.23 Because we have consistently found that the smoking behavior of women seems to be influenced less by nicotine per se and more by non-nicotine factors, relative to men24,25, potentially greater relief of negative affect from smoking in women may not be due to the resulting intake of nicotine. Laboratory studies manipulating nicotine content in cigarettes show that women, compared to men, are generally less sensitive to nicotine on self-report ratings of “reward”26,27, and more sensitive to non-nicotine factors (e.g. cues, olfactory smoke stimuli;28–30). Other research also is consistent with a lesser influence of nicotine, and greater influence of various non-nicotine smoking factors, on tobacco dependence in women.31,32
This study used a within-subjects design to assess acute effects of intermittently smoking nicotine versus denicotinized cigarettes on relief of withdrawal and negative affect in male and female dependent smokers who were abstinent overnight. Each cigarette was administered to participants blind to brand markings and nicotine content, with the amount of smoking intake (i.e. topography) controlled. Comparing nicotine versus denicotinized cigarettes under these conditions can determine nicotine inhalation effects per se, which we hypothesized would be less in women compared to men. As outlined above, this hypothesized sex difference was expected partly owing to the greater sensitivity of women to the non-nicotine aspects of smoking any cigarette, whether nicotine or denicotinized.
Method
Participants
Study participants (N = 44; 21 men, 23 women) were those who smoked at least 10 cigarettes per day for at least 1 year and met DSM-IV criteria for nicotine dependence33, according to a structured interview (updated from34). (Because nondependent smokers often do not experience withdrawal due to abstinence, virtually by definition35, testing dependent smokers was necessary here to ensure that overnight abstinence would result in withdrawal and negative affect symptoms, which could then be relieved by acute smoking.) Men and women did not differ on any characteristics, with respective means (±SD) of 14.9±4.1 versus 14.5±4.1 cigarettes/day and scores of 4.8±1.9 versus 4.3±1.6 on the Fagerstrom Test of Cigarette Dependence (previously called the Fagerstrom Test of Nicotine Dependence, FTND;36). The nicotine yield of their preferred brand was 1.0±0.2 versus 1.0±0.2mg, 67% of men versus 52% of women were nonmenthol smokers, and their respective ages were 28.6±11.5 versus 25.9±8.0 years. Participants were recruited from the surrounding community and most self-identified as Caucasian (88.6%), with 9.1% African-American, and 2.3% Asian. We excluded those currently taking medications to treat serious psychological problems (e.g. psychosis, major depression).
Withdrawal, Negative Affect
Nicotine withdrawal was assessed by the Minnesota Nicotine Withdrawal Scale (MNWS;37) using the following six items: depressed mood/sad, anxious/nervous, irritable/angry/frustrated, difficulty concentrating, restless/impatient, and drowsiness. Negative affect (NA) was measured by Diener and Emmons38 Mood Form, shown to be sensitive to a variety of mood manipulations, including overnight abstinence8. For each measure, items were rated on a 0 (“not at all”) to 100 (“extremely”) visual analog scale (VAS) and averaged across symptoms to get a total withdrawal or total NA score.
