Abstract
Music listening and music making activities are powerful tools to engage multisensory and motor networks, induce changes within these networks, and foster links between distant, but functionally related brain regions with continued and life-long musical practice. These multimodal effects of music together with music’s ability to tap into the emotion and reward system in the brain can be used to facilitate and enhance therapeutic approaches geared toward rehabilitating and restoring neurological dysfunctions and impairments of an acquired or congenital brain disorder. In this article, we review plastic changes in functional networks and structural components of the brain in response to short- and long-term music listening and music making activities. The specific influence of music on the developing brain is emphasized and possible transfer effects on emotional and cognitive processes are discussed. Furthermore, we present data on the potential of using musical tools and activities to support and facilitate neurorehabilitation. We will focus on interventions such as melodic intonation therapy and music-supported motor rehabilitation to showcase the effects of neurologic music therapies and discuss their underlying neural mechanisms.
Keywords: brain plasticity, melodic intonation therapy, music-supported training, neurologic music therapy, neurorehabilitation
1 MUSIC AS A DRIVER OF BRAIN PLASTICITY
Apollo’s gift, music, is one of the richest human emotional, sensory-motor, and cognitive experiences. It involves listening, watching, feeling, moving and coordinating, remembering, and expecting musical elements. It is frequently accompanied by strong emotions resulting in joy, happiness, and bittersweet sadness or even in overwhelming bodily reactions like tears in the eyes or shivers down the spine. A large number of cortical and subcortical brain regions are involved in music listening and music making activities (for reviews see Altenmüller and McPherson, 2007; Tramo, 2001).
Primary and secondary regions in the cerebral cortex are critical for any conscious perception of sensory information, be it auditory, visual, or somatosensory. However, music also influences and changes activity in multisensory and motor integration regions in frontal, parietal, and temporo-occipital brain regions. The frontal lobe is involved in the guidance of attention, in planning and motor preparation, in integrating auditory and motor information, and in specific human skills such as imitation and empathy. The two latter play an important role in the acquisition of musical skills and emotional expressiveness. Multisensory integration regions in the parietal lobe and temporo-occipital areas integrate different sensory inputs from the auditory, visual, and somatosensory system into a combined sensory impression; it is this multisensory brain representation, which constitutes the typical musical experience. The cerebellum is another important part of the brain that plays a critical role in musical experience. It is important for motor coordination, but it also plays an important role in various cognitive tasks especially when they include demands on timing. Typically, the cerebellum is activated in rhythm processing, or tapping in synchrony with an external pacemaker such as a metronome. Finally, the emotional network (comprising the basis and the inner surfaces of the two frontal lobes, the cingulate gyrus and brain structures in the evolutionarily old parts of the brain such as the amygdala, the hippocampus, and the midbrain) is crucial for the emotional perception of music and therefore for an individual’s motivation to listen to or to engage in any musical activity.
The brain is a highly dynamically organized structure that changes and adapts as a result of activities and demands imposed upon it by the environment. Musical activity has proven to be a powerful stimulus for this kind of brain adaptation, or brain plasticity (Wan and Schlaug, 2010). Effects of plasticity are not restricted to musical prodigies, they occur in children learning to play a musical instrument (Hyde et al., 2009) and in adult amateur musicians (Bangert and Altenmüller, 2003), albeit to a lesser extent. Thus, with the main topic of our article in mind, we suggest that brain plasticity induced through making music may produce manifold benefits. This holds not only for changing and/or restoring compromised sensorimotor brain networks, but also for influencing neurohormonal status as well as cognitive and emotional processes in healthy and neurologically diseased/disordered individuals. Thus, various sensory-motor, coordinative, or emotional disabilities can be improved with music-supported therapy (MST).
This chapter briefly reviews the literature on music-induced brain plasticity, its underlying mechanisms, and the impact that music has on emotion and neurohormones. Subsequently, we will demonstrate transfer effects of music exposure and music making to other cognitive and emotional domains and finally show examples of the potential of music making to support and facilitate neurorehabilitation. Our intent is not to provide an exhaustive review, but to focus our chapter on music-supported interventions geared toward improving the rehabilitation of speech-and limb-motor impairments following brain injury.
2 SOME MECHANISMS OF MUSIC-INDUCED BRAIN PLASTICITY
During the past decade, brain imaging has provided important insight into the enormous capacity of the human brain to adapt to complex demands. These adaptations are referred to as brain plasticity and do not only include the quality and extent of functional connections of brain networks, but also fine structures of nervous tissue and even the macroscopic gross structure of brain anatomy (Bangert and Schlaug, 2006). Brain plasticity is best observed in complex tasks, including, for example, temporospatially precise movements with high behavioral relevance. These behaviors are usually accompanied by emotional arousal and motivational activation of the reward system. Furthermore, plastic changes are more pronounced when the specific activities have started before puberty and require intense training. Obviously, continued musical activities throughout the life of a musician provide an ideal setup for brain plasticity to occur. It is therefore not surprising that the most dramatic effects of brain plasticity have been demonstrated in professional musicians (for a classic review see Münte et al., 2002; for more recent reviews Wan and Schlaug, 2010; and Altenmüller and Schlaug, 2012, 2013).
