Abstract
Posttraumatic osteoarthritis (PTOA) occurs after traumatic injury to the joint. It is most common following injuries that disrupt the articular surface or lead to joint instability. The reported risk of PTOA following significant joint trauma is as high as 75%; articular fractures can increase the risk more than 20-fold. Despite recent advances in surgical management, the incidence of PTOA following intra-articular fractures has remained relatively unchanged over the last few decades. Pathogenesis of PTOA after intra-articular fracture is likely multifactorial and may be associated with acute cartilage injury as well as chronic joint overload secondary to instability, incongruity, and malalignment. Additional studies are needed to better elucidate how these factors contribute to the development of PTOA and to develop advanced treatment algorithms that consist of both acute biologic interventions targeted to decrease inflammation and cellular death in response to injury and improved surgical methods to restore stability, congruity, and alignment.
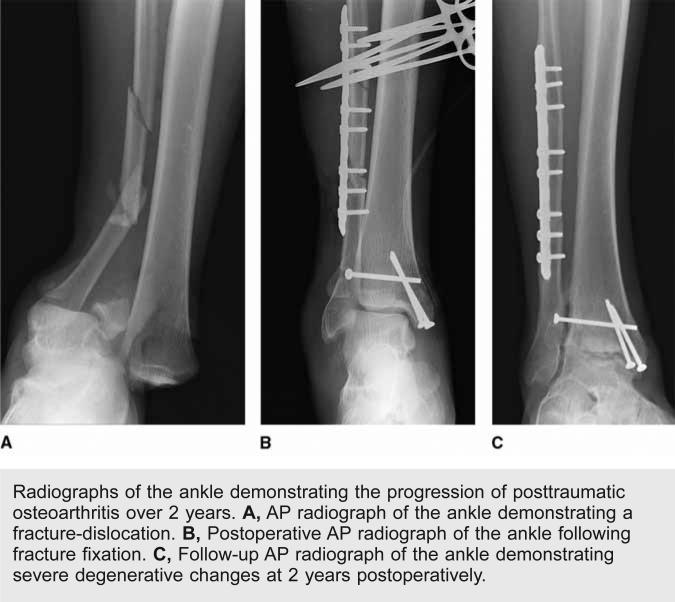
Posttraumatic osteoarthritis (PTOA) occurs after traumatic injury to the joint and is most commonly associated with fractures that disrupt the articular surface or injuries that lead to joint instability (Figure 1). Approximately 12% of the overall symptomatic osteoarthritis burden can be attributed to PTOA of the hip, knee, or ankle, and the annual healthcare costs associated with the disease in the United States is approximately $3 billion.1
Figure 1.
Radiographs of the ankle demonstrating the progression of posttraumatic osteoarthritis over 2 years. A, AP radiograph of the ankle demonstrating a fracture-dislocation. B, Postoperative AP radiograph of the ankle following fracture fixation. C, Follow-up AP radiograph of the ankle demonstrating severe degenerative changes at 2 years postoperatively.
The risk of PTOA following significant joint trauma has been reported to be as high as 75%; articular fractures can increase the risk by more than 20-fold2-9 (Table 1). Despite changes in surgical management, including improved fracture fixation and management of chondral injuries, the incidence of PTOA following intra-articular fractures has remained relatively unchanged over the last few decades.
Table 1.
Reported Risk of Posttraumatic Arthritis by Joint Involved
The mechanisms of injury and factors that contribute to the development of PTOA following intra-articular fractures are not well understood; therefore, the ability to intervene clinically and delay or prevent the progression of PTOA is limited. Current data suggest that multiple factors contribute to the development of PTOA, including acute mechanical injury to the cartilage at the time of impact, biologic response (eg, bleeding, inflammation), and chronic cartilage overload secondary to incongruity, instability, and malalignment.2 Other factors, including patient age and injury severity, also may contribute to worse clinical outcomes and progressive degeneration following intra-articular fractures.
Here, we describe the factors that contribute to the development of PTOA associated with intra-articular fracture, including articular incongruity, instability, malalignment, and the effect of injury on cartilage. Clinical interventions and research strategies, including articular reduction assessment, intraoperative imaging, and the use of biologic and tissue engineering strategies, have been proposed to forestall or halt the progression of PTOA in patients with articular injuries.
Articular Cartilage Structure, Function, and Response to Mechanical Injury
Articular cartilage is 60% to 85% water, with the solid content consisting of a dense extracellular matrix composed of collagens (primarily type II, but also types VI, IX, and XI), proteoglycans (primarily aggrecan, but also decorin, biglycan, and fibromodulin), and a cell population (chondrocytes). The composition, architecture, and remodeling of articular cartilage are uniquely adapted to enable function over a lifetime of repetitive use in a demanding mechanical environment, but, altogether, this results in tissue that is inherently limited in its response to traumatic injury. Mechanical loading of articular cartilage, such as during injury, generates a biologic response on a macro (tissue) and micro (cellular) level, activating intracellular signaling cascades through a process called mechanotransduction. Depending on the nature of the mechanical insult and the postinjury environment, cartilage may either recover or degrade, with the latter leading to PTOA.
