Abstract
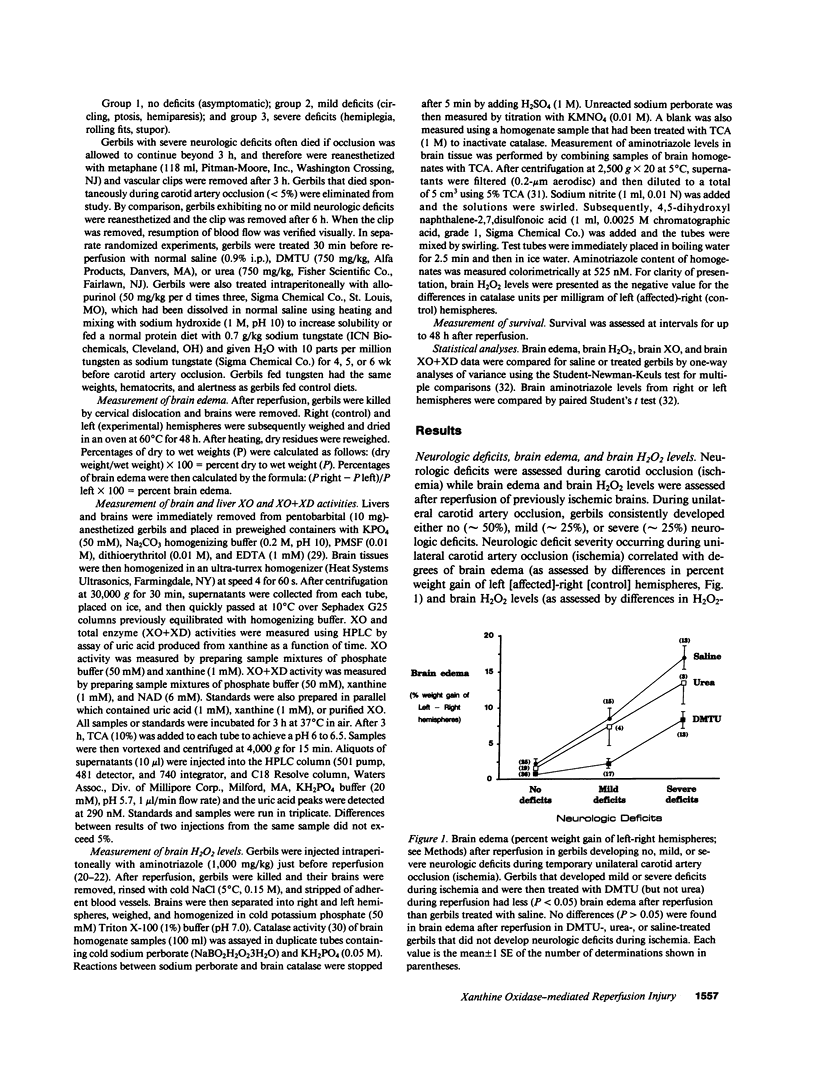
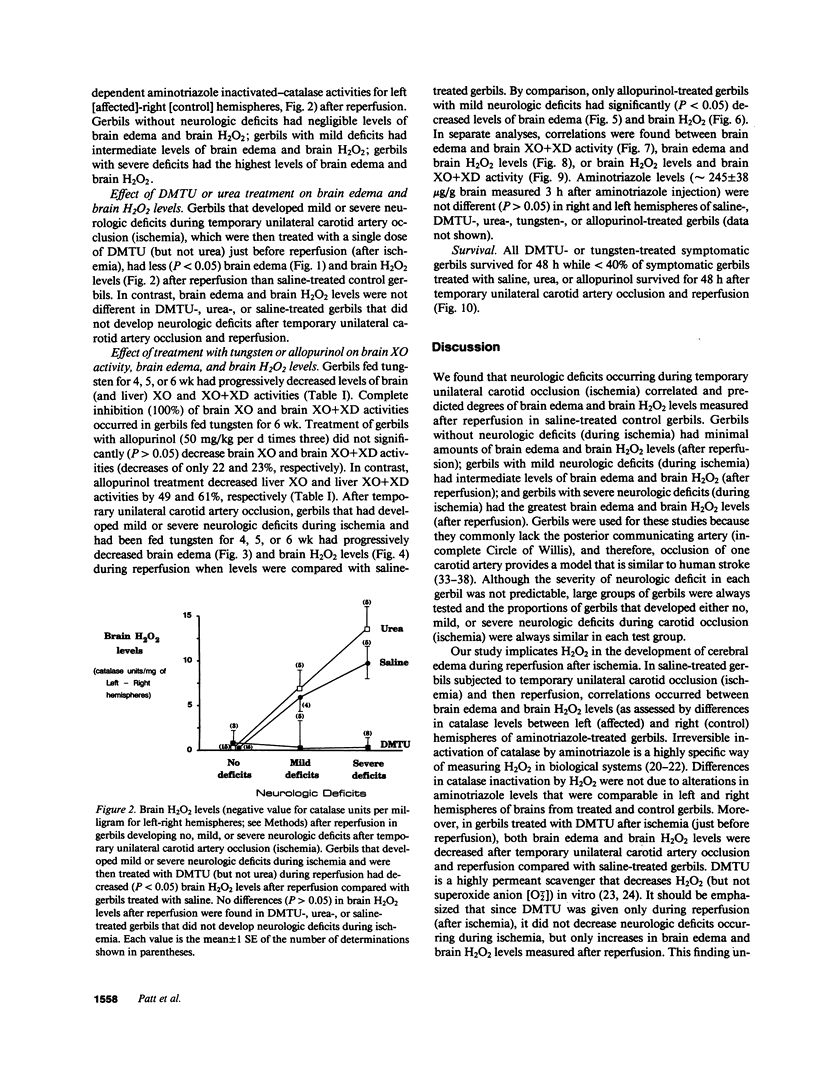
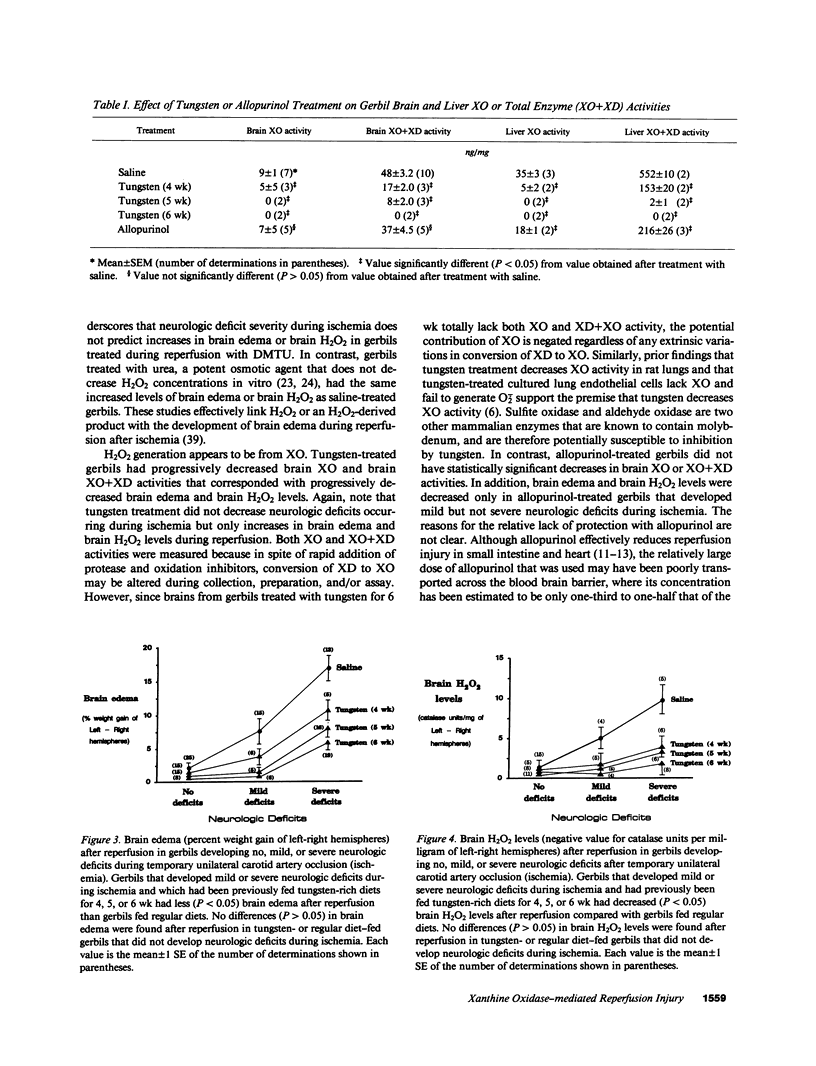
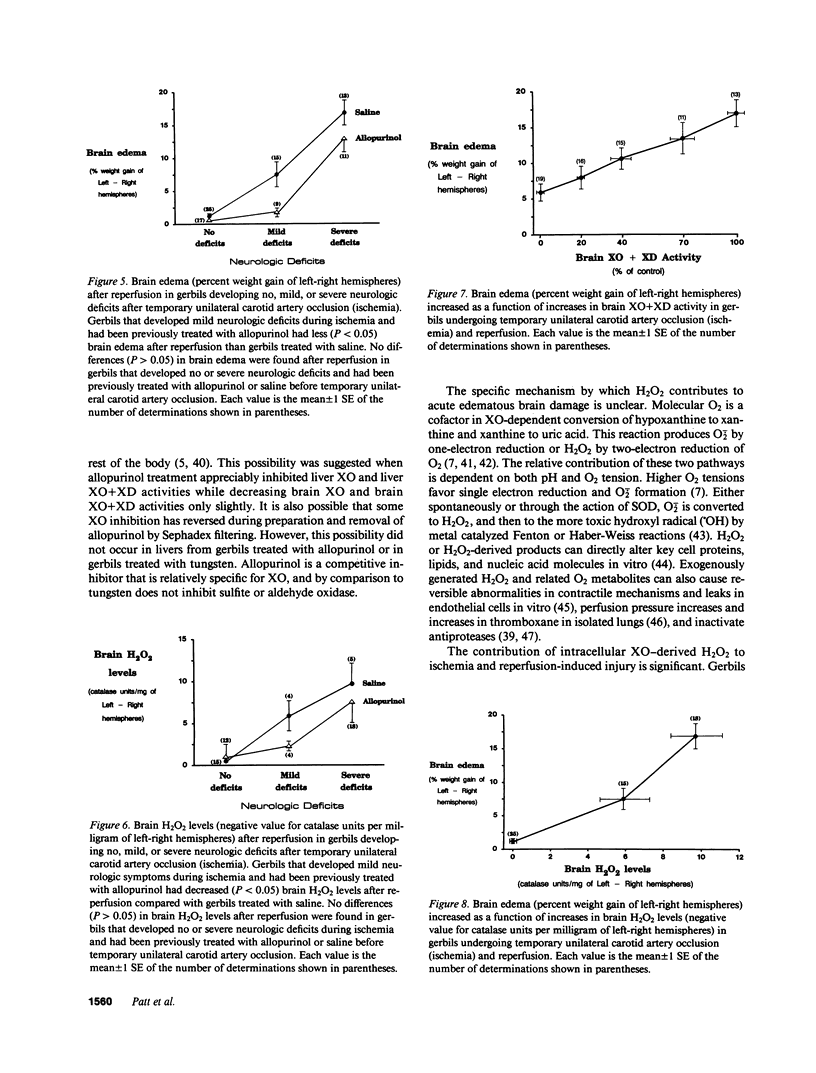
The contribution of toxic O2 metabolites to cerebral ischemia reperfusion injury has not been determined. We found that gerbils subjected to temporary unilateral carotid artery occlusion (ischemia) consistently developed neurologic deficits during ischemia with severities that correlated with increasing degrees of brain edema and brain H2O2 levels after reperfusion. In contrast, gerbils treated just before reperfusion (after ischemia) with dimethylthiourea (DMTU), but not urea, had decreased brain edema and brain H2O2 levels. In addition, gerbils fed a tungsten-rich diet for 4, 5, or 6 wk developed progressive decreases in brain xanthine oxidase (XO) and brain XO + xanthine dehydrogenase (XD) activities, brain edema, and brain H2O2 levels after temporary unilateral carotid artery occlusion and reperfusion. In contrast to tungsten-treated gerbils, allopurinol-treated gerbils did not have statistically significant decreases in brain XO or XO + XD levels, and reduced brain edema and brain H2O2 levels occurred only in gerbils developing mild but not severe neurologic deficits during ischemia. Finally, gerbils treated with DMTU or tungsten all survived, while greater than 60% of gerbils treated with urea, allopurinol, or saline died by 48 h after temporary unilateral carotid artery occlusion and reperfusion. Our findings indicate that H2O2 from XO contributes to reperfusion-induced edema in brains subjected to temporary ischemia.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery S., Crockard H. A., Russell R. R. Evolution and resolution of oedema following severe temporary cerebral ischaemia in the gerbil. J Neurol Neurosurg Psychiatry. 1984 Jun;47(6):604–610. doi: 10.1136/jnnp.47.6.