Abstract
Introduction. Previous priming with avian influenza vaccines results in more rapid and more robust neutralizing antibody responses upon revaccination, but the role CD4+ T cells play in this process is not currently known.
Methods. Human subjects previously enrolled in trials of inactivated influenza A(H5N1) vaccines and naive subjects were immunized with an inactivated subunit influenza A/Indonesia/5/05(H5N1) vaccine. Neutralizing antibody responses were measured by a microneutralization assay, and hemagglutinin (HA)-specific and nucleoprotein (NP)-specific CD4+ T-cell responses were quantified using interferon γ enzyme-linked immunosorbent spot assays.
Results. While vaccination induced barely detectable CD4+ T-cell responses specific for HA in the previously unprimed group, primed subjects had readily detectable HA-specific memory CD4+ T cells at baseline and mounted a more robust response to HA-specific epitopes after vaccination. There were no differences between groups when conserved NP-specific CD4+ T-cell responses were examined. Interestingly, neutralizing antibody responses following revaccination were significantly higher in individuals who mounted a CD4+ T-cell response to the H5 HA protein, a correlation not observed for NP-specific responses.
Conclusions. These findings suggest that prepandemic vaccination results in an enriched population of HA-specific CD4+ T cells that are recruited on rechallenge with a drifted vaccine variant and contribute to more robust and more rapid neutralizing antibody responses.
Keywords: CD4+ T cells, influenza, avian influenza, H5N1, influenza vaccine, pandemic influenza
There have been continued ongoing infections of humans with influenza A(H5N1) since 1997, with 650 reported cases of infection and 386 deaths in 15 countries as of January 2014 [1]. Although these viruses have not yet attained the ability to sustain human-to-human transmission, sporadic cases of human transmission have been reported [2–4]. Further, recent studies have suggested that transmission in ferrets could be attained with as few as 5 mutations [5] or with 4 mutations and a reassortment event [6]. As several of these mutations are already present in circulating influenza A(H5N1) viruses [7], these viruses pose an ongoing pandemic risk.
Influenza A(H5N1) vaccines tested to date have been poorly immunogenic, requiring 2 doses and either high antigen content or the use of an adjuvant to attain neutralizing antibody levels potentially associated with protection [8–14]. This, combined with the time currently required for vaccine manufacture and distribution, makes it unlikely that present vaccination strategies will be able to significantly limit a pandemic's spread [15, 16]. However, a number of studies have now demonstrated that sequential influenza A(H5N1) vaccination results in a more rapid and more robust neutralizing antibody response than is elicited in the first vaccine encounter, even when boosting occurs years after the initial vaccination [17–21]. Preexisting primed influenza A(H5N1)-specific memory B cells have been suggested to play a role in this accelerated antibody production [22, 23]. However, no studies have yet explored the importance of CD4+ T cells in this augmented antibody production, despite the known importance of CD4+ T cells in promoting neutralizing antibody responses [24–27].
Previous work in our laboratory and by others has indicated that CD4+ T-cell help may be a limiting factor in development of neutralizing antibody upon challenge with influenza vaccines [28–32]. Additionally, when we addressed whether the specificity of CD4+ T cells influences their ability to help B cells during infection or vaccination, we found evidence that CD4+ T-cell responses directed against epitopes within the hemagglutinin (HA) protein may be best able to help facilitate neutralizing antibody production [28, 33, 34]. As seasonal and avian HA proteins have only a limited degree of sequence conservation, we hypothesized that in unprimed individuals there may be a paucity of H5 HA-specific CD4+ T cells. In this situation, the initial influenza A(H5N1) vaccination would prime a population of HA-specific memory CD4+ T cells that, on revaccination years later, could be rapidly recalled to help promote neutralizing antibody production.
To further evaluate the repertoire of influenza virus–specific CD4+ T-cell responses following avian influenza immunization, we compared prevaccination and postvaccination CD4+ T-cell reactivity in subjects previously enrolled in trials of inactivated influenza A(H5N1) vaccines to a cohort with no past exposure to avian viruses. Using cytokine enzyme-linked immunosorbent spot assays with pools of overlapping synthetic peptides as recall antigen, we found that previously primed individuals had increased HA-specific CD4+ T-cell responses that correlated with increased neutralizing antibody production. These findings indicate that specific boosting of memory H5 HA-specific CD4+ T cells established during the initial vaccination may be a mechanism contributing to the robust neutralizing antibody responses seen on revaccination with a heterologous avian vaccine. Further, these studies suggest that efforts to boost memory CD4+ T cells specific for potentially cross-reactive HA epitopes may improve the immunogenicity of avian influenza vaccines.
