Abstract
Objectives Provide an updated literature review on prevalence, measurement, and correlates of disordered eating in youth with Type 1 diabetes (T1D), present a novel theoretical risk model (i.e., The Modified Dual Pathway Model) for disordered eating in youth with T1D incorporating psychosocial and physiological risk factors, and discuss clinical implications. Methods Literature review of prevalence, correlates, risk factors, and outcomes of disordered eating behavior (DEB) in youth with T1D. Results Insulin treatment, subsequent weight gain, and disruptions to hunger and satiety regulation are hypothesized disease-related mechanisms through which the treatment of T1D may increase vulnerability to development of behavior characterized as DEB. The Modified Dual Pathway Model integrates these factors with a validated psychosocial risk (body dissatisfaction, depression, and abstinence violation) model for DEB in nondiabetic youth. Conclusions The Modified Dual Pathway model of DEB in youth with T1D is a comprehensive representation of both psychosocial and T1D-related risk factors with the potential to inform future interventions for this population.
Keywords: eating disorders, Modified Dual Pathway, risk for disordered eating in T1D, Type 1 diabetes
Disordered eating behavior (DEB) consists of unhealthy weight control practices that typically onset in adolescence, and are linked to weight fluctuations and the development of eating disorders (Neumark-Sztainer, Wall, Larson, Eisenberg, & Loth, 2011). It is suggested that Type 1 diabetes (T1D), which also typically onsets in childhood or adolescence, increases risk for DEB. Posited risk factors include increased rates of depressive symptoms and changes in weight and shape associated with the disease itself (Goebel-Fabbri, 2009). Additional posited risk factors include frustration with blood glucose ranges, food preoccupation, body image dissatisfaction, and fear of weight gain (Goebel-Fabbri, 2009). Studies estimate that 20–40% of youth with T1D have used insulin manipulation for weight loss, which is associated with significant negative health outcomes including increased mortality rates (Colton, Rydall, Olmsted, Rodin, & Daneman, 2004; Goebel-Fabbri et al., 2008; Hanlan, Griffith, Patel, & Jasser, 2013; Young et al., 2013). The potential for increased DEB in youth with T1D and their unique treatment needs, coupled with adverse health outcomes, highlight the need to identify risk factors specific to this population. This article proposes an integration of a well-validated psychosocial risk model for the development of disordered eating in adolescents, with physiological influences specific to T1D. It is proposed that Stice’s Dual Pathway Model (Stice, 2001), extensively researched in adolescents without chronic illnesses, can be modified to include T1D-specific variables.
The Dual Pathway Model
Adolescents in Western cultures are exposed to messages by family, peers, and mass media about the importance of thinness and a specific physical appearance that is virtually unattainable (Stice, 2002). Repeated messages that one is not thin enough promote discontent with one’s body. Adolescent girls gain a significant amount of weight during puberty, which is a risk factor for body image dissatisfaction (Streigel-Moore & Bulik, 2007). Dissatisfaction with one’s body size, coupled with sociocultural pressure for thinness, can exacerbate preexisting genetic vulnerabilities for DEB (Stice, 2002; Trace, Baker, Peñas-Lledó, & Bulik, 2013). Body image dissatisfaction can promote both negative affect and dieting in efforts to lose weight (Stice, 2002; Streigel-Moore & Bulik, 2007). Both dieting (restraint pathway) and negative affect (negative affect pathway) are thought to predict binge eating (Allen, Byrne, & McLean, 2012). Restraint may trigger binge eating when breaking rigid dietary rules results in disinhibited eating. The relationship between binge eating and negative affect is maintained via negative reinforcement. Although the Dual Pathway Model has primarily been tested in adolescent girls, adolescent boys are also at risk for body dissatisfaction, negative affect, and DEB due to idealized images of muscularity in boys (Mond et al., 2014).
Risk for DEB in T1D
The Modified Dual Pathway model proposes three disease-based mechanisms through which DEB is potentiated in youth with T1D: (1) carbohydrate counting driving imposed food preoccupation, (2) weight fluctuations associated with variable use of insulin and subsequent body dissatisfaction, and (3) blood glucose fluctuations associated with mismatched insulin dose, excessive caloric intake secondary to hypoglycemia, and resultant weight gain. T1D involves weight loss associated with cessation of endogenous insulin production from beta cell death (Jahromi & Eisenbarth, 2007), and interruption of amylin production, which is associated with hunger and satiety regulation (Lutz, 2005). Amylin mediates several satiety mechanisms via its effects on the area postrema, an area of the brainstem that integrates hormonal and metabolic signals to regulate food intake (Mack et al., 2007). In addition to amylin, ghrelin is disrupted in youth with T1D and is posited to increase hunger and promote dysregulated eating (Prodam et al., 2014). Satiety can also be disrupted in youth with T1D as a consequence of the treatment regimen, specifically timing of meals and subsequent dosing of insulin that is not based on hunger cues.