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B. R., Cheronis J. C., Sandhaus R. A., Berger E. M., White C. W., Repine J. E. O2 metabolites and neutrophil elastase synergistically cause edematous injury in isolated rat lungs. J Appl Physiol (1985) 1986 Dec;61(6):2224–2229. doi: 10.1152/jappl.1986.61.6.2224. [DOI] [PubMed] [Google Scholar]
- Battelli M. G., Corte E. D., Stirpe F. Xanthine oxidase type D (dehydrogenase) in the intestine and other organs of the rat. Biochem J. 1972 Feb;126(3):747–749. doi: 10.1042/bj1260747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz A. L. Identification of hypoxanthine transport and xanthine oxidase activity in brain capillaries. J Neurochem. 1985 Feb;44(2):574–579. doi: 10.1111/j.1471-4159.1985.tb05451.x. [DOI] [PubMed] [Google Scholar]
- Braunwald E., Kloner R. A. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985 Nov;76(5):1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B., Berry L. C., Jr, Repine J. E. Antioxidants protect cultured bovine lung endothelial cells from injury by endotoxin. J Appl Physiol (1985) 1987 Aug;63(2):840–850. doi: 10.1152/jappl.1987.63.2.840. [DOI] [PubMed] [Google Scholar]
- Brown J. M., Terada L. S., Grosso M. A., Whitmann G. J., Velasco S. E., Patt A., Harken A. H., Repine J. E. Xanthine oxidase produces hydrogen peroxide which contributes to reperfusion injury of ischemic, isolated, perfused rat hearts. J Clin Invest. 1988 Apr;81(4):1297–1301. doi: 10.1172/JCI113448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie-Pope B. C., Palmer G. C., Chronister R. B., Callahan A. S., 3rd Adenylate cyclase and histopathological changes in the gerbil brain following prolonged unilateral ischemia and recirculation. Stroke. 1985 Jul-Aug;16(4):710–717. doi: 10.1161/01.str.16.4.710. [DOI] [PubMed] [Google Scholar]