Our understanding of the molecular and cellular mechanisms underlying these adaptations is far from complete. Brain plasticity may occur on different time scales. For example, the efficiency and size of synapses may be modified in a time window of seconds to minutes, the growth of new synapses and dendrites may require hours to days. Other changes require up to several weeks. They include an increase in gray matter density, reflecting either an enlargement of neurons, a change in synaptic density, more support structures such as capillaries and glial cells or a reduced rate of physiological cell death (apoptosis). White-matter density also changes as a consequence of musical training. This effect seems to be primarily due to an enlargement of myelin cells: The myelin cells, wrapped around the nerve fibers (axons) are contributing essentially to the velocity of the electrical impulses traveling along the nerve fiber tracts. Under conditions requiring rapid information transfer and high temporal precision, these myelin cells are growing, and, as a consequence, nerve conduction velocity will increase. The axons within these myelin sheaths can potentially sprout and form more and new connections particularly between the nodal cortical points that are connected by white tracts (for a recent publication on this see Wan et al., 2014). Finally, brain regions involved in specific tasks may also be enlarged after long-term training due to the growth of structures supporting the nervous function, for example, blood vessels/capillaries that are necessary for the oxygen and glucose transportation sustaining nervous function or glial cells as supporters of the local homeostasis.
Comparison of the brain anatomy of skilled musicians with that of nonmusicians shows that prolonged instrumental practice leads to an enlargement of the hand area in the motor cortex (Amunts et al., 1997). Furthermore, Gaser and Schlaug (2003) could demonstrate enhancement of gray matter density in cortical sensory-motor regions, auditory regions, the left dorsolateral prefrontal cortex, and in the cerebellum in professional instrumentalists as compared to nonmusicians and amateurs. Interestingly, these plastic adaptations depend on critical periods: musicians, who start early, before the age of seven do not display these structural adaptations of the brain at least in the sensory-motor cortices and the callosal fibers, however, they seem to have an “early optimized network,” which allows superior performance of motor tasks without enlarged anatomical structures (Steele et al., 2013; Vaquero et al., 2014). In contrast, relatively late starters, after age seven do show the abovementioned structural adaptations accounting for the effects observed in many morphological brain imaging studies (e.g., Bangert and Schlaug, 2006; Gärtner et al., 2013).
With respect to the larger callosal body in musicians, it seems plausible to assume that the high demands on coordination between the two hands, and the rapid exchange of information may either stimulate the nerve fiber growth—the myelination of nerve fibers that determines the velocity of nerve conduction—or prevent the physiological loss of nerve tissue during the typical pruning processes of adolescence or during aging. These between-group differences in the midsagittal size of the corpus callosum were confirmed in a longitudinal study comparing a group of children learning to play musical instruments versus a group of children without instrumental music experience (Hyde et al., 2009).
Halwani et al. (2011) recently showed another impressive adaptation of white-matter structures. They reported differences in macrostructure and microstructure of the arcuate fasciculus (AF)—a prominent white-matter tract connecting temporal and frontal brain regions—between singers, instrumentalists, and nonmusicians. Both groups of musicians had higher tract volumes in the right dorsal and ventral tracts compared to nonmusicians, but did not show a significant difference between each other. Singers had higher tract volume and different microstructures of the tract on the left side when compared to instrumental musicians and nonmusicians. This suggests that the right-hemisphere AF might show a more general effect of music making, while the left-hemisphere AF has a stronger response to the specific aspects of vocal-motor training and control that singers engage in. Microstructural parameters (i.e., fractional anisotropy) of the left dorsal branch of the arcuate fasciculus correlated with the number of years of participants’ vocal training, suggesting that long-term vocal-motor training might not only lead to an increase in volume, but also to an increase in microstructural complexity of specific white-matter tracts constituting the so-called aural–oral loop and connecting regions that are fundamental to sound perception, production, and its feed forward and feedback control. Similarly, Bengtsson et al. (2005) and recently Rueber et al. (2013) have found structural differences in the corticospinal tract, particularly in the posterior limb of the internal capsule, between musicians and nonmusicians as well as within musicians groups (keyboard players compared to string players). Between group differences were related to measures of training intensity as well as to the specific requirements of the instruments played. It is worth to mention that instrumental training does not only affect cortical regions, but subcortical structures also seem to show adaptation. For example, professional musicians in comparison to matched nonmusicians, seem to have a larger cerebellar volume or cerebellar gray matter (Gaser and Schlaug, 2003; Hutchinson et al., 2003). The cerebellum plays a role in the precise timing, accuracy, and coordination of motor actions, which is an important aspect of instrumental music activities.
In summary, instrumental music training, particularly when it starts at a young age, leads to plastic adaptations of various cortical and subcortical brain structures and functional networks. These changes can also include enlarged cortical representations of, for example, specific fingers or specific sounds or timbre of sounds (e.g., a string timbre vs. a brass timbre) within existing brain structures.
3 THE ROLE OF MUSIC-INDUCED EMOTIONS FOR BRAIN PLASTICITY
An intriguing question is why music is such a powerful driver of beneficial brain plasticity. This brings us to the specific motivational and emotional role of musical experience. Emotional responses to music are often cited when people describe why they value music and why they ascribe certain effects of music on health. Music is known to have a wide range of physiological effects on the human body including, for example, changes in heart rate, respiration, blood pressure, skin conductivity, skin temperature, muscle tension, and biochemical responses (for a review see Hodges, 2010; Kreutz et al., 2012).
Joyful musical behaviors, for example, learning to play a musical instrument or to sing is characterized by curiosity, stamina, and the ability to strive for rewarding experiences in future. This results in incentive goal directed activities over prolonged time periods, which are mainly mediated by the transmitter substance dopamine. Most nerve cells sensitive to this neurotransmitter are found in a small part of the brain, which is localized behind the basis of the frontal cortex, the so-called mesolimbic system, an important part of the “emotional” brain. Dopamine plays a dominant role in the neurobiology of reward, learning, and addiction. Virtually all drugs of abuse, including heroin, alcohol, cocaine, and nicotine activate dopaminergic systems. So-called natural rewards such as musical experiences and other positive social interactions likewise activate dopaminergic neurons and are powerful aids to attention and learning (Keitz et al., 2003). There is ample evidence that the sensitivity to dopamine in the mesolimbic brain regions is largely genetically determined resulting in the enormous variability in reward-dependent behavior. The genetic “polymorphism” of dopaminergic response explains the different motivational drives we observe in children with a similar social and educational background. It is intriguing that there is a strong link of dopaminergic activity to learning and memory, which in turn promote plastic adaptations in brain areas involved in the tasks to be learned.