MATERIALS AND METHODS
Study Design
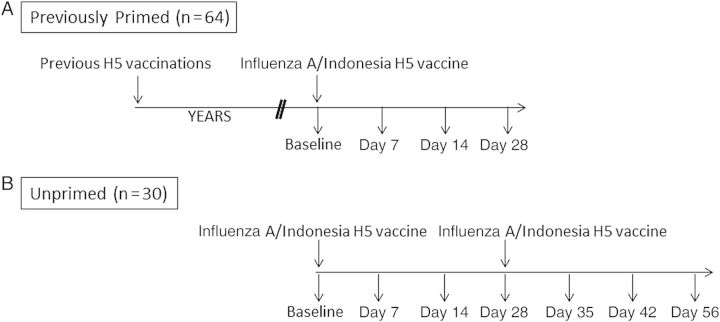
This study assessed the immune response to unadjuvanted, inactivated, subvirion influenza A/Indonesia/5/05 vaccine (clade 2.1.3) in 64 previously primed subjects and 30 unprimed healthy adults with no history of H5 vaccination who were not at risk for H5 exposure (Figure 1). The previously primed group received egg-derived, inactivated, subvirion influenza rg A/Vietnam/1203/2004 vaccine (clade 1) with HA doses of 3.75–90 µg in 2005–2006; while the majority of these subjects received unadjuvanted vaccine, 11 subjects had alum in their initial vaccination regimen. Sixteen subjects received 2 doses of baculovirus-expressed recombinant influenza A/Hong Kong/156/1997 (clade 0) H5 protein in 1997–1998 [9], followed by a single dose of unadjuvanted inactivated subvirion influenza A/Vietnam/1203/04 in 2006 [17].
Figure 1.
Schematic of study design. Previously primed (A) or unprimed (B) subjects were subdivided into groups that received either 15 µg or 90 µg of vaccine. All of the previously primed subjects received the inactivated subvirion influenza A/Vietnam/1203/04 vaccine in 2005–2006. Of these subjects, 16 had received baculovirus-expressed recombinant influenza A/Hong Kong/156/97 vaccine in 1997–1998.
Previously primed subjects received a single dose of influenza A/Indonesia/5/05 vaccine; unprimed subjects received a prime-boost regimen consisting of 2 identical vaccinations separated by 28 days. Vaccine was produced by Sanofi Pasteur (Swiftwater, PA) in eggs, using the seed influenza strain A/Indonesia/05/2005 PR8-IBCDC-RG2, and was inactivated, partially purified, and provided in unit-dose vials containing 0.5 mL of either 30 μg/mL or 180 μg/mL influenza A/(H5N1) HA protein, as determined by single radial immunodiffusion. Vaccine was administered intramuscularly in the deltoid muscle. Subjects were randomly assigned to 15-µg and 90-µg dose arms. Blood specimens were collected for serum and peripheral blood mononuclear cell (PBMC) analyses before vaccination and on days 7, 14, 28, and 56 after vaccination. Unprimed subjects also had blood specimens obtained 7 and 14 days following the second immunization.
Microneutralization (MN) and Hemagglutination Inhibition (HAI) Assays
Serum neutralizing and Hemagglutination Inhibition (HAI) antibody responses to the homologous A/Indonesia/05/2005 PR8-IBCDC-RG2 virus were measured at Southern Research Institute as previously described [9]. The neutralizing antibody response was measured by microtiter neutralization in Madin–Darby canine kidney cells, and HAI assays were performed with horse erythrocytes as indicator cells. All serum samples were tested at a starting dilution of 1:10, with negative results assigned a titer of 5 for calculation purposes. The replicate geometric mean was calculated to determine the antibody titer for each sample.
Peptides
The influenza A/Thailand/4(SP-528)/04(H5N1) HA (NR-2607) and influenza A/New York/348/2003(H1N1) nucleoprotein (NP; NR-2611) and M1 protein (M1; NR-2613) peptide sets were obtained through the National Institutes of Health (NIH) Biodefense and Emerging Infections Research Resources Repository (National Institutes of Allergy and Infectious Diseases, NIH; Bethesda, Maryland). Sequences of the influenza A/Thailand/4(SP-528)/04 and influenza A/Indonesia/5/05 H5 HA proteins were aligned, and 5 additional influenza A/Indonesia/5/05 peptides were synthesized in our facility using an Apex 396 system (AAPPTec; Louisville, Kentucky) as described previously [35] and were included in the appropriate HA pool to cover regions of sequence divergence between the 2 HA proteins.
In general, H5 HA peptides containing <6 contiguous conserved amino acids, compared with recently circulating H1N1 strains, were put into a pool termed “HA unique” (59 peptides; Supplementary Table 1). Peptides containing ≥8 continuous conserved amino acids were placed in a pool referred to as “HA conserved” (40 peptides; Supplementary Table 2). The alignments of the remaining HA peptides were individually analyzed and grouped with the appropriate pool, based on the location and degree of conservation of the substitutions present. The total H5 HA-specific response was calculated by summing the response to the H5 HA–unique and HA-conserved pools. As the influenza A/Indonesia/5/05 vaccine was on an influenza A/Puerto Rico/8/34 backbone that was conserved, compared with influenza A/New York/348/2003, all NP and M1 peptides were combined into pools termed “NP” (82 peptides) and “M1” (41 peptides), respectively.
Quantification of CD4+ T-Cell Responses
Cryopreserved PBMCs were thawed and rested overnight at 37°C and 5% CO2 in Roswell Park Memorial Institute 1640 medium containing 10% fetal bovine serum and gentamicin (Life Technologies, Carlsbad, California), with a typical yield of >70% viable cells after thawing. After resting, PBMCs were depleted of CD8+ and CD56+ cells by magnetic-activated cell sorting with positive selection using LD separation columns, as per the manufacturer's instructions (Miltenyi Biotec, Auburn, California). Enzyme-linked immunosorbent spot (ELISPOT) assays were performed as previously described [28, 36], with 250 000–350 000 CD8-depleted and CD56-depleted PBMCs cocultured with either peptide pool or tetanus toxoid (Calbiochem, Billerica, Massachusetts). Plates were analyzed using an Immunospot reader series 2A using Immunospot software, version 3.2 (Cellular Technology, Shaker Heights, Ohio). Results were normalized to peptide-specific spots per 106 cells after averaging duplicate wells and subtracting background. The change in response was calculated by subtracting the CD4+ T-cell response at day 14 from baseline, with negative changes normalized to 0.