- Cohen G., Somerson N. L. Catalase-aminotriazole method for measuring secretion of hydrogen peroxide by microorganisms. J Bacteriol. 1969 May;98(2):543–546. doi: 10.1128/jb.98.2.543-546.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Levin R. I., Belanoff J., Weissmann G., Hirschhorn R. Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J Clin Invest. 1986 Sep;78(3):760–770. doi: 10.1172/JCI112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demopoulos H. B., Flamm E. S., Pietronigro D. D., Seligman M. L. The free radical pathology and the microcirculation in the major central nervous system disorders. Acta Physiol Scand Suppl. 1980;492:91–119. [PubMed] [Google Scholar]
- Engerson T. D., McKelvey T. G., Rhyne D. B., Boggio E. B., Snyder S. J., Jones H. P. Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues. J Clin Invest. 1987 Jun;79(6):1564–1570. doi: 10.1172/JCI112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEINSTEIN R. N., BERLINER S., GREEN F. O. Mechanism of inhibition of Catalase by 3-amino-1,2,4-triazole. Arch Biochem Biophys. 1958 Jul;76(1):32–44. doi: 10.1016/0003-9861(58)90116-4. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Hernandez L. A., Granger D. N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986 Oct;251(4 Pt 1):G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- HIGGINS E. S., RICHERT D. A., WESTERFELD W. W. Molybdenum deficiency and tungstate inhibition studies. J Nutr. 1956 Aug 10;59(4):539–559. doi: 10.1093/jn/59.4.539. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Grootveld M. The measurement of free radical reactions in humans. Some thoughts for future experimentation. FEBS Lett. 1987 Mar 9;213(1):9–14. doi: 10.1016/0014-5793(87)81455-2. [DOI] [PubMed] [Google Scholar]
- Harrison M. J., Arnold J., Sedal L., Russell R. W. Ischaemic swelling of cerebral hemisphere in the gerbil. J Neurol Neurosurg Psychiatry. 1975 Dec;38(12):1194–1196. doi: 10.1136/jnnp.38.12.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearse D. J., Manning A. S., Downey J. M., Yellon D. M. Xanthine oxidase: a critical mediator of myocardial injury during ischemia and reperfusion? Acta Physiol Scand Suppl. 1986;548:65–78. [PubMed] [Google Scholar]