Serotonin is another neurotransmitter important for music-induced brain plasticity. It is commonly associated with feelings of satisfaction from expected outcomes, whereas dopamine is associated with feelings of pleasure based on novelty or newness. In a study of neurochemical responses to pleasant and unpleasant music, serotonin levels were significantly higher when subjects were exposed to music they found pleasing (Evers and Suhr, 2000). In another study with subjects exposed to pleasing music, functional and effective connectivity analyses showed that listening to music strongly modulated activity in a network of mesolimbic structures involved in reward processing including the dopaminergic nucleus accumbens and the ventral tegmental area, as well as the hypothalamus and insula. This network is believed to be involved in regulating autonomic and physiological responses to rewarding and emotional stimuli (Menon and Levitin, 2005).
Blood and Zatorre (2001) determined changes in regional cerebral blood flow (rCBF) with PET technology during intense emotional experiences involving sensations such as goose bumps or shivers down the spine whilst listening to music. Each participant listened to a piece of their own favorite music to which they usually had a chill experience. Increasing chill intensity correlated with rCBF decrease in the amygdala as well as the anterior hippocampal formation. An increase in rCBF correlating with increasing chill intensity was observed in the ventral striatum, the midbrain, the anterior insula, the anterior cingulate cortex, and the orbitofrontal cortex: Again, these latter brain regions are related to reward and positive emotional valence.
In a newer study by the same group, the neurochemical specificity of [(11)C] raclo-pride PET scanning was used to assess dopamine release on the basis of the competition between endogenous dopamine and [11C]raclopride for binding to dopamine D2 receptors (Salimpoor et al., 2011). They combined dopamine-release measurements with psychophysiological measures of autonomic nervous system activity during listening to intensely pleasurable music and found endogenous dopamine release in the striatum at peak emotional arousal during music listening. To examine the time course of dopamine release, the authors used functional magnetic resonance imaging (fMRI) with the same stimuli and listeners, and found a functional dissociation: the caudate was more involved during the anticipation and the nucleus accumbens was more involved during the experience of peak emotional responses to music. These results indicate that intense pleasure in response to music can lead to dopamine release in the striatal system. Notably, the anticipation of an abstract reward can result in dopamine release in an anatomical pathway distinct from that associated with the peak pleasure itself. Such results may well help to explain why music is of such high value across all human societies. As stated above, dopaminergic activation regulates and heightens arousal, motivation and supports memory formation in the episodic and the procedural memory (Karabanov et al., 2010) and thereby will contribute to memorization of auditory stimuli producing such strong emotional responses. In a very recent study, the authors could even demonstrate that the degree of activation and connectivity in a network comprising the nucleus accumbens, auditory cortices, amygdala, and ventromedial frontal cortex predicted the amount of money, subjects were willing to spend in an auction paradigm (Salimpoor et al., 2013).
Taken together, these powerful music-induced modulations of neurohormonal status may not only account for pleasurable experiences but may also play a role in neurologic music therapy.
4 FACILITATING RECOVERY FROM NONFLUENT APHASIA THROUGH A FORM OF SINGING
The ability to sing in humans is evident from infancy, and does not depend on formal vocal training but can be enhanced by training. Given the behavioral similarities between singing and speaking, as well as the shared and distinct neural correlates of both, researchers have begun to examine whether forms of singing can be used to treat some of the speech-motor abnormalities associated with various neurological conditions (Wan et al., 2010).
Aphasia is a common and devastating complication of stroke or other brain injuries that results in the loss of ability to produce and/or comprehend language. It has been estimated that between 24% and 52% of acute stroke patients have some form of aphasia if tested within 7 days of their stroke; 12% of survivors still have significant aphasia at 6 months after stroke (Wade et al., 1976). The nature and severity of language dysfunction depends on the location and extent of the brain lesion. Accordingly, aphasia can be classified broadly into fluent or nonfluent. Fluent aphasia often results from a lesion involving the posterior superior temporal lobe known as Wernicke’s area. Patients who are fluent exhibit articulated speech with relatively normal utterance length. However, their speech may be completely meaningless to the listener and littered with jargon. Furthermore, it may contain violations to syntactic and grammatical rules. These patients also have severe speech comprehension deficits. In contrast, nonfluent aphasia results most commonly from a lesion in the left frontal lobe, involving the left posterior inferior frontal region known as Broca’s area. Patients who are nonfluent tend to have relatively intact comprehension for conversational speech, but have marked impairments in articulation and speech production.