Procedure
Participants were first screened by telephone on their smoking and health history and scheduled for an introductory session in the laboratory to obtain written informed consent and verify eligibility. They then engaged in two 2-hr experimental sessions on separate days, each following overnight abstinence (>12hr) and differing only in the nicotine content of the cigarette made available, nicotine or denicotinized, which were presented under blind conditions and in counter-balanced order. The moderate nicotine brand was QuestR 1 (yield of 0.6mg nicotine, 9mg tar) and the denicotinized (“denic”) brand was QuestR 3 (yield < 0.05mg nicotine, 9mg tar), formerly sold commercially by Vector Group, Ltd. (Miami, FL). Menthol smokers received menthol Quests; non-menthol smokers received non-menthol Quests. All Quest cigarettes had identifiable markings covered to keep subjects blind to brand, as in prior research.39
Upon arrival to each session, participants provided expired-air CO to confirm overnight abstinence (CO < 10 ppm;40) and completed the withdrawal and NA measures to provide baseline values for effects of abstinence prior to any smoking behavior. Then, prior to each of four trials, one every 25min, participants self-administered six puffs over 3min, one puff every 30 s, on the nicotine or denic cigarette assigned for that session. All smoking was done via the portable Clinical Research Support System (“CReSS Pocket”; Borgwaldt KC, Inc., Richmond VA; http://borgwaldt.hauni.com/en/instruments/smoking-machines/smoking-topography-devices/cress-pocket.html), which assesses puff volume (in ml;41,42). Exact puff timing and duration were guided by computer-presented puffing instructions to standardize intake at about 60ml per puff (consistent with ad lib puffing;42), as in our prior studies of controlled smoke exposure, including with the CReSS8. After the first trial of smoking, subjects rated the cigarette on “how much nicotine” they perceived43, using the same 0–100 VAS as in the withdrawal and NA measures (described above), to gauge ability to perceive the cigarette’s nicotine content. NA was assessed after each smoking trial, and withdrawal was assessed after the completion of these four trials. (The post-smoking withdrawal measure is missing from one male and one female during one session each, and so they were excluded from analyses of withdrawal relief due to smoking.) Also, CO was again obtained after the last trial of each session to gauge exposure during this intermittent smoking from the nicotine and denicotinized cigarettes.
Data Analyses
All analyses were conducted using IBM SPSS 21.0. The primary dependent measures were NA and withdrawal, focusing on the change in each from baseline, prior to smoke exposure, to post-smoking of the nicotine versus denicotinized cigarettes. Preliminary analyses showed no effects of cigarette condition order between sessions, and so data were collapsed between orders in analyses of variance (ANOVAs) for these responses. The between-subjects factor was subject sex, and the within-subjects factor was cigarette condition (nicotine, denicotinized). Follow-up comparisons for significant ANOVA results were conducted with least significant difference (LSD) t-tests.44 Effect sizes for responses of particular interest are presented by partial eta-squared values (ŋp 2), which indicate the percent of variance explained.
Results
Affective Responses to Smoking
Withdrawal
Mean (SE) baseline withdrawal after overnight abstinence did not differ between men and women, 29.3±3.8 versus 35.1±3.6, respectively, F(1,42) = 1.23, p > .20, As expected, withdrawal decreased after smoking, F(1,40) = 20.60, p < .001, ŋp 2 = 0.340, with no differential decrease in withdrawal due to main effects of sex or of cigarette condition, both F(1,40) < 1. Notably, however, this withdrawal relief due to smoking was significant for the interaction of sex × cigarette condition, F(1,40) = 4.55, p < .05, ŋp 2 = 0.102. As shown in Figure 1, withdrawal decreased more following the nicotine versus denic cigarette for men, F(1,19) = 5.24, p < .05, but not for women, F(1,21) = 1.02, n.s. In follow-up tests, withdrawal decreased marginally less in women versus men after the nicotine cigarette, t(41) = 1.74, p < .10, but decreased significantly more in women versus men after the denic cigarette, t(41) = 2.64, p < .02.
Figure 1.
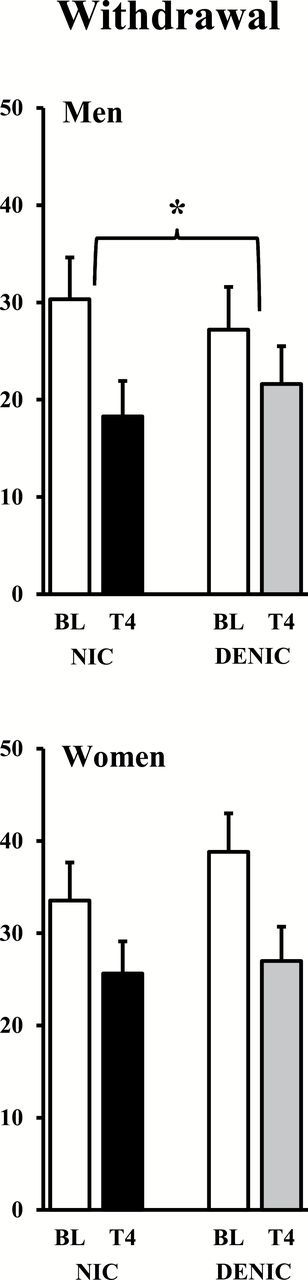
Mean (SE) withdrawal for men (top) and women (bottom) after overnight tobacco abstinence (baseline, BL) and after 24 controlled puffs from the nicotine or denicotinized (denic) cigarette through trial 4 (T4; *p < .05 for difference between cigarette conditions in decline due to smoking).