- Ito U., Ohno K., Suganuma Y., Suzuki K., Inaba Y. Effect of steroid on ischemic brain edema. Analysis of cytotoxic and vasogenic edema occurring during ischemia and after restoration of blood flow. Stroke. 1980 Mar-Apr;11(2):166–172. doi: 10.1161/01.str.11.2.166. [DOI] [PubMed] [Google Scholar]
- Jackson J. H., White C. W., Parker N. B., Ryan J. W., Repine J. E. Dimethylthiourea consumption reflects H2O2 concentrations and severity of acute lung injury. J Appl Physiol (1985) 1985 Dec;59(6):1995–1998. doi: 10.1152/jappl.1985.59.6.1995. [DOI] [PubMed] [Google Scholar]
- Jarasch E. D., Bruder G., Heid H. W. Significance of xanthine oxidase in capillary endothelial cells. Acta Physiol Scand Suppl. 1986;548:39–46. [PubMed] [Google Scholar]
- Jarasch E. D., Grund C., Bruder G., Heid H. W., Keenan T. W., Franke W. W. Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell. 1981 Jul;25(1):67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Rajagopalan K. V., Cohen H. J. Molecular basis of the biological function of molybdenum. Effect of tungsten on xanthine oxidase and sulfite oxidase in the rat. J Biol Chem. 1974 Feb 10;249(3):859–866. [PubMed] [Google Scholar]
- Joó F. The blood-brain barrier. New aspects to the function of the cerebral endothelium. Nature. 1986 May 15;321(6067):197–198. doi: 10.1038/321197a0. [DOI] [PubMed] [Google Scholar]
- Kontos H. A., Wei E. P., Povlishock J. T., Dietrich W. D., Magiera C. J., Ellis E. F. Cerebral arteriolar damage by arachidonic acid and prostaglandin G2. Science. 1980 Sep 12;209(4462):1242–1245. doi: 10.1126/science.7403881. [DOI] [PubMed] [Google Scholar]