The general consensus is that there are two routes to recovery from aphasia. In patients with small lesions in the left hemisphere, there tends to be recruitment of both left-hemispheric, perilesional cortices with variable involvement of right-hemispheric homologous regions during the recovery process (Heiss and Thiel, 2006; Heiss et al., 1999; Hillis, 2007; Rosen et al., 2000). In patients with large left-hemispheric lesions involving language-related regions of the frontotemporal lobes, the only path to recovery may be through recruitment of homologous language and speech-motor regions in the right hemisphere (Geschwind, 1971; Schlaug et al., 2008). It has been suggested that recovery via the right hemisphere may be less efficient than recovery via the left hemisphere (Hillis, 2007), possibly because patients with relatively large left-hemispheric lesions that encompass all of the relevant speech-motor regions of the left hemisphere are generally more impaired and recover to a lesser degree than patients with smaller left-hemisphere lesions. Nevertheless, activation of right-hemispheric regions during speech/language fMRI tasks has been reported in patients with aphasia, irrespective of their lesion size (Rosen et al., 2000). For patients with large lesions that cover the language-relevant regions on the left, therapies that specifically engage or stimulate the homologous right-hemispheric regions have the potential to facilitate the language recovery process beyond the limitations of natural recovery (Gerstman, 1964; Keith and Aronson, 1975). Based on clinical observations of patients with severe nonfluent aphasia and their ability to sing lyrics better than they can speak the same words (Albert et al., 1973; Schlaug et al., 2010; Sparks and Holland, 1976), an intonation-based therapy called Melodic Intonation Therapy (MIT) that would emphasize melody and contour and engage a sensorimotor network of articulation on the unaffected hemisphere through rhythmic tapping was developed (Albert et al., 1973; Schlaug et al., 2010). The two unique components of MIT are first the intonation of words and simple phrases using a melodic contour that follows the prosody of speech, and second the rhythmic tapping of the left hand that accompanies the production of each syllable and serves as a catalyst for fluency.
To date, studies using MIT have produced positive outcomes in patients with nonfluent aphasia. These outcomes range from improvements on the Boston Diagnostic Aphasia Examination (BDAE; Goodglass and Kaplan, 1983), to improvements in articulation and phrase production (Bonakdarpour et al., 2000; Wilson et al., 2006) after treatment. The effectiveness of this intervention is further demonstrated in a recent study that examined transfer of language skills to untrained contexts. Schlaug et al. (2008) compared the effects of MIT with a control intervention (speech repetition) on picture naming performance and measures of propositional speech. After 40 daily sessions, both therapy techniques resulted in significant improvement on all outcome measures, but the extent of this improvement was far greater for the patient who underwent MIT compared to the one who underwent the control therapy.
The therapeutic effect of MIT is also evident in neuroimaging studies that show reorganization of brain functions. MIT resulted in increased activation in a right-hemisphere network involving the premotor, inferior frontal, and temporal lobes (Schlaug et al., 2008), as well as increased fiber number and volume of the arcuate fasciculus in the right hemisphere (Schlaug et al., 2009; Wan et al., 2014). These findings demonstrate that intensive experimental therapies such as MIT—when applied over a longer period of time in chronic stroke patients—can induce functional and structural brain changes in a right-hemisphere vocal-motor network, and these changes are related to speech output improvements (Wan et al., 2014).
The mechanisms that are underlying the recovery-enhancing effects of MIT are not completely clear. However, it has been argued that we suggest that there are four possible mechanisms by which MIT’s therapeutic effect is achieved (for details, see Schlaug et al., 2008): (1) reduction of speed to approximately one syllable/sec. which may specifically engage right-hemisphere perceptual and perception–action coupling on the right hemisphere, as the right hemisphere has been shown to integrate sensory information over a larger time window than the left (Abrams et al., 2008; Poeppel, 2003); (2) syllable lengthening that isolates/emphasizes individual phonemes even as they remain part of the continuously voiced words/phrases; and (3) “chunking” that not only combines prosodic information with meaningful content to facilitate production of longer, more fluent phrases, but has also been shown to lead to more right- than left-hemisphere activation in healthy subjects (Meyer et al., 2002; Ozdemir et al., 2006; Zatorre and Belin, 2001). Given that, patients with right-hemisphere lesions have greater difficulty with global processing tasks (e.g., melody and contour processing) than those with left-hemisphere lesions (Peretz, 1990; Schuppert et al., 2000), it is possible that the melodic element of MIT does, indeed engage the right hemisphere, particularly the right temporal lobe, more than therapies that do not make use of tonal information or melodic contour, and again intervention that would integrate information over a larger timescale favoring right over left-hemispheric processing (Poeppel, 2003). The fourth mechanism—Left-hand tapping (one tap/syllable, one syllable/sec.)—is likely to play an important role in engaging a right-hemispheric, sensorimotor network capable of providing an impulse for verbal production in much the same way that a metronome has been shown to serve as a “pacemaker” when rhythmic motor activities prime and/or entrain sensorimotor networks (Thaut and Abiru, 2010; Thaut et al., 1999). In addition, research suggesting that hand movements and articulatory movements may share neural correlates (Gentilucci et al., 2000; Meister et al., 2003; Tokimura et al., 1996; Uozumi et al., 2004) further supports the notion that hand tapping is critically important for facilitating the coupling of sounds to orofacial and articulatory actions (Lahav et al., 2007). Since concurrent speech and hand use occurs in daily life, and gestures are frequently used to emphasize/accompany important and/or elusive concepts in speech, rhythmic hand movements, in synchrony with articulatory movements, may have similarly beneficial effects on speech production and in particular in relearning of speech-motor functions after a stroke.