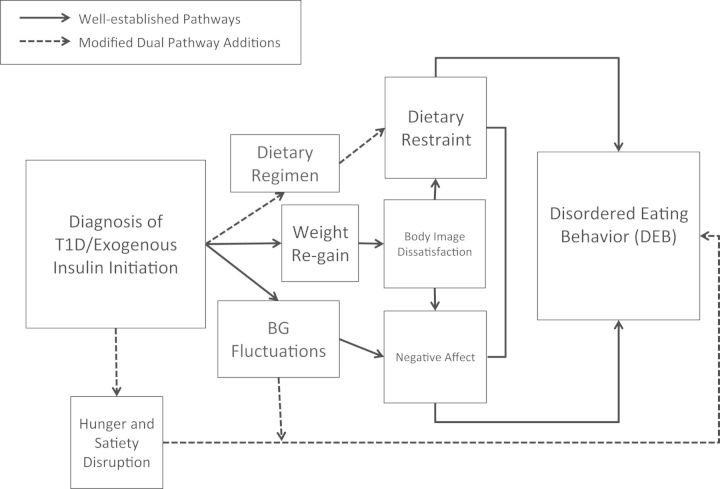
On diagnosis and exogenous insulin treatment, individuals experience significant weight gain, which is often perceived as undesirable, and may promote body dissatisfaction, particularly in girls (Mellin, Neumark-Sztainer, Patterson, & Sockalosky, 2004; Russell-Jones & Khan, 2007). Further, many adolescents struggle with episodes of hypoglycemia and subsequent dysregulated eating, which can promote guilt and prompt insulin omission (Merwin et al., 2014). Although validated in nondiabetic youth, Stice’s model does not account for hunger and satiety disruptions, or other T1D-related factors such as implementation of a dietary regimen. Youth with T1D are charged with monitoring blood sugar level and matching insulin dose to carbohydrate intake to maintain glycemic control (Bantle et al., 2008). Thus, having diabetes and engaging in treatment potentiates risk for DEB, above and beyond risk that an individual without diabetes might experience. Symptoms may derive from very different sources: weight concerns, psychopathology, poor psychosocial adjustment to the illness, and/or disrupted physiology (Young-Hyman & Davis, 2010). The proposed model of risk for DEB integrates T1D treatment, psychological, and physiological variables (Figure 1).
Figure 1.
The Modified Dual Pathway Model.
Factors in T1D That Influence DEB
Initiation of Insulin, Weight Gain, and Body Dissatisfaction
Initiation of insulin treatment on T1D diagnosis results in weight rebound and accelerates weight gain due to increased metabolic efficiency in children and adolescents (Russell-Jones & Khan, 2007). Overweight status is a risk factor for the development of DEB in youth with T1D (Engstrom et al., 1999). Further, adolescents with T1D are often concurrently managing the physical changes that occur as a result of pubertal development, a process that can also increase body fat (Tremblay & Lariviere, 2009). Thus, the integrated model suggests that weight gain secondary to treatment with initiation of exogenous insulin increases risk for DEB via body image dissatisfaction, especially in girls (Figure 1). Adolescent girls may desire weight loss, while adolescent boys may desire an increase in muscle mass rather than an increase in body fat.
Dietary Regimen and Dietary Restraint
The Dual Pathway Model proposes that body dissatisfaction as a result of thin ideal internalization during pubertal development then leads to dieting behavior (Stice, 2001). One modification made by the proposed model is that both weight gain and the T1D insulin dosing algorithm (i.e., counting carbohydrates, the foundation of the dietary regimen) may have similarities to self-imposed diets in a nondiabetic population. Previous studies have found that some patients use carbohydrate counting in a more extreme manner with excessive concerns about food composition and planning meals, which leads to food preoccupation and even DEBs (Colton et al., 2004; Pereira & Alvarenga, 2007). Finally, youth may resort to dieting rather than exercise to manage weight, as exercise can increase risk for hypoglycemia and, hence, the need to eat (Yardley et al., 2010).