Pathogenesis of PTOA
Acute Articular Injury
Acute articular injury is one of the proposed mechanisms of PTOA following intra-articular fracture. Acute insult to cartilage triggers chondrocyte death or dysfunction, resulting in subsequent dysfunction of cartilage metabolism that may lead to degeneration of the entire joint (Table 2). Tissues explanted after intra-articular calcaneal fracture have been shown to have significantly lower chondrocyte viability than that of control specimens (73% versus 95%, P = 0.005).10 In a recent study, Tochigi et al11 simulated an intra-articular tibial plafond fracture by delivering a direct impact to fresh human cadaver ankles. The authors observed a reproducible pattern of plafond injury and chondrocyte death, with significantly more chondrocyte death adjacent to the fracture lines than distant from the fracture (25.9% and 8.6%, respectively). Chondrocyte death progressed over 48 hours after the initial injury.11 In animal models, chondrocyte death reportedly occurs at the fracture site following impaction injuries, with more chondrocyte death found in fractured specimens than in specimens with subfracture impaction injuries; this is likely attributed to the supraphysiologic forces associated with actual fracture of the articular surface.12
Table 2.
Phases of Acute Joint Injury and Biologic Response
| Early phase: Cell death and inflammation |
| Necrosis |
| Apoptosis |
| Matrix degradation |
| Elevated caspase, proinflammatory cytokines, nitric oxide, reactive oxygen species, basic fibroblast growth factor, matrix metalloproteinases, aggrecanases, release of matrix fragments |
| Intermediate phase: Balance of catabolic and anabolic phases |
| Late phase: Matrix formation |
| Initial anabolic response |
| Matrix synthesis |
| Elevated anabolic growth factors |
Several in vitro studies have sought to examine how chondrocytes die following cartilage impact, including the pattern of cell death by apoptosis (programmed cell death) or necrosis (premature cell death, accompanied by an inflammatory response). Mechanically induced cell death in vitro has been shown to depend on load duration and magnitude.13,14 Several studies have found early signs of necrosis13-15 in explanted specimens after cartilage impact, whereas others have found markers of apoptosis in explanted human cartilage specimens following intra-articular fracture.16,17 How this early cell death may lead to the cascade of PTOA is not known.
One of the proposed mechanisms that trigger this cascade is the release of reactive oxygen species and/or proinflammatory mediators following injury, which may lead to progressive chondrocyte damage and matrix degeneration. Some in vitro studies of impact injuries on cartilage explants showed that injury induced the release of oxygen free radicals from chondrocytes, possibly secondary to mitochondrial injury,18 which led to chondrocyte death and matrix degeneration.18,19 Severe high-impact injuries have been shown to result in greater local tissue damage (as measured by a higher proportion of cells releasing reactive oxygen species) and a higher rate of chondrocyte death and matrix disruption than that associated with less severe injuries.19,20 Intra-articular fracture has also been shown to result in elevated synovial levels of proinflammatory cytokines and mediators, including tumor necrosis factor-α, interleukin (IL)-1, nitrous oxide, matrix metalloproteinases, and fibronectin fragments that can stimulate further cell and matrix degradation.19,21,22
Recent studies using animal models have demonstrated that cellular events related to initial impact injuries are associated with the progression of PTOA. Borrelli et al23 showed that a single impact injury in a rabbit knee decreased markers of cartilage metabolism (procollagen type II and bone morphogenetic protein [BMP]-2) and disrupted the extracellular matrix. Other studies that used this model demonstrated worsening changes in the viscoelastic properties of the cartilage over time and a corresponding increase in subchondral bone formation beneath the articular changes.24 Furman et al25 observed degenerative changes, including loss of bone density and increases in subchondral bone thickness, as early as 8 weeks following untreated closed impaction injuries to the tibial plateau in mice, with severe cartilage loss observed by 50 weeks.12,19 In addition, joint changes were accompanied by rapid changes in proinflammatory cytokines and cartilage biomarkers in the serum and synovial fluid.25 In another animal model, the authors compared the injury patterns in standard mice with those in a breed of mice (MRL/MpJ) that produce a decreased inflammatory response to injury via decreased production of proinflammatory cytokines (IL-1) and increased production of anti-inflammatory cytokines (IL-4 and IL-10). This model demonstrated decreased joint inflammatory response to intra-articular injury, with relative protection from PTOA,26 suggesting that clinically decreasing the inflammatory response may decrease the severity of PTOA in patients with intra-articular fractures.
Chronic Joint Overload
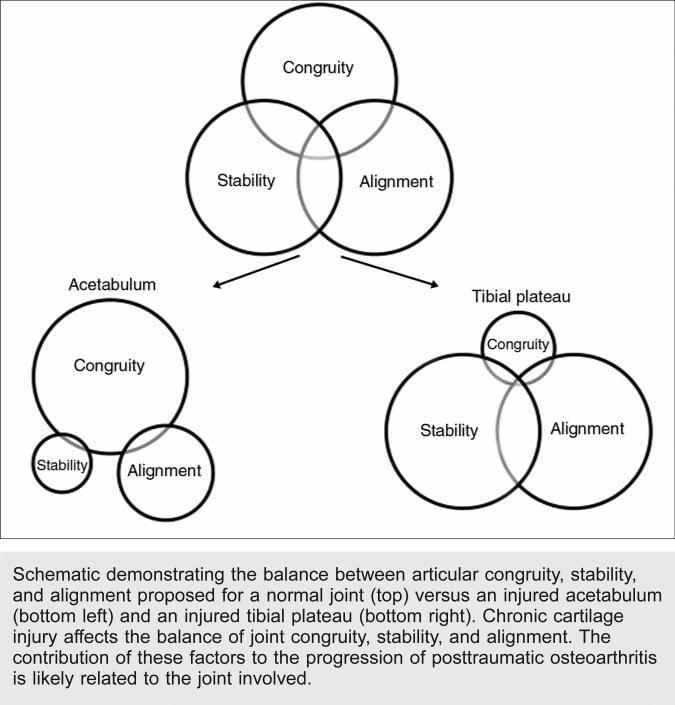
Early surgical intervention for intra-articular fractures is recommended to achieve anatomic reduction of the articular surface and absolute fixation of the articular fragments. Articular incongruity, joint instability, and joint malalignment likely play a role in the development of PTOA; however, the relative contribution of these factors to the subsequent progression of arthritis has not been well characterized and is likely related to the joint involved (Figure 2). Although articular reduction within 2 mm of normal articular congruity is recommended, much greater incongruities are well-tolerated in some joints.27
Figure 2.