Statistical Analysis
CD4+ T-cell responses against vaccine were compared between prevaccination and postvaccination time points, using the Wilcoxon signed rank test. The Mann–Whitney U test was used to compare unpaired groups. Correlations between groups were examined using Spearman rank correlation. Statistical analyses were performed using SAS, version 9.3, or GraphPad Prism 5. All P values of < .05 were considered statistically significant.
Ethical Statement
The University of Rochester Research Subjects Review Board approved this study protocol, and human experimentation guidelines of the Department of Health and Human Services and the University of Rochester were followed. Study procedures were in accordance with the ethical standards of the Declaration of Helsinki. All subjects provided written informed consent before study participation.
RESULTS
Study Subjects
Ninety-five healthy adult subjects were enrolled into the study and received the influenza A/Indonesia/5/05 vaccine between October 2010 and July 2011; 64 subjects were in the primed group, and 31 were in the unprimed group. One subject in the unprimed group was excluded because of a serious adverse event not related to the study vaccine, leaving 30 subjects for analysis in this group (Figure 1). The median age in both groups was 48 years, with no significant differences in age between the groups. Eleven subjects (35%) in the unprimed group and 25 subjects (39%) in the primed group were male.
Humoral Immune Responses
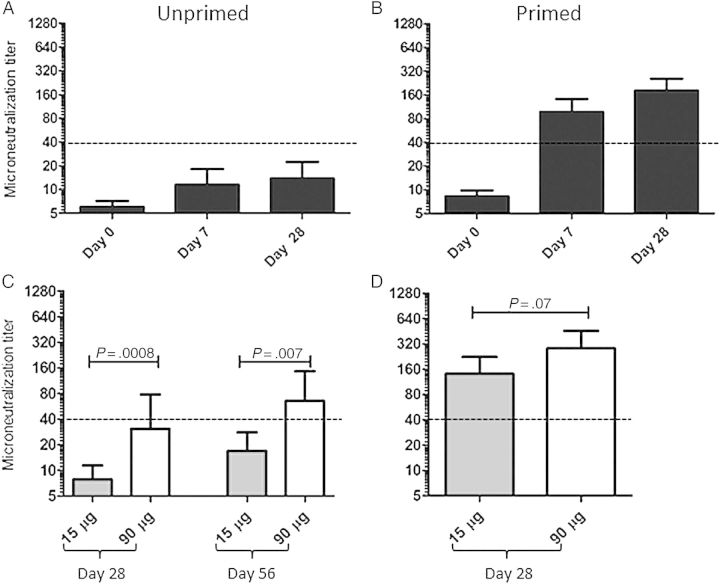
HAI and Microneutralization (MN) titers were determined at all visits, with a strong correlation observed between results of these 2 assays (r = 0.91, P < .0001 at day 28). However, as the MN titer is a more sensitive assay [37], these data are reported here. Antibody titers against the influenza A/Indonesia/5/05 virus were low at study initiation, with a geometric mean titer (GMT) of 6 in the unprimed group and 8.2 in the previously primed group (P = .01). Unprimed individuals had overall weak responses to a single dose of vaccine, achieving a GMT of only 13.8 (range, 5–1280) at 28 days following the initial vaccine dose (Figure 2A). Consistent with previous publications [17–20], revaccination of primed subjects with an antigenically distinct H5 vaccine resulted in a markedly more robust neutralizing antibody response (Figure 2B), with a GMT of 181.2 (range, 10–1280) at day 28 after vaccination. Also noted was that the kinetics of the antibody response in previously primed subjects were accelerated relative to those for the unprimed group, with 73% of subjects achieving a neutralizing antibody titer of ≥40 and 75% of subjects exhibiting at least a 4-fold rise in antibody titers by 7 days after vaccination.
Figure 2.
Previous influenza A(H5N1) vaccination primes subjects for a robust and accelerated neutralizing antibody response upon subsequent revaccination. Microneutralization assays were performed using the influenza A/Indonesia/5/2005 vaccine strain. A and B, The microneutralization titer at various time points after vaccination in previously unprimed subjects (A) and subjects with a history of prior vaccination (B). C and D, Microneutralization titers, by vaccine dose administered in unprimed (C) and previously primed (D) subjects. Bars represent the geometric mean of the antibody titer, with error bars showing the 95% confidence interval. P values were calculated using the Mann–Whitney U test.
Next, the effect of vaccine dose on the neutralizing antibody response was examined. As demonstrated in Figure 2C, a higher vaccine dose was needed to elicit a potentially protective neutralizing antibody response in unprimed subjects, with a greater titer achieved in the cohort given 90 µg of vaccine. Vaccine dose did not have as marked an impact in the primed group, with no significant differences between the antibody response at day 28 in the 15-µg and 90-µg dose cohorts (Figure 2D). Overall, these data demonstrate that a history of previous influenza A(H5N1) vaccination primes subjects for a more robust and more accelerated neutralizing antibody response on revaccination, even when a lower dose of antigenically drifted vaccine is administered.