- Levine S., Sohn D. Cerebral ischemia in infant and adult gerbils. Relation to incomplete circle of Willis. Arch Pathol. 1969 Mar;87(3):315–317. [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A., SCHEJTER A. Irreversible reaction of 3-amino-1:2:4-triazole and related inhibitors with the protein of catalase. Biochem J. 1960 Feb;74:339–348. doi: 10.1042/bj0740339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McCord J. M. The superoxide free radical: its biochemistry and pathophysiology. Surgery. 1983 Sep;94(3):412–414. [PubMed] [Google Scholar]
- Michelson A. M., Puget K. Cell penetration by exogenous superoxide dismutase. Acta Physiol Scand Suppl. 1980;492:67–80. [PubMed] [Google Scholar]
- Nakai K., Welch K. M., Meyer J. S. Critical cerebral blood flow for production of hemiparesis after unilateral carotid occlusion in the gerbil. J Neurol Neurosurg Psychiatry. 1977 Jun;40(6):595–599. doi: 10.1136/jnnp.40.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J. S., Ballow D. P., Palmer G., Massey V. The reaction of xanthine oxidase with molecular oxygen. J Biol Chem. 1974 Jul 25;249(14):4350–4362. [PubMed] [Google Scholar]
- Parker N. B., Berger E. M., Curtis W. E., Muldrow M. E., Linas S. L., Repine J. E. Hydrogen peroxide causes dimethylthiourea consumption while hydroxyl radical causes dimethyl sulfoxide consumption in vitro. J Free Radic Biol Med. 1985;1(5-6):415–419. doi: 10.1016/0748-5514(85)90155-2. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N., Hamilton S. R., McCord J. M. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982 Jan;82(1):9–15. [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N. Role of oxygen-derived free radicals in digestive tract diseases. Surgery. 1983 Sep;94(3):415–422. [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- Patt A., Rutherford R. B., Pearce W. H., Repine J. E. Cerebral ischemia-reperfusion injury in the gerbil. J Surg Res. 1987 May;42(5):462–466. doi: 10.1016/0022-4804(87)90019-9. [DOI] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Cheronis J. C., Rodell T. C., Linas S. L., Patt A. Pulmonary oxygen toxicity and ischemia-reperfusion injury. A mechanism in common involving xanthine oxidase and neutrophils. Am Rev Respir Dis. 1987 Aug;136(2):483–485. doi: 10.1164/ajrccm/136.2.483. [DOI] [PubMed] [Google Scholar]