Schematic demonstrating the balance between articular congruity, stability, and alignment proposed for a normal joint (top) versus an injured acetabulum (bottom left) and an injured tibial plateau (bottom right). Chronic cartilage injury affects the balance of joint congruity, stability, and alignment. The contribution of these factors to the progression of posttraumatic osteoarthritis is likely related to the joint involved.
Some experimental and finite element studies of contact stress in cadaver specimens have reported increased contact stresses associated with articular surface incongruity.28-30 A study of contact stresses associated with articular step-offs in cadaver ankles reported increases in contact stresses of up to 300%.28 The combination of instability and articular surface incongruity has been shown to cause disproportionate increases in contact stress rates and magnitudes29,30 and can anatomically shift the articular surface loading pattern, resulting in joint contact outside the typical uniform, central location found in intact controls.31 This shift in loading pattern may be crucial to subsequent progression of osteoarthritis; the new point of contact may be maladapted for cartilage loading and may be unable to rapidly accommodate the transition to load bearing via remodeling, leading to degeneration of the whole joint.
In contrast, several experimental models of intra-articular fractures have shown relatively mild increases in articular surface contact stresses, even in the setting of large articular incongruities.27,32 In a canine cadaver model, 7-mm full-thickness osteochondral defects in the medial femoral condyle were statically loaded and showed mean increases in contact stress of only 10% to 30%.32 These results are likely affected by the fact that the specimens in most studies are statically loaded across a fixed joint position without motion. Results of these early studies on contact stresses should be interpreted cautiously because many biomechanical testing methods cannot detect transiently elevated contact stresses and cumulative stresses that occur during motion. In addition, many of these methods cannot account for the effects of joint instability. Methods of assessing in vitro and in vivo dynamic contact pressures are improving.33 Well-designed studies that assess the effects of postfixation articular incongruity and instability are needed to better elucidate the effect of these factors on the progression of PTOA and on outcomes following intra-articular fracture management.
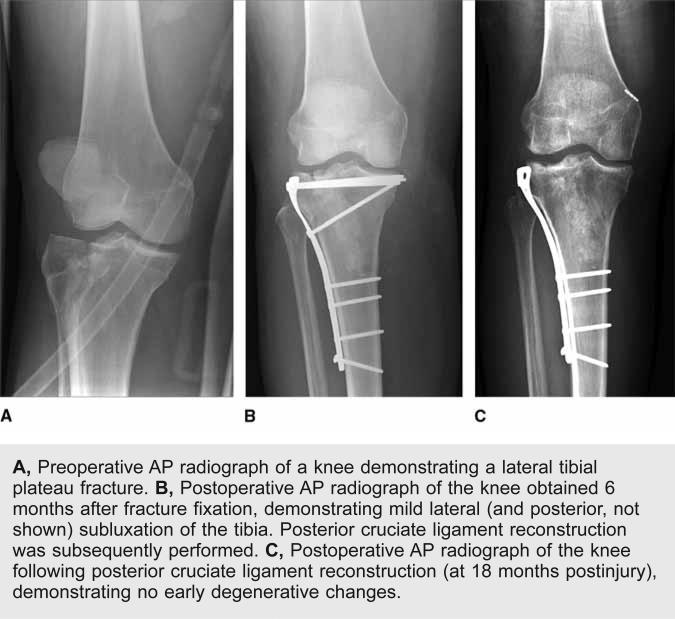
Recently, Giannoudis et al27 conducted a systematic review of the literature to examine the connection between articular step-off and the risk of PTOA following management of intra-articular fractures. The risk of PTOA seems to vary based on the joint involved (Table 1). In studies of PTOA associated with injury to the distal radius, articular step-offs and gaps have been associated with a higher incidence of radiographic evidence of PTOA, but no definite link has been found between poor articular reduction or degenerative changes on radiography and worse long-term clinical outcomes.6,34 In a meta-analysis of PTOA following surgical management of acetabular fractures, the authors reported that restoration of the superior weight-bearing dome of the acetabulum decreased the rate of PTOA and improved clinical outcomes; however, fracture of the posterior wall, a proposed marker of instability, was a negative prognostic factor, likely independent of articular reduction.9 Finally, two studies on fractures of the tibial plateau reported that successful articular reduction of severe bicondylar injuries has been associated with better functional outcomes;4,35 however, compared with fractures of other anatomic locations, articular incongruities in the knee appear to be relatively well tolerated; other factors, including joint stability, retention of the meniscus, and coronal alignment, were proposed to be potentially more important for development of PTOA of the knee (Figure 3). No consensus has been reached on the maximal acceptable articular step-off. The relative tolerance of imperfect reduction may be related to the thickness of the tibial plateau cartilage compared with that of other anatomic regions.