5 MUSIC-SUPPORTED MOTOR THERAPY IN STROKE PATIENTS
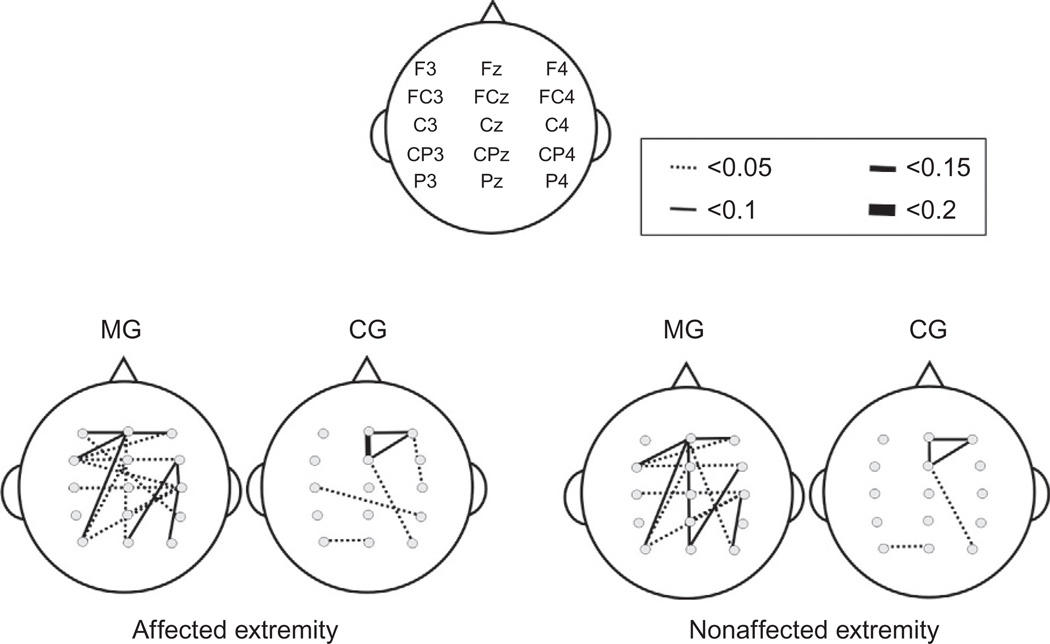
MST in the rehabilitation of fine motor hand skills was first systematically investigated by Schneider et al. (2007). Patients were encouraged to play melodies with the paretic hand on a piano, or to tap with the paretic arm on eight electronic drum pads that emitted piano tones. It was demonstrated that these patients regained faster their motor abilities, and improved in timing, precision, and smoothness of fine motor skills. Along with fine motor recovery, an increase in neuronal connectivity between sensory-motor and auditory regions was demonstrated by means of EEG-coherence measures (Altenmüller et al., 2009; Rojo et al., 2012; Schneider et al., 2010). Therefore, establishing an audio-sensory-motor co-representation may support the rehabilitation process (see Fig. 1). This notion is corroborated by findings in a patient who underwent music-supported training 20 months after suffering a stroke. Along with clinical improvement, fMRI follow up provided evidence for the establishment of an auditory-sensory-motor network due to the training procedure (Rojo et al., 2012). Recently, in a larger group of 20 stroke patients, changes in motor cortex excitability following a 4 weeks intervention were demonstrated with the transcranial magnetic stimulation technique. These changes were accompanied with marked improvements of fine motor skills (Amengual et al., 2013).
FIGURE 1.
Topographic task-related coherence maps for the Music group (MG) compared to the control group (CG) during self-paced arm movements for the drum pad condition in the beta band (18–22 Hz). Statistically significant increases in task-related coherence during the motor performance after 3 weeks and 15 sessions of music-supported therapy on sonified drum pads are displayed.
From Altenmüller and Schlaug (2013) with permission.
Music-supported training is undoubtedly efficient and seems to be even more helpful than functional motor training using no auditory feedback, but otherwise similar fine motor training. A randomized prospective study comprising all three groups is presently under the way and will clarify the differential effects of functional motor training and music-supported training. With respect to the underlying mechanisms, a number of open questions still remain. First, the role of motivational factors must be clarified. From the patients’ informal descriptions of their experience with the music-supported training, it appears that this was highly enjoyable and a highlight of their rehabilitation process. Thus, motivational and emotional factors might have contributed to the success of the training program. Furthermore, according to a study by Särkämö et al. (2008), music listening activates a widespread bilateral network of brain regions related to attention, semantic processing, memory, motor functions, and emotional processing. Särkämö and colleagues showed that music exposure significantly enhances cognitive functioning in the domains of verbal memory and focused attention in stroke patients. The music group also experienced less depressed mood than the control groups. These mechanisms may also hold true for the music-supported training we applied.
Another issue is related to the auditory feedback mechanisms. Up to now, it has not been clear whether any auditory feedback (e.g., simple beep tones) would have a similar effect on fine motor rehabilitation or whether explicit musical parameters such as a sophisticated pitch and time structure are prerequisites for the success of the training. This has to be addressed in a study comparing the effects of musical feedback compared to simple acoustic feedback. With respect to the latter, according to a study by Thaut et al. (2002), simple rhythmic cueing with a metronome significantly improves the spatiotemporal precision of reaching movements in stroke patients.
Furthermore, it is not clear, whether timing regularity and predictability is crucial for the beneficial effect of MST using key-board playing or tapping on drumpads. Although it has been argued that the effectiveness of this therapy relies on the fact that the patient’s brain receives a time-locked auditory feedback with each movement, new results challenge this viewpoint. In a recent study, 15 patients in early stroke rehabilitation with no previous musical background learned to play simple finger exercises and familiar children’s songs on the piano. The participants were assigned to one of two groups: in the normal group, the keyboard emitted a tone immediately at keystroke, in the delay group, the tone was delayed by a random time interval between 100 and 600 ms. To assess recovery, we performed standard clinical tests such as the nine-hole-pegboard test and index finger tapping speed and regularity. Surprisingly, patients in the delay group improved more in the nine-hole-pegboard test, whereas patients in the normal group did not. In finger tapping rate and regularity both groups showed similar marked improvements. The normal group showed reduced depression whereas the delay group did not (van Vugt et al., 2014). Here, we conclude that music therapy on a randomly delayed keyboard can improve motor recovery after stroke. We hypothesize that the patients in the delayed feedback group implicitly learn to be independent of the auditory feedback and therefore outperform those in the normal condition.