Negative Affect
Baseline NA also did not differ between men and women, 21.1±3.6 versus 25.0±3.4, respectively, F(1,42) < 1. Very similar to the results for withdrawal, NA decreased after acute smoking, F(1,42) = 33.01, p < .001, ŋp 2 = 0.440, and this decrease in NA did not differ due to main effects of sex, F(1,42) = 1.03, n.s., or of cigarette condition, F(1,42) < 1. Yet, again, the decrease in NA due to smoking was significantly influenced by the interaction of sex × cigarette condition, F(1,42) = 4.17, p < .05, ŋp 2 = 0.090. As shown in Figure 2, NA decreased from pre-smoking baseline following the nicotine versus denic cigarette to a marginally greater extent for men, F(1,20) = 4.07, p < .06, but not at all for women, F(1,22) < 1. Follow-up tests showed that NA decreased significantly less in women versus men after the nicotine cigarette, t(41) = 3.28, p < .005, but NA decreased similarly in women and men after the denic cigarette, t(41) < 1.
Figure 2.
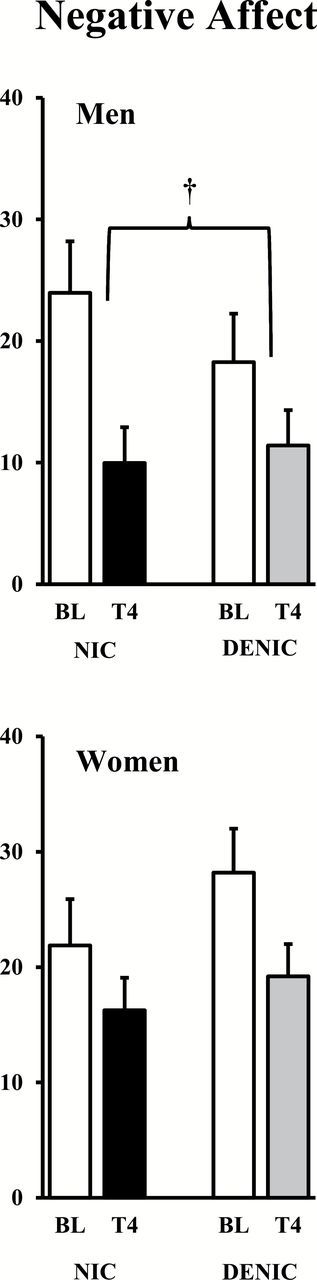
Mean (SE) negative affect ratings for men and women after overnight abstinence (baseline, BL) and after the controlled puffs from the nicotine or denicotinized (deic) cigarette through trial 4 (T4; †p < .06 for difference between cigarette conditions in decline due to smoking).
For all subjects, NA decreased significantly from baseline even after just the first trial of six puffs (perhaps simulating a brief lapse), whether from the nicotine, −7.0±1.6, t(42) = 3.82, p < .001, or denic cigarette, −5.0±1.5, t(42) = 2.74, p < .01. The interaction of sex × cigarette condition, F(1,42) = 3.14, p < .10, ŋp 2 = 0.069, was marginal, however, as the respective decline in NA from baseline due to six puffs tended to be less in women than men (−4.7 vs. −9.4) following the nicotine cigarette but more in women than men (−6.0 vs. −3.8) following the denic cigarette. Thus, the pattern of sex differences in NA response to very brief smoking was somewhat similar to the interaction for NA change from baseline over all four trials of smoking (Figure 2 and above).