- Rodell T. C., Cheronis J. C., Ohnemus C. L., Piermattei D. J., Repine J. E. Xanthine oxidase mediates elastase-induced injury to isolated lungs and endothelium. J Appl Physiol (1985) 1987 Nov;63(5):2159–2163. doi: 10.1152/jappl.1987.63.5.2159. [DOI] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Oxidant injury of cells. DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J Clin Invest. 1986 Apr;77(4):1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstätter I. U., Revak S. D., Cochrane C. G. Proteases and oxidants in experimental pulmonary inflammatory injury. J Clin Invest. 1984 Apr;73(4):1175–1184. doi: 10.1172/JCI111303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shasby D. M., Lind S. E., Shasby S. S., Goldsmith J. C., Hunninghake G. W. Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood. 1985 Mar;65(3):605–614. [PubMed] [Google Scholar]
- Stirpe F., Della Corte E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O). J Biol Chem. 1969 Jul 25;244(14):3855–3863. [PubMed] [Google Scholar]
- Tate R. M., Morris H. G., Schroeder W. R., Repine J. E. Oxygen metabolites stimulate thromboxane production and vasoconstriction in isolated saline-perfused rabbit lungs. J Clin Invest. 1984 Aug;74(2):608–613. doi: 10.1172/JCI111458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei E. P., Lamb R. G., Kontos H. A. Increased phospholipase C activity after experimental brain injury. J Neurosurg. 1982 May;56(5):695–698. doi: 10.3171/jns.1982.56.5.0695. [DOI] [PubMed] [Google Scholar]
- Worcester J. The statistical method. N Engl J Med. 1966 Jan 6;274(1):27–36. doi: 10.1056/NEJM196601062740106. [DOI] [PubMed] [Google Scholar]