Finally, the stability of improvements needs to be assessed in further studies, and the length and number of training sessions might be manipulated in future research. Additionally, the effect of training in chronic patients suffering from motor impairments following a stroke for more than a year will be assessed.
6 CONCLUSIONS
Emerging research over the past decade has shown that long-term music training and the associated sensorimotor skill learning can be a strong stimulant for neuroplastic changes in the developing as well as in the adult brain, affecting both white and gray matter as well as cortical and subcortical structures. Making music including singing and dancing leads to a strong coupling of perception and action mediated by sensory, motor, and multimodal brain regions and affects either in a top-down or bottom-up fashion important relay stations in the brainstem and thalamus. Furthermore, listening to music and making music provokes motions and emotions, increases between-subject communications and interactions and—mediated via neurohormons such as serotonin and dopamine—is experienced as joyous and rewarding through activity changes in amygdala, ventral striatum, and other components of the limbic system. Making music makes rehabilitation more enjoyable and can remediate impaired neural processes or neural connections by engaging and linking brain regions with each other that might otherwise not be linked together.
As other experimental interventions, music-based experimental interventions need to be grounded on a neurobiological understanding of how and why particular brain systems could be affected. The efficacy of these experimental interventions should be assessed quantitatively and in an unbiased way. A neuroscientific basis for music-based interventions and data derived from randomized clinical trials are important steps in establishing neurologically based music therapies that might have the power to enhance brain recovery processes, ameliorate the effects of developmental brain disorders, and neuroplasticity in general.
ACKNOWLEDGMENTS
G. S. gratefully acknowledges support from NIH (1RO1 DC008796, 3R01DC008796-02S1, R01 DC009823-01), the family of Rosalyn and Richard Slifka, and the Matina R. Proctor Foundation.
Parts of this Review Article are containing an updated version of a previous review, which appeared 2013 in the Journal “Music and Medicine.”
REFERENCES
- Abrams DA, Nicol T, Zecker S, Kraus N. Right-hemisphere auditory cortex is dominant for coding syllable patterns in speech. J. Neurosci. 2008;28:3958–3965. doi: 10.1523/JNEUROSCI.0187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert ML, Sparks RW, Helm NA. Melodic intonation therapy for aphasia. Arch. Neurol. 1973;29:130–131. doi: 10.1001/archneur.1973.00490260074018. [DOI] [PubMed] [Google Scholar]
- Altenmüller E, McPherson G. Motor learning and instrumental training. In: Gruhn FR, editor. Neurosciences in Music Pedagogy. New York, NY: Nova Science Publisher; 2007. pp. 145–155. [Google Scholar]
- Altenmüller E, Schlaug G. Music, brain, and health: exploring biological foundations of music’s health effects. In: MacDonald R, Kreutz G, Mitchell L, editors. Music, Health, and Wellbeing. Oxford: Oxford University Press; 2012. pp. 12–24. [Google Scholar]
- Altenmüller E, Schlaug G. Neurobiological aspects of neurologic music therapy. Music Med. 2013;5:210–216. [Google Scholar]
- Altenmüller E, Schneider S, Marco-Pallares PW, Münte TF. Neural reorganization underlies improvement in stroke induced motor dysfunction by music supported therapy. Ann. N.Y. Acad. Sci. 2009;1169:395–405. doi: 10.1111/j.1749-6632.2009.04580.x. [DOI] [PubMed] [Google Scholar]
- Amengual JL, Rojo N, Veciana de las Heras M, Marco-Pallarés J, Grau J, Schneider S, Vaquero L, Juncadella M, Montero J, Mohammadi B, Rubio F, Rueda N, Duarte E, Grau E, Altenmüller E, Münte TF, Rodríguez-Fornells A. Sensorimotor plasticity after music-supported therapy in chronic stroke patients revealed by transcranial magnetic stimulation. PLoS One. 2013;8:e61883. doi: 10.1371/journal.pone.0061883. http://dx.doi.org/10.1371/journal.pone.0061883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Schlaug G, Jäncke L, Steinmetz H, Schleicher A, Dabringhaus A, Zilles K. Motor cortex and hand motor skills: structural compliance in the human brain. Hum. Brain Mapp. 1997;5:206–215. doi: 10.1002/(SICI)1097-0193(1997)5:3<206::AID-HBM5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bangert M, Altenmüller E. Mapping perception to action in piano practice: a longitudinal DC-EEG-study. BMC Neurosci. 2003;4:26–36. doi: 10.1186/1471-2202-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangert M, Schlaug G. Specialization of the spezialized in features of external brain morphology. Eur. J. Neurosci. 2006;24:1832–1834. doi: 10.1111/j.1460-9568.2006.05031.x. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc. Natl. Acad. Sci. U.S.A. 2001;198:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonakdarpour B, Eftekharzadeh A, Ashayeri H. Preliminary report on the effects of melodic intonation therapy in the rehabilitation of Persian aphasic patients. Iran. J. Med. Sci. 2000;25:156–160. [Google Scholar]
- Evers S, Suhr B. Changes of the neurotransmitter serotonin but not of hormones during short time music perception. Eur. Arch. Psychiatry Clin. Neurosci. 2000;250:144–147. doi: 10.1007/s004060070031. [DOI] [PubMed] [Google Scholar]