Control Over Smoke Intake
Mean (SE) puff volume from the six puffs on each trial was less on the nicotine versus denicotinized cigarette smoking sessions, 296±12 versus 354±13ml, respectively, F(1,42) = 26.83, p < .001, ŋp 2 = 0.390. Yet, there was no difference in puff volume across the 4 smoking trials, F(3,40) = 1.02, p > .20, or due to the interaction of trial × nicotine/denicotinized cigarette, F(3,40) < 1. Although the main effect of sex was significant, F(1,42) = 10.15, p < 0.005, ŋp 2 = 0.195, there was no interaction of sex × nicotine/denicotinized cigarette, F(1,42) < 1, or trial × sex × cigarette condition, F(3,40) = 1.51, p > .20. Puff volume across trials was comparably smaller for the nicotine versus denicotinized cigarette, respectively, in both men (328±17 vs. 390±18ml) and women (264±16 vs. 317±18ml).
In contrast, CO increased from baseline more after smoking the nicotine cigarette, from 5.5±0.3 to 23.5±1.1 ppm, compared to the denic cigarette, from 5.4±0.3 to 19.4±1.1 ppm, F(1,42) = 14.35, p < .001, ŋp 2 = .255. Importantly, though, there were no significant effects on CO of sex, F(1,42) = 1.98, p > .10 or interaction of sex × cigarette condition, F(1,42) < 1. Very similarly, mean perceived ratings of “how much nicotine” were higher for the nicotine versus denic cigarette, 56.8±3.8 versus 32.4±3.7, F(1,42) = 20.14, p < .001, ŋp 2 = .324, as would be expected, but again there were no significant effects of sex or the interaction of sex × cigarette condition, both F(1,42) < 1.
In sum, puff volume was less but CO boost and perceived nicotine amount were greater for the nicotine versus denic cigarette. Yet, men and women did not differ at all in these relative responses to the two different cigarettes, in sharp contrast to their differential affective responses.
Discussion
These results indicate that acute nicotine intake from cigarette smoking after overnight abstinence differentially relieves withdrawal and reduces negative affect in men versus women. For men, acute smoking of a regular nicotine cigarette reduced withdrawal and (marginally) negative affect more than smoking a denic cigarette, while for women these responses did not differ due to the nicotine content of the cigarette smoked. Similarly, in between-subjects comparisons, women responded to the nicotine cigarette with marginally less withdrawal relief and significantly less NA relief compared to men, while women responded to the denic cigarette with significantly more withdrawal relief relative to men. Thus, these affective responses to smoking in women were influenced less by nicotine intake per se and more by non-nicotine stimuli, compared to men’s responses. The consistency between our measures in the pattern of sex difference results may reflect the emphasis on negative affect symptoms among these MNWS items37, perhaps suggesting a more specific sex difference in nicotine effects on affect regulation per se during abstinence and not necessarily a broader sex difference in subjective responses to nicotine via smoking.
In any case, our findings are very consistent with prior research on sex differences in the relative reinforcing effects of nicotine versus non-nicotine factors in smoking30 and extend these sex differences to affective responses to abstinence-induced symptoms. However, the current study may clarify the nature of these sex differences in that men and women did not differ in ability to perceive the different nicotine amounts between these cigarettes, based on their ratings of “how much nicotine”. Thus, it likely was not a relative insensitivity to the interoceptive stimulus effects of nicotine that attenuated the affective responses of women to the nicotine cigarette.45 Rather, intake of nicotine per se appeared to simply not relieve these affective symptoms of abstinence as much in women compared to men.