CD4+ T-Cell Response Following H5 Vaccination
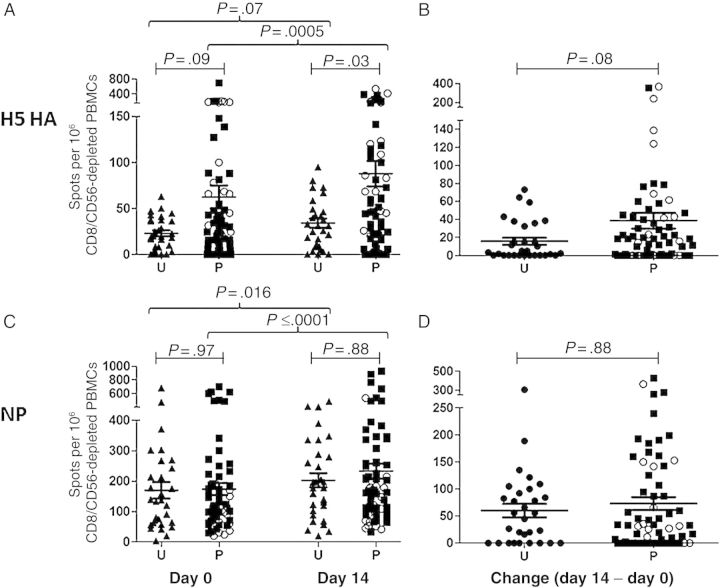
Although the effect of prepriming on the B-cell response has been previously evaluated [22, 23], the impact on the CD4+ T-cell repertoire has not yet been characterized. Consequently, we sought to gain insight into the status of the CD4+ T-cell repertoire in subjects before and after vaccination. IFN-γ ELISPOT assays were used to quantify CD4+ T-cell responses following restimulation with large, overlapping peptide pools to allow determination of anti–influenza virus CD4+ T-cell specificity at various time points after vaccination. Figure 3 shows the results of this analysis, in which the number of influenza virus–reactive cells per million CD8- and CD56-depleted PBMCs is displayed. When responses were directly compared between the previously unprimed cohort and subjects with a history of prior vaccination, striking and selective changes in the CD4+ T-cell repertoire were seen. First, previously primed individuals had a higher baseline number of HA-specific CD4+ T cells (Figure 3A), indicating that the original encounter with avian vaccine led to population of the long-term memory compartment with CD4+ T cells specific for H5 HA epitopes. The difference between groups became greater and reached statistical significance at day 14 after vaccination (Figure 3A). Previous priming was also associated with a trend toward greater expansion of HA-specific CD4+ T cells between days 0 and 14 (Figure 3B), a parameter that was correlated with improved neutralizing antibody responses in our previous work [28]. Importantly, subjects with a history of prior vaccination did not demonstrate enhanced reactivity to the repeatedly encountered NP (Figure 3C) or M1 (Supplementary Figure 1) proteins present within the vaccine, and greater expansion of NP-specific CD4+ T cells was not observed (Figure 3D). This suggests that priming with avian vaccines leads to selective enhancement of avian HA-specific cells in the CD4+ T-cell repertoire.
Figure 3.
Previous vaccination poises subjects for an improved CD4+ T-cell response to the hemagglutinin (HA) protein. Peripheral blood mononuclear cells (PBMCs) were depleted of CD8+ T cells and natural killer cells and then restimulated with HA and nucleoprotein (NP) peptide pools in an interferon γ enzyme-linked immunosorbent spot assay. H5 HA–specific (A and B) and NP-specific (C and D) CD4+ T-cell responses were compared between unprimed (U) and previously primed (P) groups. A and C, Responses to H5 HA (A) and NP (C) peptide pools are demonstrated at day 0 (left) and day 14 (right) after vaccination. B and D, Change in the HA-specific (B) or NP-specific (D) CD4+ T-cell responses between day 14 and baseline, with negative changes normalized to 0. Lines represent the mean CD4+ T-cell responses, with error bars depicting the standard error of the mean. Subjects who received a baculovirus-derived recombinant HA protein vaccine in 1997–1998 are shown as open circles. Unpaired P values were calculated using the Mann–Whitney U test; P values for paired data were calculated using the Wilcoxon rank sum test.
When responses were examined over time, our studies revealed that previously unprimed subjects had little gain in H5 HA reactivity between baseline and day 14 (P = .07; Figure 3A). In contrast, the subjects primed years earlier displayed a significant increase in H5 HA–specific responses between days 0 and 14 (P = .0005; Figure 3A). Interestingly, only previously primed individuals were able to mount a response to peptides expected to be unique to H5 HA, while both groups displayed increased reactivity to peptides likely to be conserved compared with recently circulating seasonal HA proteins (Supplementary Figure 2). Both groups exhibited statistically significant increases in the CD4+ T-cell response to conserved NP peptide-epitopes to which they had been repeatedly exposed (Figure 3C), demonstrating that the vaccine elicited comparable expansion of NP-specific CD4+ T cells (Figure 3D). Of note, when the subjects that received a baculovirus-expressed recombinant HA vaccine in 1997–1998 were examined separately, there was even greater H5 HA–specific CD4+ T-cell reactivity (Figure 3A and 3B), suggesting a possible advantage for repeated, intentional priming with different clades of H5 vaccine or perhaps a benefit to using recombinant HA protein for initial priming.