- Gärtner H, Minnerop M, Pieperhoff P, Schleicher A, Zilles K, Altenmüller E, Amunts K. Brain morphometry shows effects of long-term musical practice in middle-aged keyboard players. Front. Psychol. 2013;4:636. doi: 10.3389/fpsyg.2013.00636. http://dx.doi.org/10.3389/fpsyg.2013.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J. Neurosci. 2003;23:9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentilucci M, Benuzzi F, Bertolani L, Daprati E, Gangitano M. Language and motor control. Exp. Brain Res. 2000;133:468–490. doi: 10.1007/s002210000431. [DOI] [PubMed] [Google Scholar]
- Gerstman HL. A case of aphasia. J. Speech Hear. Disord. 1964;29:89–91. doi: 10.1044/jshd.2901.89. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Current concepts: aphasia. N. Engl. J. Med. 1971;284:654–656. doi: 10.1056/NEJM197103252841206. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination. second ed. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- Halwani GF, Loui P, Rüber T, Schlaug G. Effects of practice and experience on the arcuate fasciculus: comparing singers, instrumentalists, and non-musicians. Front. Psychol. 2011;2:156. doi: 10.3389/fpsyg.2011.00156. http://dx.doi.org/10.3389/fpsyg.2011.001561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98:118–123. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann. Neurol. 1999;45:430–438. doi: 10.1002/1531-8249(199904)45:4<430::aid-ana3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hillis AE. Aphasia: progress in the last quarter of a century. Neurology. 2007;69:200–213. doi: 10.1212/01.wnl.0000265600.69385.6f. [DOI] [PubMed] [Google Scholar]
- Hodges DA. Psychophysiological measures. In: Juslin PN, Sloboda JA, editors. Handbook of Music and Emotion. Oxford: Oxford University Press; 2010. pp. 279–311. [Google Scholar]
- Hutchinson S, Lee LHL, Gaab N, Schlaug G. Cerebellar volume: gender and musicianship effects. Cereb. Cortex. 2003;13:943–949. doi: 10.1093/cercor/13.9.943. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Lerch J, Norton A, Forgeard M, Winner E, Evans AC, Schlaug G. Musical training shapes structural brain development. J. Neurosci. 2009;29:3019–3025. doi: 10.1523/JNEUROSCI.5118-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabanov A, Cervenka S, de Manzano O, Forssberg H, Farde L, Ullén F. Dopamine D2 receptor density in the limbic striatum is related to implicit but not explicit movement sequence learning. Proc. Natl. Acad. Sci. 2010;107:7574–7579. doi: 10.1073/pnas.0911805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith RL, Aronson AE. Singing as therapy for apraxia of speech and aphasia: report of a case. Brain Lang. 1975;2:483–488. doi: 10.1016/s0093-934x(75)80085-x. [DOI] [PubMed] [Google Scholar]
- Keitz M, Martin-Soelch C, Leenders KL. Reward processing in the brain: a prerequisite for movement preparation? Neural Plast. 2003;1:121–128. doi: 10.1155/NP.2003.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutz G, Quiroga Murcia C, Bongard S. Psychoneuroendocrine research on music and health. In: MacDonald R, Kreutz G, Mitchell L, editors. Music, Health and Wellbeing. Oxford: Oxford University Press; 2012. pp. 457–476. An overview. [Google Scholar]
- Lahav A, Saltzman E, Schlaug G. Action representation of sound: audiomotor recognition network while listening to newly acquired actions. J. Neurosci. 2007;27:308–314. doi: 10.1523/JNEUROSCI.4822-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister IG, Boroojerdi B, Foltys H, Sparing R, Huber W, Topper R. Motor cortex hand area and speech: implications for the development of language. Neuropsychologia. 2003;41:401–406. doi: 10.1016/s0028-3932(02)00179-3. [DOI] [PubMed] [Google Scholar]
- Menon V, Levitin DJ. The rewards of music listening: response and physiological connectivity of the mesolimbic system. NeuroImage. 2005;28:175–184. doi: 10.1016/j.neuroimage.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Meyer M, Alter K, Friederici AD, Lohmann G, von Cramon DY. FMRI reveals brain regions mediating slow prosodic modulations in spoken sentences. Hum. Brain Mapp. 2002;17:73–88. doi: 10.1002/hbm.10042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münte TF, Altenmüller E, Jäncke L. The musician’s brain as a model of neuroplasticity. Nat. Neurosci. 2002;3:473–478. doi: 10.1038/nrn843. [DOI] [PubMed] [Google Scholar]
- Ozdemir E, Norton A, Schlaug G. Shared and distinct neural correlates of singing and speaking. NeuroImage. 2006;33:628–635. doi: 10.1016/j.neuroimage.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Peretz I. Processing of local and global musical information by unilateral brain-damaged patients. Brain. 1990;113:1185–1205. doi: 10.1093/brain/113.4.1185. [DOI] [PubMed] [Google Scholar]
- Poeppel D. The analysis of speech in different temporal integration windows: cerebral lateralization as “asymmetric sampling in time”. Speech Comm. 2003;41:245–255. [Google Scholar]
- Rojo N, Amengual J, Juncadella M, Rubio F, Camara E, Marco-Pallares J, Schneider S, Veciana M, Montero J, Mohammadi B, Altenmüller E, Grau C, Münte TF, Rodriguez-Fornells A. Music-supported therapy induces plasticity in the sensorimotor cortex in chronic stroke: a single-case study using multimodal imaging (fMRI-TMS) Brain Inj. 2012;25:787–793. doi: 10.3109/02699052.2011.576305. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Petersen SE, Linenweber MR. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55:1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- Rueber T, Lindenberg R, Schlaug G. Differential adaptation of descending motor pathways in musicians. Cereb. Cortex. 2013 doi: 10.1093/cercor/bht331. http://dx.doi.org/10.1093/cercor/bht331 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat. Neurosci. 2011;14:257–262. doi: 10.1038/nn.2726. [DOI] [PubMed] [Google Scholar]