Consequently, non-nicotine aspects of cigarette smoking may be more effective in providing affective relief from overnight abstinence in women. We are unaware of prior research examining sex differences in non-nicotine cigarette stimuli on affective responses to abstinence. Yet, we previously conducted a somewhat similar study that varied non-nicotine stimuli while holding constant nicotine intake29, the reverse of the manipulation in the current study, in which non-nicotine stimuli were held constant while varying nicotine intake. In the earlier study, we found that blocking the ability of a smoker to taste or smell cigarette smoke while smoking decreased reward ratings and smoking behavior more in women than in men, but blocking ability to see the lit cigarette did not differentially affect responses29. Therefore, at least in acute tests of responses to smoking after overnight abstinence, a sex difference in non-nicotine stimulus effects may be specific to olfactory/taste stimuli of smoking. Perhaps relevant here, somewhat similar sex differences have been found in rodent research on nicotine self-administration, as nicotine-associated cues (light or tone) increase responding in female rats more than in male rats.46
Our results may have implications for clinical research on smoking persistence in men versus women, especially during abstinence-induced negative affect. It would be important to determine the degree to which these sex differences generalize to the natural environment, where other factors, such as stressors, concurrent drug use, activity requirements, etc., could minimize or enhance these differences.13,30,47 Whether comparable sex differences would be found with completely ad lib smoking of these nicotine and denicotinized cigarettes would also be important, although distinguishing between differential responsivity to a similar amount of smoke intake (as shown here) versus differential amounts of smoke intake could be challenging. Also important would be assessing the duration of these sex differences after abstinence, since they may wane over time; a shift in the relative impact of nicotine and non-nicotine factors on subsequent affective responses to acute smoking in women could eventually eliminate these differences due to sex.
Yet, findings also could help explain why women have greater difficulty maintaining cessation than men, especially soon after abstaining, although the participants in this study were not trying to quit smoking. Less affective relief from nicotine per se during early abstinence may further account for observations of less efficacy with most formulations of nicotine replacement medications in women versus men, as outlined in the clinical literature.13,14,16,32,48 For example, Vogel et al.32 recently showed better quit rates after NRT patch treatment in men versus women, but better quit rates after very low nicotine cigarette use in women versus men, although these 6-week conditions were not placebo-controlled or presented in blind fashion. This finding appears very consistent with the notion that nicotine per se is more influential for reinforcing smoking behavior in men, while non-nicotine stimuli from smoking are more influential for women.24 Importantly, the Vogel et al.32 observations extend this notion to efficacy results in clinical outcome research.
Finally, the results suggest other directions for research aimed at improving quit success in women smokers. New treatments aimed at relieving initial negative affect during abstinence may especially benefit women trying to quit smoking20, and non-medication strategies may warrant attention. For example, denicotinized cigarettes may be viewed as the ultimate sensory substitute for nicotine cigarettes, since they match almost all the effects of smoking other than nicotine intake.49 Therefore, recent development of very low nicotine cigarettes50 and other sensory substitutes may provide easier ways to gradually transition from dependence on nicotine cigarettes to complete tobacco cessation.51 Such an approach may be a particularly fruitful area of research into novel aids for improving smoking cessation rates52, especially in women.32
Funding
This research was supported by NIH Grants DA35774 and DA35968 (KAP).
Declaration of Interests
None declared.
Acknowledgment
Data from this paper were presented at a Pre-Conference Workshop at the Society for Research on Nicotine and Tobacco Annual Meeting; February 25, 2015; Philadelphia, Pennsylvania, USA.