Relationship Between HA-Specific CD4+ T Cells and Neutralizing Antibody Production
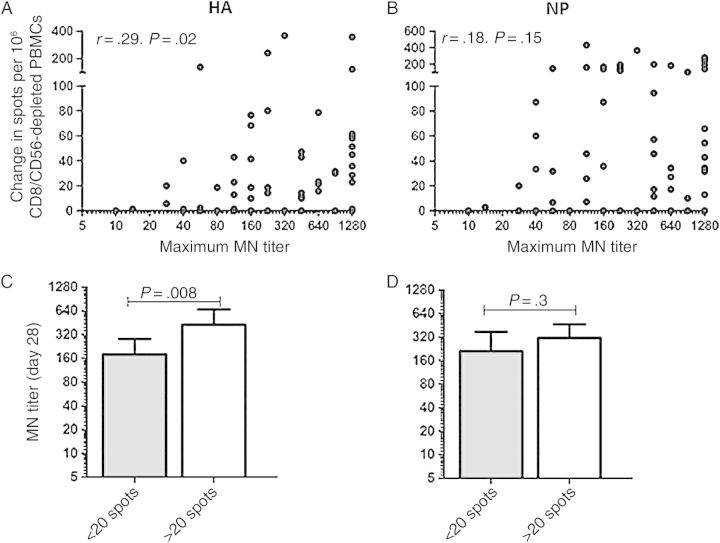
CD4+ T-cell help is critical for the production of high-affinity class-switched neutralizing antibody, and we and others have demonstrated that the specificities of responding CD4+ T cells and B cells may be linked [33, 34, 38]. Thus, we next wanted to determine whether increases in the CD4+ T-cell response directed against H5 HA peptide epitopes were associated with an enhanced neutralizing antibody response to vaccination. As our previous studies have shown that expansion of CD4+ T cells (as measured by the change in response between day 14 and baseline) predicts the development of a neutralizing antibody response following influenza vaccination [28], we examined that parameter here. In previously vaccinated subjects, there was a correlation between expansion of HA-specific CD4+ T cells between days 0 and 14 and the maximum neutralizing antibody titer attained (Figure 4A) that was not present when expansion of NP-specific (Figure 4B) or M1-specific (r = 0.09, P = .49; data not shown) CD4+ T cells was examined. To further explore this finding, we subgrouped individuals into those who had a detectable response to epitopes within the H5 HA protein and those who did not, using a difference of ≥20 spots between the day 14 and prevaccination time points as a breakpoint. This cutoff was chosen because it represented a change of at least 5 spots per well and divided the subjects into approximately equal groups. Interestingly, subjects who had a response to epitopes within the H5 HA protein achieved a neutralizing antibody GMT of 426, while those whose CD4+ T cells failed to respond had a geometric mean titer of 180 (P = .008; Figure 4C). This pattern was not seen when subjects were subgrouped on the basis of the magnitude of their response to NP epitopes (P = .3; Figure 4D). Overall, these results suggest that HA-specific B cells may preferentially recruit cognate help from HA-specific CD4+ T cells owing to selective uptake of HA protein through the B-cell receptor.
Figure 4.
Increases in CD4+ T-cell reactivity to hemagglutinin (HA) correlate with higher microneutralization (MN) titers. A and B, Correlation between the change in HA-specific (A) or nucleoprotein (NP)-specific (B) CD4+ T cells and the maximum neutralizing antibody titer measured. CD4+ T-cell responses with a negative change between day 14 and baseline were normalized to 0. r and P values were calculated using the Spearman rank correlation test. C and D, Subjects were subgrouped based on their CD4+ T-cell response to the HA (C) or NP (D) peptide pools, with a positive response defined as an increase of >20 spots in CD4+ T-cell reactivity. Bars represent the geometric mean MN titer, with error bars showing 95% confidence intervals. P values were calculated using the Mann–Whitney U test. Abbreviation: PBMC, peripheral blood mononuclear cell.