Figure 3.
A, Preoperative AP radiograph of a knee demonstrating a lateral tibial plateau fracture. B, Postoperative AP radiograph of the knee obtained 6 months after fracture fixation, demonstrating mild lateral (and posterior, not shown) subluxation of the tibia. Posterior cruciate ligament reconstruction was subsequently performed. C, Postoperative AP radiograph of the knee following posterior cruciate ligament reconstruction (at 18 months postinjury), demonstrating no early degenerative changes.
There is a paucity of controlled studies that systematically identify the relative contribution of articular step-off, joint incongruity, and joint instability to accelerated PTOA. Based on the previously described literature reviews, PTOA seems to be triggered by different “failures” in different joints (eg, the tibial plateau seems to be relatively spared from articular step-off incongruencies); however, the development of PTOA in the knee seems to be dependent on alignment and ligamentous/meniscus preservation for improved outcomes. Until these factors are better elucidated, the best clinical approach for management of articular injuries is to restore all three factors—congruity, stability, and alignment—to decrease the likelihood of PTOA.
Assessment of Articular Reduction
Plain radiography, CT, and fluoroscopy can be used for preoperative, intraoperative, and postoperative assessment of articular fracture reduction. One study evaluated the interrater reliability among five orthopaedic traumatologists in measuring the articular incongruity of 56 tibial plateau fractures on plain radiographs. When the traumatologists assessed the maximal depression of the fracture, the agreement in quantifying articular depression was poor (±12 mm).36
CT is often used to improve diagnostic assessment of articular surface disruption and step-off by aiding in surgical decision making during preoperative planning of reduction and fixation strategies. CT is also useful for postoperative assessment of fracture reduction and hardware placement. With regard to preoperative assessment, CT has been shown to have better sensitivity than plain radiography for measurement of articular surface gapping, comminution, and distal radioulnar joint involvement; in a small case series, CT findings led to treatment plan modifications for patients with intra-articular radius fractures.37 In a study of the influence of three-dimensional CT findings on management of distal radius fractures, four observers assessed a total of 120 fractures using radiographs and two- and three-dimensional CTs.38 Assessment of three-dimensional CTs of the fractures resulted in alterations to treatment plans in 57 cases (48%), with a greater number of decisions for an open approach as well as combined dorsal and volar approaches.
Several studies have compared postoperative assessment of fracture reduction with plain radiography or CT and have found that CT offers superior detection of gap and step-off.39,40 Moed et al39 compared the quality of fracture reduction on plain radiography and CT in 67 patients following open reduction and internal fixation of posterior wall acetabular fractures. In 65 patients, the reduction was deemed “anatomic” based on standard plain postoperative radiographs of the pelvis; 11 of those were found to have >2 mm of articular incongruency and 52 had a fracture gap of >2 mm on CT. These studies found that small differences in articular congruity cannot be reliably assessed with plain radiography, and CT improved diagnostic capacity for both small and large joint articular injuries.
Similarly, the incorporation of three-dimensional fluoroscopy may improve immediate visualization of articular reduction and hardware placement.41 Intraoperative imaging may reduce the need to return to the operating room to address articular malreductions and/or intra-articular hardware penetration missed on standard intraoperative fluoroscopy and later discovered on postoperative CT. Intraoperative imaging technology has progressed with the use of intraoperative CT, particularly for guided placement of pedicle screws in spine fusions; however, this is not standard practice for reduction of articular fractures.
Two major issues exist with regard to imaging and management of articular injuries. First, despite the debate about the optimal amount of articular step-off that a joint can tolerate, unless there is direct visualization of every part of the articular surface, we are only as good as our intraoperative imaging. Based on our review of the literature, assessment of articular step-off can be off by up to 12 mm with plain radiography. Second, many of the historical studies that exclusively used plain radiography to evaluate the effects of articular step-off on PTOA should be interpreted with caution because of high rates of interobserver variability.
Intra-articular Fracture Management and Its Impact on PTOA
The best management of intra-articular fracture focuses on optimizing articular congruity, stability, and alignment with the goal of decreasing the future risk of PTOA. Several studies have described the inclusion of biologics and proposed tissue engineering strategies to further diminish or halt the progression of PTOA in patients with intra-articular fractures.
Improvement of Articular Reduction
Because imaging has been shown to be inaccurate in determining the degree of articular step-off, investigators have searched for alternative methods of improving articular reduction. Several published case series have described techniques for improving joint reduction of intra-articular fractures with arthroscopic assistance.42-46 In a recent systematic review of studies on arthroscopic-assisted fracture fixation, Atesok et al42 noted that arthroscopic-assisted reduction and fixation have been used for fractures of the tibial plateau, tibial eminence, malleolus, pilon, calcaneus, femoral head, glenoid, greater tuberosity, distal clavicle, radial head, coronoid, distal radius, and scaphoid. Potential benefits of arthroscopic-assisted fixation include direct visualization of the articular surface; decreased invasiveness; and the ability to diagnose and treat associated osteochondral and ligamentous injuries, remove joint debris, and perform joint lavage. However, this technique is technically demanding, uses additional resources that result in higher costs, and may be associated with an increased risk of compartment syndrome secondary to fluid extravasation.