The proposed model suggests that increases in body dissatisfaction associated with treatment-related weight gain may increase risk for dietary restriction. Cross-sectional and longitudinal studies have demonstrated associations between weight gain, body image dissatisfaction, and dietary restraint in youth with T1D (Mellin et al., 2004). However, in the proposed modified model, dietary changes are not necessarily directly linked to internalization of the thin ideal. Thus, youth may adhere to a dietary regimen as part of the T1D regimen or use adherence to the dietary regimen to mask excessive restriction of eating.
Additionally, it is not known if failing to adhere to carbohydrate treatment guidelines when managing T1D represents similar risk for abstinence violation-driven binge eating (i.e., nonadherence is perceived as breaking dietary rules). The abstinence–violation effect (AVE) posits that a lapse in restrained eating produces the AVE, which fuels the likelihood of an increase in binge eating such that the greater the AVE intensity, the greater the likelihood of an increase in that behavior after a lapse (Merwin et al., 2014; Polivy & Herman, 1985). Thus, cognitive and affective consequences of failure to adhere to dietary regimen behaviors may promote binge eating via maladaptive affect regulation mechanisms. For example, an adolescent may feel guilt and frustration regarding failure to adhere to treatment guidelines, which could promote emotionally driven eating. In this case, the adolescent may not have been attempting to manage diet for weight loss purposes, but rather, experienced difficulty with adherence and regulation of blood sugars, resulting in poor regulation of negative emotion.
Depression and DEB Risk in Youth With T1D
In the general population, dietary restraint can promote depressive symptoms due to potential failed efforts at dieting (Stice, 2001). This is especially salient in youth with T1D who have increased rates of depressive symptoms and for whom weight is elevated (Kanner, Hamrin, & Grey, 2003). Studies have reported higher rates of depression in youth with both DEB and T1D (Colton, Olmsted, Daneman, & Rodin, 2013). The proposed model suggests that for youth who have depressive symptoms before onset of T1D, the effects of receiving the diagnosis, demands of managing the treatment regimen, and difficulty controlling weight gain associated with insulin therapy may exacerbate depression. For youth with preexisting body image concerns, the effects of weight gain could exacerbate body dissatisfaction, which can increase depressive symptoms. Additionally, depressive symptoms may develop due to the challenges of managing a chronic illness. Depressive symptoms are postulated to subsequently increase risk for binge eating via negative reinforcement, or increase risk for manipulation of insulin/dietary regimen for weight loss.
Integration of Physiological and Psychological Pathways to Overeating
Episodes of overeating in response to mismatched blood sugar level and insulin dose, and hypoglycemia, may be related to disruptions to appetite regulation in the hypothalamus (Engstrom et al., 1999). A recent study found that 98% of individuals with T1D engage in disinhibited eating during perceived episodes of hypoglycemia (Merwin et al., 2014). Interestingly, this study did not distinguish between actual hypoglycemic episodes and perceived hypoglycemic episodes. Thus, inaccurate insulinization, actual hypoglycemia, and perceived hypoglycemia could potentially drive episodes of loss of control over eating. The proposed model suggests that treatment-imposed food preoccupation enhances the risk for binge eating through both mechanisms of dietary restraint (abstinence violation and affect regulation attempts) and disruptions in hunger and satiety caused by hormonal dysregulation. Hunger and satiety dysregulation is not mediated by dietary restraint, negative affect, or body image dissatisfaction in the modified model because physiological mechanisms may directly contribute to what appears to be DEB.
Clinical Implications
There is limited research on interventions for youth with T1D and DEB. One intervention provided psychoeducation on the relationship between DEB and T1D, the detrimental effects of insulin omission, the effects of DEB on glycemic control, and encouraged patients to approach eating with a balance, nondeprivational approach (Olmsted, Daneman, Rydall, Lawson, & Rodin, 2002). Although there were reductions in DEB, there were no significant effects on glycemic control, adherence, or insulin omission (Olmsted et al., 2002). This highlights the need for interventions to address disease- and treatment-related variables.