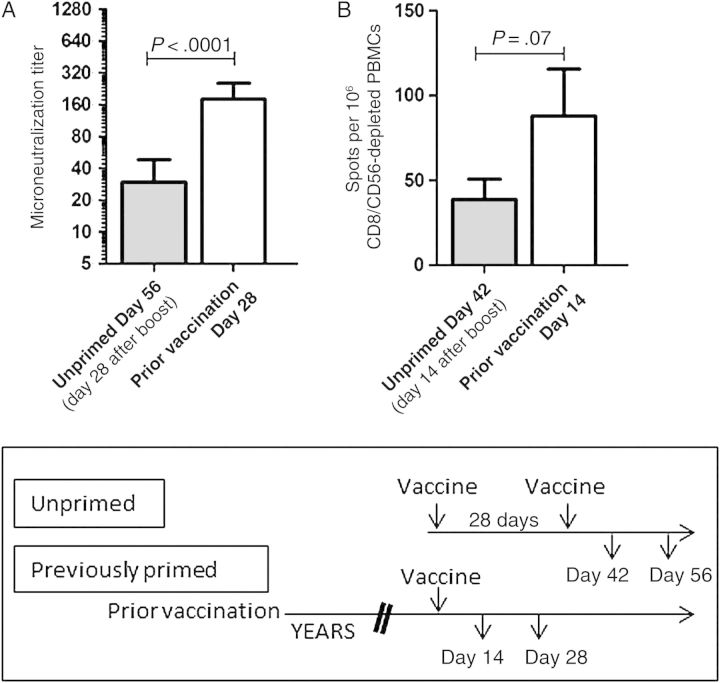
Effect of a Booster Vaccine Dose in Previously Unprimed Subjects
One factor complicating this study was that only unprimed subjects received a booster dose of vaccine. To determine whether a single booster dose 28 days after primary vaccination allowed unprimed subjects to achieve responses similar to those in the primed cohort, we compared antibody and HA-specific CD4+ T-cell responses at 28 and 14 days after booster administration, respectively, to responses in the primed cohort after a single vaccine dose. Both the neutralizing antibody response (Figure 5A) and the HA-specific CD4+ T-cell response (Figure 5B) were greater in subjects with previous vaccination, even after the unprimed cohort was given a second vaccine dose, with the difference in neutralizing antibody titer achieving statistical significance. This suggests that the advantages conferred by prepandemic vaccination are not replicated by a simple prime-boost vaccination regimen.
Figure 5.
The advantage of prior influenza A(H5N1) vaccination persists despite boosting of unprimed subjects. Neutralizing antibody and HA-specific CD4+ T-cell responses were examined after previously unprimed subjects were administered a second dose of influenza A/Indonesia/5/05 vaccine. Neutralizing antibody titers (A) and hemagglutinin (HA)-specific CD4+ T-cell responses (B) remained higher in the primed subjects even after unprimed subjects received a booster immunization. Lines show either the geometric mean titer (A) or the mean number of spots per 106 CD8-depleted and CD56-depleted peripheral blood mononuclear cells (PBMCs) (B), with error bars showing the 95% confidence interval. P values were calculated using the Mann–Whitney U test.
DISCUSSION
The experiments presented here demonstrate that subjects previously vaccinated years earlier with an influenza A(H5N1) vaccine have a selective increase in their H5 HA-specific CD4+ T-cell reactivity at day 0 and after challenge with a drifted influenza A(H5N1) vaccine variant. This greater H5 HA-specific CD4+ T-cell response is associated with an improved neutralizing antibody response, with a positive correlation between the ability to respond to epitopes within the H5 HA protein on revaccination and gains in the neutralizing antibody titer. Together, these findings suggest that, on reimmunization with even a drifted influenza A(H5N1) vaccine, an enriched population of HA-specific CD4+ T cells is available that, on recruitment, can contribute to more robust neutralizing antibody production.
This study argues for HA-specific CD4+ T cells having an important role in facilitating antibody responses on revaccination with an unadjuvanted avian influenza vaccine. It is possible that the previous vaccination primed a population of HA-specific CD4+ T cells that persisted in circulating memory and could be recruited into the evolving germinal center, facilitating HA-specific B-cell activation and the formation of memory B cells and long-lived plasma cells [25, 26, 39, 40]. This possibility is supported by previous studies that have reported a correlation between CD4+ T-cell responses and neutralizing antibody titers following adjuvanted avian influenza vaccination [29, 30], although these studies did not address the specificity of the CD4+ T-cell repertoire or how it is altered upon heterologous influenza A(H5N1) revaccination.
In addition to enhancing the HA-specific memory compartment, it is clear that previous influenza A(H5N1) vaccination could also prime cross-reactive long-lasting memory B cells that are rapidly recalled on subsequent H5 immunization [22, 23]. The slight increase in prevaccination neutralizing antibody seen in our study does support concurrent priming of the B-cell memory compartment. Although we did not evaluate the repertoire of H5 HA-specific B cells in this study, our results indicate that HA-specific CD4+ T cells may independently contribute to increases in neutralizing antibody titer following avian influenza vaccination. Further study is needed to understand the relative importance of B-cell and CD4+ T-cell priming in the improved vaccine responses that develop on rechallenge with a drifted influenza A(H5N1) vaccine variant.
While previous work has reported that healthy adults have CD4+ T cells that cross-react with avian HA proteins, including H5 [41–45], the number of HA-specific CD4+ T cells available to be recruited into the immune response on exposure to an avian HA protein is less than that on exposure to a seasonal virus [36] or to a pandemic virus more closely related to seasonal viruses, such as the 2009 pandemic influenza A(H1N1) strain [28]. This could help to explain the overall poor immunogenicity of avian influenza vaccines [8, 9, 13, 46, 47] and provides evidence to support improved vaccination strategies that optimize the avian HA-specific CD4+ T-cell response. The finding that HA-specific and not NP-specific CD4+ T-cell expansion correlates with the magnitude of the neutralizing antibody response could be consistent with a lack of physical association between the vaccine proteins and thus the lack of display of peptides derived from the internal virion proteins on the surface of HA-specific B cells. CD4+ T cells of broader specificity, including specificity to NP, polymerase, and M1, that are typically highly represented in the human CD4+ memory compartment may be able to contribute to other effector functions, such as recruitment of innate effectors to the lung or direct cytotoxicity. Indeed, Wilkinson et al have shown that preexisting NP-specific and M1-specific cells correlate with protection from infection in human subjects [48]. Future experiments by our group and others will lead to more complete characterization of the functional potential of responding CD4+ T cells, potentially enabling future vaccines to optimize priming of the cellular subsets best able to promote neutralizing antibody and protect against heterosubtypic infection.