References
- 1. Rose JE, Ananda S, Jarvik ME. Cigarette smoking during anxiety-provoking and monotonous tasks. Addict Behav. 1983;8:353–359. [DOI] [PubMed] [Google Scholar]
- 2. Conklin CA, Perkins KA. Subjective and reinforcing effects of smoking during negative mood induction. J Abnormal Psychol. 2005;114:153–164. [DOI] [PubMed] [Google Scholar]
- 3. Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. [DOI] [PubMed] [Google Scholar]
- 4. Gehricke J-G, Loughlin SE, Whalen CK, et al. Smoking to self-medicate attentional and emotional dysfunctions. Nicotine and Tobacco Res. 2007;9 (suppl 4):S523–S536. [DOI] [PubMed] [Google Scholar]
- 5. Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: contrasting affective and physical models of dependence. J Consult Clin Psychol. 2002;70:216–227. [PubMed] [Google Scholar]
- 6. Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev 2004;111:33–51. [DOI] [PubMed] [Google Scholar]
- 7. Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol. 2004;72:192–201. [DOI] [PubMed] [Google Scholar]
- 8. Perkins KA, Karelitz JL, Conklin CA, Sayette MA, Giedgowd GE. Acute negative affect relief from smoking depends on the affect measure and situation, but not on nicotine. Biol Psychiatry. 2010;67:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilbert DG. Smoking: Individual Differences, Psychopathology, and Emotion. London: Taylor and Francis; 1995. [Google Scholar]
- 10. Xu J, Azizian A, Monterosso J, et al. Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob Res. 2008;10:1653–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carpenter CM, Wayne GF, Connolly GN. The role of sensory perception in the development and targeting of tobacco products. Addiction. 2007;102:136–147. [DOI] [PubMed] [Google Scholar]
- 12. Borrelli B, Papandonatos G, Spring B, Hitsman B, Niaura R. Experimenter-defined quit rates for smoking cessation: adherence improves outcomes for women but not for men. Addiction. 2004;99:378–385. [DOI] [PubMed] [Google Scholar]
- 13. Perkins KA. Smoking cessation in women: special considerations. CNS Drugs. 2001;15:391–411. [DOI] [PubMed] [Google Scholar]
- 14. Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res. 2008;10:1245–1250. [DOI] [PubMed] [Google Scholar]
- 15. Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR. Addiction (Abingdon, England). 2004;99:1462–1469. [DOI] [PubMed] [Google Scholar]
- 16. Wetter D, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. J Consult Clin Psychol. 1999;67:555–562. [DOI] [PubMed] [Google Scholar]
- 17. Brandon TH, Baker TB. The smoking consequences questionnaire: The subjective expected utility of smoking in college students. Psychol Assess. 1991;3:484–491. [Google Scholar]
- 18. Weinberger AH, McKee SA. Mood and smoking behavior: the role of expectancy accessibility and gender. Addict Behav. 2012;37:1349–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borland R. Slip-ups and relapse in attempts to quit smoking. Addict Behav. 1990;15:235–245. [DOI] [PubMed] [Google Scholar]
- 20. Cofta-Woerpel L, McClure JB, Li Y, Urbauer D, Cinciripini PM, Wetter DW. Early cessation success or failure among women attempting to quit smoking: trajectories and volatility of urge and negative mood during the first postcessation week. J Abnormal Psychol. 2011;120:596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McKee SA, O’Malley SS, Salovey P, Krishnan-Sarin S, Mazure C. Perceived risk and benefits of smoking cessation: Gender-specific predictors of motivation and treatment outcome. Addict Behav. 2005;30:423–425. [DOI] [PubMed] [Google Scholar]
- 22. Weinberger AH, Mazure CM, Morlett A, McKee SA. Two decades of smoking cessation treatment research on smokers with depression: 1990–2010. Nicotine Tob Res. 2013;15:1014–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benowitz NL, Hatsukami DK. Gender differences in the pharmacology of nicotine addiction. Addict Biol. 1998;3:383–404. [DOI] [PubMed] [Google Scholar]
- 24. Perkins KA. Sex differences in nicotine versus non-nicotine reinforcement as determinants of tobacco smoking. Exp Clin Psychopharmacol. 1996; 4:166–177. [Google Scholar]
- 25. Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–315. [DOI] [PubMed] [Google Scholar]
- 26. Perkins KA, Jacobs L, Sanders M, Caggiula AR. Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology. 2002;163:194–201. [DOI] [PubMed] [Google Scholar]