Several studies have compared functional and radiographic outcomes in patients treated with arthroscopic-assisted procedures versus standard open procedures. Two studies examined functional outcomes in patients with intra-articular distal radius fractures: one compared the outcomes of arthroscopic-assisted reduction and fluoroscopy-assisted reduction,43 while the second compared outcomes of arthroscopic-assisted techniques and traditional open reduction and internal fixation.44 Range of motion and radiographic alignment were improved in the arthroscopic-assisted groups in both studies; however, the authors found that a functional benefit associated with arthroscopic-assisted reduction was not conclusive.43,44
In two studies that compared outcomes of arthroscopic-assisted management of tibial plateau fractures with those of traditional open techniques, the authors found that arthroscopic-assisted management was associated with a shorter hospital stay, decreased time to full weight bearing, and improved early range of motion and reduction; however, evidence of functional long-term benefit was inconclusive.45,46 Additional well-designed prospective studies are needed to better elucidate the effect of arthroscopic-assisted techniques on the long-term development of PTOA following intra-articular fractures.
Biologic Intervention and Future Research
Development of biologic interventions for treatment of osteoarthritis is a major focus of current basic science and clinical research. Most of this research is focused on forestalling osteoarthritis in the mid- to late-term. Knowledge of the phases of acute injury and biologic response to injury offers a unique opportunity for early biologic intervention in patients with intra-articular fractures to prevent or delay PTOA (Table 2). Biologic therapies for intra-articular fractures can be directed at any of the three overlapping phases of acute joint injury and response to injury: early, intermediate (balance between catabolic and anabolic phases), and late (limited repair, remodeling, matrix formation).2
The early phase of acute joint injury involves cell death and inflammation (necrosis/apoptosis) as well as elevation of proinflammatory cytokines, enzymes, and reactive oxygen species that cause further joint destruction. In an in vitro study of acute mechanical cartilage injury, local delivery of a known inhibitor of chondrocyte apoptosis has been shown to decrease mechanically induced chondrocyte death.47 Administration of P188 surfactant, a cell membrane stabilizer and inhibitor of stress-related p38 mitogen-activating protein, can limit cell necrosis after impact loading of cartilage.48 Blocking the fibronectin pathway has been shown to decrease progressive cell damage and matrix degeneration.49 Local administration of antioxidants (eg, N-acetylcysteine, superoxide dismutase, vitamin E) within hours of traumatic injury to cartilage has been shown to prevent progressive, mechanically induced chondrocyte damage and matrix degeneration in vitro.15,19 Administration of dexamethasone also has been shown to limit injury-induced catabolism by preventing proteoglycan degradation and restoring biosynthesis.50 Although these studies offer promising trends in the laboratory, the effects of these agents in vivo and the effects of systemic versus local drug delivery need to be evaluated to determine possible therapeutic benefit.
In clinical practice, both hyaluronic acid (HA) derivatives and BMP-7 (osteogenic protein-1) are available for therapeutic intervention and have been proposed as biologic interventions to delay or prevent the progression of PTOA.51-53 HA is believed to act in the early phase of joint injury by attenuating the catabolic activities of fibroblast-like synoviocytes, which release proinflammatory cytokines and enzymes. In an in vitro study of cells derived from the synovium of patients with tibial plateau fractures, HA had anti-inflammatory and chondroprotective effects.51 However, an in vivo study of patients with PTOA of the elbow treated with intra-articular injections of HA reported that the treatment was not effective.52 Finally, BMP-7 has been shown to have a chondroprotective effect in vitro and in vivo when administered up to 3 to 4 weeks after injury.53 The paucity of data and lack of consistent clinical success indicate the need for new molecules for therapeutic care, which are most efficiently derived via high-throughput screening methods.
In addition to biologic interventions, tissue engineering strategies are being investigated to “fill the cracks” of acute articular cartilage injury and/or replace focal defects and whole damaged joints.54 These strategies are beyond the scope of this review, but they will be the focus of future research efforts for management of the posttraumatic sequelae of intra-articular fractures.
Summary
The development of PTOA after intra-articular fracture is likely multifactorial and may be the combined result of initial cartilage injury and the associated chondrocyte death, matrix disruption, and release of proinflammatory cytokines and reactive oxygen species as well as chronic joint overload secondary to instability, incongruity, and malalignment. Additional studies are needed to better elucidate the relative contributions of these factors in the development of PTOA and to develop advanced treatment algorithms. Based on available clinical data, future treatment modalities may consist of acute biologic interventions targeted at decreasing inflammation and cellular death in response to injury as well as improved surgical methods to better restore stability, congruity, and alignment following intra-articular fractures to reduce the individual and societal burden of PTOA.