Overall, this study provides strong support in favor of prepandemic vaccination for avian viruses. Although the strain of virus that will cause the next pandemic is uncertain, ongoing human infections with influenza A(H5N1) strains, sporadic cases of human-to-human transmission [2–4], and the need for only a small number of mutations to achieve sustained respiratory droplet transmission in a mammalian host [5–7] demonstrate the pandemic potential of influenza A(H5N1) infection. While rapid vaccination is the most potent way to contain an incipient pandemic [49, 50], stockpiled vaccines are unlikely to be an exact match for an emerging pandemic virus. By priming cross-reactive HA-specific CD4+ T-cell–mediated immunity, prepandemic immunization with an avian vaccine will allow for neutralizing antibodies to be generated more rapidly upon immunization with a single lower dose of matched vaccine and may also potentiate the antibody response in the setting of an active infection. Such a strategy may possibly mitigate both the extent of viral spread and morbidity and mortality during a pandemic due to a highly pathogenic virus such as influenza A(H5N1).
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the National Institutes of Health (contract HHSN266200700008C and grants 1K08AI106954 and 1K12HD068373-01).
Potential conflicts of interest. J. J. T. has professional relationships or consultancies with the following: Novartis Vaccines and Immune Targeting Systems, Abt Associates, Farmak PJSC, Novartis, Sanofi, GlaxoSmithKline, Pfizer, Takeda Pharmaceutical, Romark Laboratories, and Protein Sciences. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003–2014. http://www.who.int/influenza/human_animal_interface/EN_GIP_20140124CumulativeNumberH5N1cases.pdf?ua=1 Accessed 19 June 2014.
- 2.Wang H, Feng Z, Shu Y, et al. Probable limited person-to-person transmission of highly pathogenic avian influenza A (H5N1) virus in China. Lancet 2008; 371:1427–34. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Halloran ME, Sugimoto JD, Longini IM., Jr Detecting human-to-human transmission of avian influenza A (H5N1). Emerg Infect Dis 2007; 13:1348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med 2005; 352:333–40. [DOI] [PubMed] [Google Scholar]
- 5.Herfst S, Schrauwen EJ, Linster M, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 2012; 336:1534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imai M, Watanabe T, Hatta M, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 2012; 486:420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell CA, Fonville JM, Brown AE, et al. The potential for respiratory droplet-transmissible A/H5N1 influenza virus to evolve in a mammalian host. Science 2012; 336:1541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med 2006; 354:1343–51. [DOI] [PubMed] [Google Scholar]
- 9.Treanor JJ, Wilkinson BE, Masseoud F, et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine 2001; 19:1732–7. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein DI, Edwards KM, Dekker CL, et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis 2008; 197:667–75. [DOI] [PubMed] [Google Scholar]
- 11.Vesikari T, Forsten A, Herbinger KH, et al. Safety and immunogenicity of an MF59((R))-adjuvanted A/H5N1 pre-pandemic influenza vaccine in adults and the elderly. Vaccine 2012; 30:1388–96. [DOI] [PubMed] [Google Scholar]
- 12.Vesikari T, Karvonen A, Tilman S, et al. Immunogenicity and safety of MF59-adjuvanted H5N1 influenza vaccine from infancy to adolescence. Pediatrics 2010; 126:e762–70. [DOI] [PubMed] [Google Scholar]
- 13.Manzoli L, Salanti G, De Vito C, Boccia A, Ioannidis JP, Villari P. Immunogenicity and adverse events of avian influenza A H5N1 vaccine in healthy adults: multiple-treatments meta-analysis. Lancet Infect Dis 2009; 9:482–92. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson KG, Colegate AE, Podda A, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 2001; 357:1937–43. [DOI] [PubMed] [Google Scholar]
- 15.Stohr K. Vaccinate before the next pandemic? Nature 2010; 465:161. [DOI] [PubMed] [Google Scholar]
- 16.Stohr K, Bucher D, Colgate T, Wood J. Influenza virus surveillance, vaccine strain selection, and manufacture. Methods Mol Biol 2012; 865:147–62. [DOI] [PubMed] [Google Scholar]
- 17.Goji NA, Nolan C, Hill H, et al. Immune responses of healthy subjects to a single dose of intramuscular inactivated influenza A/Vietnam/1203/2004 (H5N1) vaccine after priming with an antigenic variant. J Infect Dis 2008; 198:635–41. [DOI] [PubMed] [Google Scholar]
- 18.Belshe RB, Frey SE, Graham I, et al. Safety and immunogenicity of influenza A H5 subunit vaccines: effect of vaccine schedule and antigenic variant. J Infect Dis 2011; 203:666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talaat KR, Luke CJ, Khurana S, et al. A Live Attenuated Influenza A(H5N1) Vaccine Induces Long-Term Immunity in the Absence of a Primary Antibody Response. J Infect Dis 2014; 209:1860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephenson I, Nicholson KG, Hoschler K, et al. Antigenically distinct MF59-adjuvanted vaccine to boost immunity to H5N1. N Engl J Med 2008; 359:1631–3. [DOI] [PubMed] [Google Scholar]