- Salimpoor VN, van den Bosch I, Kovacevicm N, McIntoshm AR, Dagher A, Zatorre RJ. Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science. 2013;340:216–219. doi: 10.1126/science.1231059. [DOI] [PubMed] [Google Scholar]
- Särkämö T, Tervaniemi M, Laitinen S, Forsblom A, Soinila S, Mikkonen M. Music listening enhances cognitive recovery and mood after middle Cerebral artery stroke. Brain. 2008;131:866–876. doi: 10.1093/brain/awn013. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Norton A. From singing to speaking: why patients with Broca’s aphasia can sing and how that may lead to recovery of expressive language functions. Music. Percept. 2008;25:315–323. doi: 10.1525/MP.2008.25.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca’s aphasia undergoing intense intonation-based speech therapy. Ann. N.Y. Acad. Sci. 2009;1169:385–394. doi: 10.1111/j.1749-6632.2009.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Norton A, Marchina S, Zipse L, Wan CY. From singing to speaking: facilitating recovery from nonfluent aphasia. Future Neurol. 2010;5:657–665. doi: 10.2217/fnl.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Schönle PW, Altenmüller E, Münte TF. Using musical instruments to improve motor skill recovery following a stroke. J. Neurol. 2007;254:1339–1346. doi: 10.1007/s00415-006-0523-2. [DOI] [PubMed] [Google Scholar]
- Schneider S, Münte TF, Rodriguez-Fornells A, Sailer M, Altenmüller E. Music supported training is more efficient than functional motor training for recovery of fine motor skills in stroke patients. Music. Percept. 2010;27:271–280. [Google Scholar]
- Schuppert M, Munte TF, Wieringa BM, Altenmuller E. Receptive amusia: evidence for cross-hemispheric neural networks underlying music processing strategies. Brain. 2000;123:546–559. doi: 10.1093/brain/123.3.546. [DOI] [PubMed] [Google Scholar]
- Sparks RW, Holland AL. Method: melodic intonation therapy for aphasia. J. Speech Hear. Disord. 1976;41:287–297. doi: 10.1044/jshd.4103.287. [DOI] [PubMed] [Google Scholar]
- Steele CJ, Bailey JA, Zatorre RJ, Penhune VB. Early musical training and white-matter plasticity in the corpus callosum: evidence for a sensitive period. J. Neurosci. 2013;33:1282–1290. doi: 10.1523/JNEUROSCI.3578-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaut MH, Abiru M. Rhythmic auditory stimulation in rehabilitation of movement disoders: a review of current research. Music. Percept. 2010;27:263–269. [Google Scholar]
- Thaut MH, Kenyon GP, Schauer ML, McIntosh GC. The connection between rhythmicity and brain function. IEEE Eng. Med. Biol. Mag. 1999;18:101–108. doi: 10.1109/51.752991. [DOI] [PubMed] [Google Scholar]
- Thaut MH, Kenyon GP, Hurt CP, McIntosh GC, Hoemberg V. Kinematic optimization of spatiotemporal patterns in paretic arm training with stroke patients. Neuropsychologia. 2002;40:1073–1081. doi: 10.1016/s0028-3932(01)00141-5. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Tokimura Y, Oliviero A, Asakura T, Rothwell JC. Speech-induced changes in corticospinal excitability. Ann. Neurol. 1996;40:628–634. doi: 10.1002/ana.410400413. [DOI] [PubMed] [Google Scholar]
- Tramo MJ. Biology and music. Music of the hemispheres. Science. 2001;291:54–56. doi: 10.1126/science.10.1126/science.1056899. [DOI] [PubMed] [Google Scholar]
- Uozumi T, Tamagawa A, Hashimotor T, Tsuji S. Motor hand representation in cortical area 44. Neurology. 2004;62:757–761. doi: 10.1212/01.wnl.0000113731.75479.25. [DOI] [PubMed] [Google Scholar]
- van Vugt FT, Kafczyk T, Kuhn W, Rollnik JD, Tillmann B, Altenmüller E. The role of auditory feedback in music-supported stroke rehabilitation: a single-blinded randomised controlled intervention. Brain Inj. 2014 doi: 10.3233/RNN-150588. (Submitted) [DOI] [PubMed] [Google Scholar]
- Vaquero L, Hartmann K, Ripollés P, Rojo N, Sierpowska J, Cámara E, Mohammadi B, Samii A, Münte TF, Rodriguez-Fornells A, Altenmüller E. The earlier, the smaller: neuroplastic changes in professional pianists depend on age of onset. NeuroImage. 2014 (Submitted) [Google Scholar]
- Wade DT, Hewer RL, David RM, Enderby PM. Aphasia after stroke: natural history and associated deficits. J. Neurol. Neurosurg. Psychiatry. 1976;49:11–16. doi: 10.1136/jnnp.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Schlaug G. Music making as a tool for promoting brain plasticity across the life-span. Neuroscientist. 2010;16:566–577. doi: 10.1177/1073858410377805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Rüber T, Hohmann A, Schlaug G. The therapeutic effects of singing in neurological disorders. Music. Percept. 2010;27:287–295. doi: 10.1525/mp.2010.27.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan C, Zheng X, Marchina S, Norton A, Schlaug G. Intensive therapy induces contralateral white matter changes in chronic stroke patients with Broca’s aphasia. Brain Lang. 2014;136:1–7. doi: 10.1016/j.bandl.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Parsons K, Reutens DC. Preserved singing in aphasia: a case study of the efficacy of the melodic intonation therapy. Music. Percept. 2006;24:23–36. [Google Scholar]
- Zatorre RJ, Belin P. Spectral and temporal processing in human auditory cortex. Cereb. Cortex. 2001;11:946–953. doi: 10.1093/cercor/11.10.946. [DOI] [PubMed] [Google Scholar]