- 27. Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, Caggiula AR. Sex differences in the influence of nicotine and dose instructions on subjective and reinforcing effects of smoking. Psychopharmacology. 2006;184:600–607. [DOI] [PubMed] [Google Scholar]
- 28. Doran N. Sex differences in smoking cue reactivity: craving, negative affect, and preference for immediate smoking. Am J Addict. 2014;23: 211–217. [DOI] [PubMed] [Google Scholar]
- 29. Perkins KA, Gerlach D, Vender J, Grobe JE, Meeker J, Hutchison S. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res. 2001;3:141–150. [DOI] [PubMed] [Google Scholar]
- 30. Perkins KA. Sex differences in nicotine reinforcement and reward: influences on the persistence of tobacco smoking. In: Bevins R, Caggiula AR, eds. The Motivational Impact of Nicotine and its Role in Tobacco Use. New York: Springer; 2009a: 143–169. [DOI] [PubMed] [Google Scholar]
- 31. Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Gender differences in quit rates following smoking cessation with combination nicotine therapy: influence of baseline smoking behavior. Nicotine Tob Res. 2003;5: 111–116. [DOI] [PubMed] [Google Scholar]
- 32. Vogel RI, Hertsgaard LA, Dermody SS, et al. Sex differences in response to reduced nicotine content cigarettes. Addict Behav. 2014;39: 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. American Psychiatric Association (APA). Diagnostic and Statistical Manual-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 34. Breslau N, Kilbey MM, Andreski P. DSM-IIIR nicotine dependence in young adults: prevalence, correlates and associated psychiatric disorders. Addiction. 1994;89:743–754. [DOI] [PubMed] [Google Scholar]
- 35. Shiffman S. Tobacco “chippers”—individual differences in tobacco dependence. Psychopharmacology. 1989;97:539–547. [DOI] [PubMed] [Google Scholar]
- 36. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 37. Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. [DOI] [PubMed] [Google Scholar]
- 38. Diener E, Emmons RA. The independence of positive and negative affect. J Personal Social Psychol. 1984;47:1105–1117. [DOI] [PubMed] [Google Scholar]
- 39. Perkins KA, Karelitz JL. Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology. 2013; 228:479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. SRNT Committee. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. [DOI] [PubMed] [Google Scholar]
- 41. Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB. Smoking topography: reliability and validity in dependent smokers. Nicotine Tob Res. 2003;5:673–679. [DOI] [PubMed] [Google Scholar]
- 42. Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette. Nicotine Tob Res. 2012;14:490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacol Biochem Behav. 1996;53:309–315. [DOI] [PubMed] [Google Scholar]
- 44. Huitema B. Analysis of Covariance and Alternatives. New York: John Wiley & Sons; 1980. [Google Scholar]
- 45. Perkins KA. Nicotine discrimination in men and women. Pharmacol Biochem Behav. 1999;64:295–299. [DOI] [PubMed] [Google Scholar]
- 46. Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology. 2005;180:258–266. [DOI] [PubMed] [Google Scholar]
- 47. Perkins KA.) Treatment of nicotine dependence in women. In Brady K, Greenfield S, eds. Women and Addiction: A Comprehensive Textbook. New York: Guilford Press; 2009b:360–378. [Google Scholar]
- 48. Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: differences between men and women. J Consult Clin Psychol. 2004;72:712–722. [DOI] [PubMed] [Google Scholar]
- 49. Pickworth WB, Fant RV, Nelson RA, Rohrer MS, Henningfield JE. Pharmacodynamic effects of new de-nicotinized cigarettes. Nicotine Tob Res. 1999;1:357–364. [DOI] [PubMed] [Google Scholar]
- 50. Hatsukami DK, Heishman SJ, Vogel RI, et al. Dose-response effects of spectrum research cigarettes. Nicotine Tob Res. 2013;15:1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hatsukami DK, Benowitz NL, Donny E, Henningfield J, Zeller MR. Nicotine reduction: strategic research plan. Nicotine Tob Res. 2013;15:1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hatsukami DK, Perkins KA, LeSage MG, et al. Nicotine reduction revisited: science and future directions. Tobacco Control. 2010;19:436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]