- 21.Khurana S, Coyle EM, Dimitrova M, et al. Heterologous prime-boost vaccination with MF59-adjuvanted H5 vaccines promotes antibody affinity maturation towards the hemagglutinin HA1 domain and broad H5N1 cross-clade neutralization. PLoS One 2014; 9:e95496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galli G, Hancock K, Hoschler K, et al. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci U S A 2009; 106:7962–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baer J, Santiago F, Yang H, et al. B cell responses to H5 influenza HA in human subjects vaccinated with a drifted variant. Vaccine 2010; 28:907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 2011; 29:621–63. [DOI] [PubMed] [Google Scholar]
- 25.Nutt SL, Tarlinton DM. Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nat Immunol 2011; 12:472–7. [DOI] [PubMed] [Google Scholar]
- 26.Ramiscal RR, Vinuesa CG. T-cell subsets in the germinal center. Immunol Rev 2013; 252:146–55. [DOI] [PubMed] [Google Scholar]
- 27.Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med 2012; 209:1241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nayak JL, Fitzgerald TF, Richards KA, Yang H, Treanor JJ, Sant AJ. CD4 T-cell expansion predicts neutralizing antibody responses to monovalent inactivated pandemic H1N1 influenza vaccine. J Infect Dis 2013; 207:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galli G, Medini D, Borgogni E, et al. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc Natl Acad Sci U S A 2009; 106:3877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen GK, Madhun AS, Breakwell L, et al. T-Helper 1 Cells Elicited by H5N1 Vaccination Predict Seroprotection. J Infect Dis 2012; 206:158–66. [DOI] [PubMed] [Google Scholar]
- 31.Bentebibel SE, Lopez S, Obermoser G, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med 2013; 5:176ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spensieri F, Borgogni E, Zedda L, et al. Human circulating influenza-CD4+ ICOS1+IL-21+ T cells expand after vaccination, exert helper function, and predict antibody responses. Proc Natl Acad Sci U S A 2013; 110:14330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nayak JL, Alam S, Sant AJ. Cutting edge: Heterosubtypic influenza infection antagonizes elicitation of immunological reactivity to hemagglutinin. J Immunol 2013; 191:1001–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alam S, Knowlden ZA, Sangster MY, Sant AJ. CD4 T cell help is limiting and selective during the primary B cell response to influenza virus infection. J Virol 2014; 88:314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alam S, Sant AJ. Infection with seasonal influenza virus elicits CD4 T cells specific for genetically conserved epitopes that can be rapidly mobilized for protective immunity to pandemic H1N1 influenza virus. J Virol 2011; 85:13310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards KA, Topham D, Chaves FA, Sant AJ. Cutting edge: CD4 T cells generated from encounter with seasonal influenza viruses and vaccines have broad protein specificity and can directly recognize naturally generated epitopes derived from the live pandemic H1N1 virus. J Immunol 2010; 185:4998–5002. [DOI] [PubMed] [Google Scholar]
- 37.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999; 37:937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sette A, Moutaftsi M, Moyron-Quiroz J, et al. Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity 2008; 28:847–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev 2012; 247:52–63. [DOI] [PubMed] [Google Scholar]
- 40.McHeyzer-Williams M, Okitsu S, Wang N, McHeyzer-Williams L. Molecular programming of B cell memory. Nat Rev Immunol 2012; 12:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roti M, Yang J, Berger D, Huston L, James EA, Kwok WW. Healthy human subjects have CD4+ T cells directed against H5N1 influenza virus. J Immunol 2008; 180:1758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jameson J, Cruz J, Terajima M, Ennis FA. Human CD8+ and CD4+ T lymphocyte memory to influenza A viruses of swine and avian species. J Immunol 1999; 162:7578–83. [PubMed] [Google Scholar]
- 43.Richards KA, Chaves FA, Sant AJ. Infection of HLA-DR1 transgenic mice with a human isolate of influenza a virus (H1N1) primes a diverse CD4 T-cell repertoire that includes CD4 T cells with heterosubtypic cross-reactivity to avian (H5N1) influenza virus. J Virol 2009; 83:6566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cusick MF, Wang S, Eckels DD. In vitro responses to avian influenza H5 by human CD4 T cells. J Immunol 2009; 183:6432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee LY, Ha do LA, Simmons C, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest 2008; 118:3478–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Couch RB, Patel SM, Wade-Bowers CL, Nino D. A randomized clinical trial of an inactivated avian influenza A (H7N7) vaccine. PLoS One 2012; 7:e49704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cox RJ, Madhun AS, Hauge S, et al. A phase I clinical trial of a PER.C6 cell grown influenza H7 virus vaccine. Vaccine 2009; 27:1889–97. [DOI] [PubMed] [Google Scholar]
- 48.Wilkinson TM, Li CK, Chui CS, et al. Preexisting influenza-specific CD4(+) T cells correlate with disease protection against influenza challenge in humans. Nat Med 2012; 18:274–80. [DOI] [PubMed] [Google Scholar]
- 49.Germann TC, Kadau K, Longini IM, Jr, Macken CA. Mitigation strategies for pandemic influenza in the United States. Proc Natl Acad Sci U S A 2006; 103:5935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature 2006; 442:448–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.