An integrated approach for treatment of DEB in youth with T1D would target dietary restriction and binge eating in the context of disease processes, the diabetes treatment regimen, and psychological factors. Given the demonstrated effectiveness of cognitive-behavioral therapy (CBT) for the treatment of bulimia nervosa (Hay, 2013), and that the most commonly reported disordered eating symptoms in youth with T1D include binge eating and purging (Colton et al., 2004), techniques drawn from CBT for bulimia could be modified to target disease-specific treatment factors and symptoms in the T1D population, making sure to identify those which are within the control of the patient and those which are disease process-based. Consideration of co-occurring depressive symptoms must be made in terms of case conceptualization and intervention. CBT interventions can be tailored to address depressive symptoms, and if severe, psychiatric medications may be considered in the context of treatment.
Consistent with the Modified Dual Pathway model, providers would need to take into consideration the insulin regimen, disruptions to hunger and satiety cues, and the likelihood of weight gain consequent to good glycemic control. Implementation of regular meals and snacks, support of adherence to an agreed-upon insulin regimen based on the adolescent’s lifestyle, and education to difficulty with satiety cues could be helpful in terms of decreasing risk for binge eating or dysregulated eating in response to blood glucose changes. Finally, the use of diabetes-specific screening tools such as the Diabetes Eating Problems Survey—Revised (Markowitz et al., 2010) to identify maladaptive misuse of insulin versus nonadherence is an important step in accurately identifying true DEB in youth with T1D.
DEB tends to worsen over time and youth with co-occurring T1D and DEB have higher dropout rates from psychological therapies (Custal et al., 2014). Thus, prevention efforts and early intervention with a multidisciplinary team involving an endocrinologist, diabetes educator, and both a dietician and mental health professional proficient in the treatment of eating disorders and experience with diabetes are warranted (Goebel-Fabbri, 2009; Olmsted et al., 2002).
Diabetes education should be modified to better educate all patients to expected disruptions to hunger and satiety as a preventive step, and to educate patients about the negative health consequences of insulin manipulation. Further, it is important that members of the treatment team are careful to not introduce the idea of insulin manipulation (rather, asking open-ended questions). Given the possibility for body image dissatisfaction resulting from weight gain, targeting current and preexisting cognitive distortions associated with food, weight, and shape concerns consistent with Phase 2 of CBT for bulimia nervosa by a mental health professional is indicated (Colton et al., 2004; Hay, 2013).
Future Directions
The Modified Dual Pathway model of DEB in youth with T1D is a comprehensive representation of both psychosocial and T1D-related risk factors. Prospective studies are needed to assess the relationships between glycemic control, weight, and depression in newly diagnosed youth with T1D and the development of DEB, as well as age-related changes in psychosocial risk factors. Examination of potential changes in DEB over time as youth progress with T1D management can provide insight into the role of the risk factors theorized by the Modified Dual Pathway Model. For example, the proposed model is limited by its focus on weight gain following initiation of insulin treatment as a risk factor for the initial development of DEB. However, DEB may develop over time as a result of other factors identified in the model—repeated challenges to adherence and thus weight, hunger and satiety fluctuations, or the growing importance of weight and shape as a child ages. Identification of insulin misuse for weight control versus other reasons such as nonadherence or physiological factors such as dysregulation of hunger and satiety is an important distinction to be made in future research. Such distinctions have the potential to inform different clinical interventions. Future studies should incorporate assessment of hunger and satiety and proprioception of hypoglycemia, in addition to assessment of traditional eating disorder symptoms such as body image dissatisfaction and depression to further delineate risks in this population and identify specific pathways to DEB. This model has the potential to inform future interventions for youth with T1D and co-occurring DEB, which would significantly improve physical and psychological health outcomes.
Conflicts of interest: None declared.