Acknowledgments
Dr. Schenker or an immediate family member has received research or institutional support from AO North America. Dr. Ahn or an immediate family member is a member of a speakers’ bureau or has made paid presentations on behalf of Synthes; serves as a paid consultant to Merck, Synthes, and U&I Corporation; and serves and as a board member, owner, officer, or committee member of the American Academy of Orthopaedic Surgeons, the Foundation for Orthopedic Trauma, the American Physician Scientists Association, the National Board of Medical Examiners, and the Orthopaedic Trauma Association. Dr. Mehta or an immediate family member is a member of a speakers’ bureau or has made paid presentations on behalf of Zimmer, Smith & Nephew, and AO North America; serves as a paid consultant to Smith & Nephew and Synthes; has received research or institutional support from Amgen, Medtronic, and Smith & Nephew; and serves as a board member, owner, officer, or committee member of the Pennsylvania Orthopaedic Society. Neither Dr. Mauck nor any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article.
References
Evidence-based Medicine: Levels of evidence are described in the table of contents. In this article, references 1, 43, and 44 are level II studies. References 39, 45, and 46 are level III studies. References 3-9, 27, 34, 35, 37, 38, and 40 are level IV studies. References 2, 22, and 42 are level V expert opinion.
References printed in bold type are those published within the past 5 years.
- 1.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: A first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20(10):739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DD, Chubinskaya S, Guilak F, et al. Post-traumatic osteoarthritis: Improved understanding and opportunities for early intervention. J Orthop Res. 2011;29(6):802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh JL, Weigel DP, Dirschl DR. Tibial plafond fractures: How do these ankles function over time? J Bone Joint Surg Am. 2003;85(2):287–295. [PubMed] [Google Scholar]
- 4.Weigel DP, Marsh JL. High-energy fractures of the tibial plateau: Knee function after longer follow-up. J Bone Joint Surg Am. 2002;84(9):1541–1551. doi: 10.2106/00004623-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Rademakers MV, Kerkhoffs GM, Sierevelt IN, Raaymakers EL, Marti RK. Operative treatment of 109 tibial plateau fractures: Five- to 27-year follow-up results. J Orthop Trauma. 2007;21(1):5–10. doi: 10.1097/BOT.0b013e31802c5b51. [DOI] [PubMed] [Google Scholar]
- 6.Lutz M, Arora R, Krappinger D, Wambacher M, Rieger M, Pechlaner S. Arthritis predicting factors in distal intraarticular radius fractures. Arch Orthop Trauma Surg. 2011;131(8):1121–1126. doi: 10.1007/s00402-010-1211-3. [DOI] [PubMed] [Google Scholar]
- 7.Rademakers MV, Kerkhoffs GM, Sierevelt IN, Raaymakers EL, Marti RK. Intra-articular fractures of the distal femur: A long-term follow-up study of surgically treated patients. J Orthop Trauma. 2004;18(4):213–219. doi: 10.1097/00005131-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Doornberg JN, van Duijn PJ, Linzel D, et al. Surgical treatment of intra-articular fractures of the distal part of the humerus: Functional outcome after twelve to thirty years. J Bone Joint Surg Am. 2007;89(7):1524–1532. doi: 10.2106/JBJS.F.00369. [DOI] [PubMed] [Google Scholar]
- 9.Giannoudis PV, Grotz MR, Papakostidis C, Dinopoulos H. Operative treatment of displaced fractures of the acetabulum: A meta-analysis. J Bone Joint Surg Br. 2005;87(1):2–9. [PubMed] [Google Scholar]
- 10.Ball ST, Jadin K, Allen RT, Schwartz AK, Sah RL, Brage ME. Chondrocyte viability after intra-articular calcaneal fractures in humans. Foot Ankle Int. 2007;28(6):665–668. doi: 10.3113/FAI.2007.0665. [DOI] [PubMed] [Google Scholar]
- 11.Tochigi Y, Buckwalter JA, Martin JA, et al. Distribution and progression of chondrocyte damage in a whole-organ model of human ankle intra-articular fracture. J Bone Joint Surg Am. 2011;93(6):533–539. doi: 10.2106/JBJS.I.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Backus JD, Furman BD, Swimmer T, et al. Cartilage viability and catabolism in the intact porcine knee following transarticular impact loading with and without articular fracture. J Orthop Res. 2011;29(4):501–510. doi: 10.1002/jor.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinn TM, Allen RG, Schalet BJ, Perumbuli P, Hunziker EB. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: Effects of strain rate and peak stress. J Orthop Res. 2001;19(2):242–249. doi: 10.1016/S0736-0266(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 14.Chen CT, Bhargava M, Lin PM, Torzilli PA. Time, stress, and location dependent chondrocyte death and collagen damage in cyclically loaded articular cartilage. J Orthop Res. 2003;21(5):888–898. doi: 10.1016/S0736-0266(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 15.Martin JA, McCabe D, Walter M, Buckwalter JA, McKinley TO. N-acetylcysteine inhibits post-impact chondrocyte death in osteochondral explants. J Bone Joint Surg Am. 2009;91(8):1890–1897. doi: 10.2106/JBJS.H.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Lima DD, Hashimoto S, Chen PC, Colwell CW, Jr, Lotz MK. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage. 2001;9(8):712–719. doi: 10.1053/joca.2001.0468. [DOI] [PubMed] [Google Scholar]