References
- Allen K L, Byrne S M, McLean M J. The dual-pathway and cognitive-behavioural models of binge eating: Prospective evaluation and comparison. European Child and Adolescent Psychiatry. 2012;21:51–62. doi: 10.1007/s00787-011-0231-z. [DOI] [PubMed] [Google Scholar]
- Bantle J P, Wylie-Rosett J, Albright A L, Apovian C M, Clark N G, Franz M J, Hoogwerf B J, Lichtenstein A H, Mayer-Davis E, Mooradian A D, Wheeler M L. Nutrition recommendations and interventions for diabetes: A position statement of the American Diabetes Association. Diabetes Care. 2008;31:S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- Colton P A, Olmsted M P, Daneman D, Rodin GM. Depression, disturbed eating behavior, and metabolic control in teenage girls with type 1 diabetes. Pediatric Diabetes. 2013;14:372–376. doi: 10.1111/pedi.12016. [DOI] [PubMed] [Google Scholar]
- Colton P, Rydall A, Olmsted M, Rodin G, Daneman D. Disturbed eating behavior and eating disorders in preteen and early teenage girls with type 1 diabetes. Diabetes Care. 2004;27:1654–1659. doi: 10.2337/diacare.27.7.1654. [DOI] [PubMed] [Google Scholar]
- Custal N, Arcelus J, Agüera Z, Bove F I, Wales J, Granero R, Jiménez-Murcia S, Sánchez I, Riesco N, Alonso P, Crespo J M, Virgili N, Menchón J M, Fernandez-Aranda F. Treatment outcome of patients with comorbid type 1 diabetes and eating disorders. BMC Psychiatry. 2014;14:140. doi: 10.1186/1471-244X-14-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom I, Kroon M, Arvidsson C G, Segnestam K, Snellman K, Aman J. Eating disorders in adolescent girls with insulin-dependent diabetes mellitus: A population-based case-control study. Acta Paediatrica. 1999;88:175–180. doi: 10.1080/08035259950170358. [DOI] [PubMed] [Google Scholar]
- Goebel-Fabbri A E. Disturbed eating behaviors and eating disorders in type 1 diabetes: Clinical significance and treatment recommendations. Current Diabetes Reports. 2009;9:133–139. doi: 10.1007/s11892-009-0023-8. [DOI] [PubMed] [Google Scholar]
- Goebel-Fabbri A E, Fikkan J, Franko D L, Pearson K, Anderson B J, Weinger K. Insulin restriction and associated morbidity and mortality in women with type 1 diabetes. Diabetes Care. 2008;31:415–419. doi: 10.2337/dc07-2026. [DOI] [PubMed] [Google Scholar]
- Hanlan M E, Griffith J, Patel N, Jaser S S. Eating disorders and disordered eating in type 1 diabetes: Prevalence, screening, and treatment options. Current Diabetes Reports. 2013;13:909–916. doi: 10.1007/s11892-013-0418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay P. A systematic review of evidence for psychological treatments in eating disorders: 2005–2012. International Journal of Eating Disorders. 2013;46:462–469. doi: 10.1002/eat.22103. [DOI] [PubMed] [Google Scholar]
- Jahromi M M, Eisenbarth G S. Cellular and molecular pathogenesis of type 1A diabetes. Cellular and Molecular Life Sciences. 2007;64:865–872. doi: 10.1007/s00018-007-6469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner S, Hamrin V, Grey M. Depression in adolescents with diabetes. Journal of Child and Adolescent Psychiatric Nursing. 2003;16:15–24. doi: 10.1111/j.1744-6171.2003.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Lutz T A. Pancreatic amylin as a centrally acting satiating hormone. Current Drug Targets. 2005;6:181–189. doi: 10.2174/1389450053174596. [DOI] [PubMed] [Google Scholar]
- Mack C, Wilson J, Athanacio J, Reynolds J, Laugero K, Guss S, Vu C, Roth J, Parkes D. Pharmacological actions of the peptide hormone amylin in the long-term regulation of food intake, food preference, and body weight. Regulatory, Integrative, & Comparative Physiology. American Journal of Physiology. 2007;293:1855–1863. doi: 10.1152/ajpregu.00297.2007. [DOI] [PubMed] [Google Scholar]
- Markowitz J T, Butler D A, Volkening L K, Antisdel J E, Anderson B J, Laffel L M. Brief screening tool for disordered eating in diabetes internal consistency and external validity in a contemporary sample of pediatric patients with type 1 diabetes. Diabetes Care. 2010;33:495–500. doi: 10.2337/dc09-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellin A E, Neumark-Sztainer D, Patterson J, Sockalosky J. Unhealthy weight management behavior among adolescent girls with type 1 diabetes mellitus: The role of familial eating patterns and weight-related concerns. Journal of Adolescent Health. 2004;35:278–289. doi: 10.1016/j.jadohealth.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Merwin R M, Moskovich A A, Dmitrieva N O, Pieper C F, Honeycutt L K, Zucker N L, Surwitt R S, Buhi L. Disinhibited eating and weight-related insulin mismanagement among individuals with type 1 diabetes. Appetite. 2014;81:123–130. doi: 10.1016/j.appet.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J, Hall A, Bentley C, Harrison C, Gratwick-Sarll K, Lewis V. Eating-disordered behavior in adolescent boys: Eating disorder examination questionnaire norms. International Journal of Eating Disorders. 2014;47:335–341. doi: 10.1002/eat.22237. [DOI] [PubMed] [Google Scholar]
- Neumark-Sztainer D, Wall M, Larson N I, Eisenberg M E, Loth K. Dieting and disordered eating behaviors from adolescence to young adulthood: Findings from a 10 year longitudinal study. Journal of the American Dietetic Association. 2011;111:1004–1011. doi: 10.1016/j.jada.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted M P, Daneman D, Rydall A C, Lawson M L, Rodin G. The effects of psychoeducation on disturbed eating attitudes and behavior in young women with type 1 diabetes mellitus. International Journal of Eating Disorders. 2002;32:230–239. doi: 10.1002/eat.10068. [DOI] [PubMed] [Google Scholar]
- Pereira R F, Alvarenga M. Disordered eating: Identifying, treating, preventing, and differentiating it from eating disorders. Diabetes Spectrum. 2007;20:141–148. [Google Scholar]
- Polivy J, Herman C P. Dieting and bingeing: A causal analysis. American Psychologist. 1985;40:193–201. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- Prodam F, Cadario F, Bellone S, Trovato L, Moia S, Pozzi E, Savastio S, Bona G. Obestatin levels are associated with c-peptide and antiinsulin antibodies at the onset, whereas unacylated and acylated ghrelin levels are not predictive of long-term metabolic control in children with type 1 diabetes. The Journal of Clinical Endocrinology & Metabolism. 2014;99(4):599–607. doi: 10.1210/jc.2013-3294. [DOI] [PubMed] [Google Scholar]
- Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes: Causes, effects and coping strategies. Diabetes Obesity and Metabolism. 2007;9:799–812. doi: 10.1111/j.1463-1326.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- Sigal R J. Greater fluctuations in blood glucose seen both during and after aerobic exercise as compared to resistance exercise or no exercise in type 1 diabetes: A study using continuous glucose monitoring. Applied Physiology, Nutrition, & Metabolism. 2010;35:669–675. [Google Scholar]
- Stice E. A prospective test of the dual-pathway model of bulimic pathology: Mediating effects of dieting and negative affect. Journal of Abnormal Psychology. 2001;110:124–135. doi: 10.1037//0021-843x.110.1.124. [DOI] [PubMed] [Google Scholar]
- Stice E. Risk and maintenance factors for eating pathology: A meta-analytic review. Psychological Bulletin. 2002;128:825–848. doi: 10.1037/0033-2909.128.5.825. [DOI] [PubMed] [Google Scholar]
- Streigel-Moore R H, Bulik C M. Risk factors for eating disorders. American Psychologist. 2007;62:181–198. doi: 10.1037/0003-066X.62.3.181. [DOI] [PubMed] [Google Scholar]
- Trace S E, Baker J H, Peñas-Lledó E, Bulik C M. The Genetics of Eating Disorders. Annual Review of Clinical Psychology. 2013;9:589–620. doi: 10.1146/annurev-clinpsy-050212-185546. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Lariviere M. The influence of puberty onset, body mass index, and pressure to be thin on disordered eating behaviors in children and adolescents. Eating Behaviors. 2009;10:75–83. doi: 10.1016/j.eatbeh.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Yardley J E, Kenny G P, Perkins B A, Riddell M C, Malcolm J S, Sigal R J. Greater fluctuations in blood glucose seen both during and after aerobic exercise as compared to resistance exercise or no exercise in type 1 diabetes: A study using continuous glucose monitoring. Applied Physiology, Nutrition, & Metabolism. 2010;35:669–675. [Google Scholar]
- Young-Hyman D L, Davis C L. Disordered eating behavior in individuals with diabetes. Importance of context, evaluation, and classification. Diabetes Care. 2010;33:683–689. doi: 10.2337/dc08-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young V, Eiser C, Johnson B, Brierley S, Epton T, Elliott J, Heller S. Eating problems in adolescents with type 1 diabetes: A systematic review with meta-analysis. Diabetic Medicine. 2013;30:189–198. doi: 10.1111/j.1464-5491.2012.03771.x. [DOI] [PubMed] [Google Scholar]