- 17.Kim HT, Lo MY, Pillarisetty R. Chondrocyte apoptosis following intraarticular fracture in humans. Osteoarthritis Cartilage. 2002;10(9):747–749. doi: 10.1053/joca.2002.0828. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin W, McCabe D, Sauter E, et al. Rotenone prevents impact-induced chondrocyte death. J Orthop Res. 2010;28(8):1057–1063. doi: 10.1002/jor.21091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin JA, Buckwalter JA. Post-traumatic osteoarthritis: The role of stress induced chondrocyte damage. Biorheology. 2006;43(3-4):517–521. [PubMed] [Google Scholar]
- 20.Beecher BR, Martin JA, Pedersen DR, Heiner AD, Buckwalter JA. Antioxidants block cyclic loading induced chondrocyte death. Iowa Orthop J. 2007;27:1–8. [PMC free article] [PubMed] [Google Scholar]
- 21.Green DM, Noble PC, Ahuero JS, Birdsall HH. Cellular events leading to chondrocyte death after cartilage impact injury. Arthritis Rheum. 2006;54(5):1509–1517. doi: 10.1002/art.21812. [DOI] [PubMed] [Google Scholar]
- 22.Guilak F, Fermor B, Keefe FJ, et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004;423:17–26. doi: 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- 23.Borrelli J, Jr, Silva MJ, Zaegel MA, Franz C, Sandell LJ. Single high-energy impact load causes posttraumatic OA in young rabbits via a decrease in cellular metabolism. J Orthop Res. 2009;27(3):347–352. doi: 10.1002/jor.20760. [DOI] [PubMed] [Google Scholar]
- 24.Borrelli J, Jr, Zaegel MA, Martinez MD, Silva MJ. Diminished cartilage creep properties and increased trabecular bone density following a single, sub-fracture impact of the rabbit femoral condyle. J Orthop Res. 2010;28(10):1307–1314. doi: 10.1002/jor.21122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. Joint degeneration following closed intraarticular fracture in the mouse knee: A model of posttraumatic arthritis. J Orthop Res. 2007;25(5):578–592. doi: 10.1002/jor.20331. [DOI] [PubMed] [Google Scholar]
- 26.Ward BD, Furman BD, Huebner JL, Kraus VB, Guilak F, Olson SA. Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis Rheum. 2008;58(3):744–753. doi: 10.1002/art.23288. [DOI] [PubMed] [Google Scholar]
- 27.Giannoudis PV, Tzioupis C, Papathanassopoulos A, Obakponovwe O, Roberts C. Articular step-off and risk of post-traumatic osteoarthritis: Evidence today. Injury. 2010;41(10):986–995. doi: 10.1016/j.injury.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 28.McKinley TO, Rudert MJ, Tochigi Y, et al. Incongruity-dependent changes of contact stress rates in human cadaveric ankles. J Orthop Trauma. 2006;20(10):732–738. doi: 10.1097/01.bot.0000211150.00919.0e. [DOI] [PubMed] [Google Scholar]
- 29.McKinley TO, Tochigi Y, Rudert MJ, Brown TD. The effect of incongruity and instability on contact stress directional gradients in human cadaveric ankles. Osteoarthritis Cartilage. 2008;16(11):1363–1369. doi: 10.1016/j.joca.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goreham-Voss CM, McKinley TO, Brown TD. A finite element exploration of cartilage stress near an articular incongruity during unstable motion. J Biomech. 2007;40(15):3438–3447. doi: 10.1016/j.jbiomech.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Anderson DD, Goldsworthy JK, Marsh JL, Brown TD. Patient-specific finite element analysis of chronic contact stress exposure after intraarticular fracture of the tibial plafond. J Orthop Res. 2008;26(8):1039–1045. doi: 10.1002/jor.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown TD, Pope DF, Hale JE, Buckwalter JA, Brand RA. Effects of osteochondral defect size on cartilage contact stress. J Orthop Res. 1991;9(4):559–567. doi: 10.1002/jor.1100090412. [DOI] [PubMed] [Google Scholar]
- 33.Bedi A, Kelly NH, Baad M, et al. Dynamic contact mechanics of the medial meniscus as a function of radial tear, repair, and partial meniscectomy. J Bone Joint Surg Am. 2010;92(6):1398–1408. doi: 10.2106/JBJS.I.00539. [DOI] [PubMed] [Google Scholar]
- 34.Goldfarb CA, Rudzki JR, Catalano LW, Hughes M, Borrelli J., Jr Fifteen-year outcome of displaced intra-articular fractures of the distal radius. J Hand Surg Am. 2006;31(4):633–639. doi: 10.1016/j.jhsa.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Barei DP, Nork SE, Mills WJ, Coles CP, Henley MB, Benirschke SK. Functional outcomes of severe bicondylar tibial plateau fractures treated with dual incisions and medial and lateral plates. J Bone Joint Surg Am. 2006;88(8):1713–1721. doi: 10.2106/JBJS.E.00907. [DOI] [PubMed] [Google Scholar]
- 36.Martin J, Marsh JL, Nepola JV, Dirschl DR, Hurwitz S, DeCoster TA. Radiographic fracture assessments: Which ones can we reliably make? J Orthop Trauma. 2000;14(6):379–385. doi: 10.1097/00005131-200008000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Katz MA, Beredjiklian PK, Bozentka DJ, Steinberg DR. Computed tomography scanning of intra-articular distal radius fractures: Does it influence treatment? J Hand Surg Am. 2001;26(3):415–421. doi: 10.1053/jhsu.2001.22930a. [DOI] [PubMed] [Google Scholar]
- 38.Harness NG, Ring D, Zurakowski D, Harris GJ, Jupiter JB. The influence of three-dimensional computed tomography reconstructions on the characterization and treatment of distal radial fractures. J Bone Joint Surg Am. 2006;88(6):1315–1323. doi: 10.2106/JBJS.E.00686. [DOI] [PubMed] [Google Scholar]
- 39.Moed BR, Carr SE, Gruson KI, Watson JT, Craig JG. Computed tomographic assessment of fractures of the posterior wall of the acetabulum after operative treatment. J Bone Joint Surg Am. 2003;85(3):512–522. doi: 10.2106/00004623-200303000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Borrelli J, Jr, Ricci WM, Steger-May K, Totty WG, Goldfarb C. Postoperative radiographic assessment of acetabular fractures: A comparison of plain radiographs and CT scans. J Orthop Trauma. 2005;19(5):299–304. [PubMed] [Google Scholar]
- 41.Weil YA, Liebergall M, Mosheiff R, Singer SB, Joskowicz L, Khoury A. Assessment of two 3-D fluoroscopic systems for articular fracture reduction: A cadaver study. Int J Comput Assist Radiol Surg. 2011;6(5):685–692. doi: 10.1007/s11548-011-0548-6. [DOI] [PubMed] [Google Scholar]
- 42.Atesok K, Doral MN, Whipple T, et al. Arthroscopy-assisted fracture fixation. Knee Surg Sports Traumatol Arthrosc. 2011;19(2):320–329. doi: 10.1007/s00167-010-1298-7. [DOI] [PubMed] [Google Scholar]
- 43.Varitimidis SE, Basdekis GK, Dailiana ZH, Hantes ME, Bargiotas K, Malizos K. Treatment of intra-articular fractures of the distal radius: Fluoroscopic or arthroscopic reduction? J Bone Joint Surg Br. 2008;90(6):778–785. doi: 10.1302/0301-620X.90B6.19809. [DOI] [PubMed] [Google Scholar]
- 44.Doi K, Hattori Y, Otsuka K, Abe Y, Yamamoto H. Intra-articular fractures of the distal aspect of the radius: Arthroscopically assisted reduction compared with open reduction and internal fixation. J Bone Joint Surg Am. 1999;81(8):1093–1110. doi: 10.2106/00004623-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Fowble CD, Zimmer JW, Schepsis AA. The role of arthroscopy in the assessment and treatment of tibial plateau fractures. Arthroscopy. 1993;9(5):584–590. doi: 10.1016/s0749-8063(05)80410-4. [DOI] [PubMed] [Google Scholar]
- 46.Ohdera T, Tokunaga M, Hiroshima S, Yoshimoto E, Tokunaga J, Kobayashi A. Arthroscopic management of tibial plateau fractures: Comparison with open reduction method. Arch Orthop Trauma Surg. 2003;123(9):489–493. doi: 10.1007/s00402-003-0510-3. [DOI] [PubMed] [Google Scholar]
- 47.D'Lima DD, Hashimoto S, Chen PC, Lotz MK, Colwell CW., Jr Prevention of chondrocyte apoptosis. J Bone Joint Surg Am. 2001;83(pt 1, suppl 2):25–26. doi: 10.2106/00004623-200100021-00006. [DOI] [PubMed] [Google Scholar]
- 48.Rundell SA, Baars DC, Phillips DM, Haut RC. The limitation of acute necrosis in retro-patellar cartilage after a severe blunt impact to the in vivo rabbit patello-femoral joint. J Orthop Res. 2005;23(6):1363–1369. doi: 10.1016/j.orthres.2005.06.001.1100230618. [DOI] [PubMed] [Google Scholar]
- 49.Ding L, Heying E, Nicholson N, et al. Mechanical impact induces cartilage degradation via mitogen activated protein kinases. Osteoarthritis Cartilage. 2010;18(11):1509–1517. doi: 10.1016/j.joca.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu YC, Evans CH, Grodzinsky AJ. Effects of short-term glucocorticoid treatment on changes in cartilage matrix degradation and chondrocyte gene expression induced by mechanical injury and inflammatory cytokines. Arthritis Res Ther. 2011;13(5):R142. doi: 10.1186/ar3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang TL, Hsu HC, Yang KC, Yao CH, Lin FH. Effect of different molecular weight hyaluronans on osteoarthritis-related protein production in fibroblast-like synoviocytes from patients with tibia plateau fracture. J Trauma. 2010;68(1):146–152. doi: 10.1097/TA.0b013e3181a92cf8. [DOI] [PubMed] [Google Scholar]
- 52.van Brakel RW, Eygendaal D. Intraarticular injection of hyaluronic acid is not effective for the treatment of post-traumatic osteoarthritis of the elbow. Arthroscopy. 2006;22(11):1199–1203. doi: 10.1016/j.arthro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 53.Hurtig M, Chubinskaya S, Dickey J, Rueger D. BMP-7 protects against progression of cartilage degeneration after impact injury. J Orthop Res. 2009;27(5):602–611. doi: 10.1002/jor.20787. [DOI] [PubMed] [Google Scholar]
- 54.Schlichting KE, Copeland-Johnson TM, Goodman M, et al. Synthesis of a novel photopolymerized nanocomposite hydrogel for treatment of acute mechanical damage to cartilage. Acta Biomater. 2011;7(8):3094–3100. doi: 10.1016/j.actbio.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]