Abstract
Gestational protein restriction causes hypertension in the adult offspring. Very little is known about the food intake regulation and ghrelin signaling in pregnant dams fed a low-protein (LP) diet. We hypothesized that diet intake and ghrelin signaling are altered in pregnant rats fed the low-protein diet. Sprague–Dawley rats were fed a control (CT) or LP diet from Day 3 of pregnancy. Diet intake and body weight were monitored daily. Expression of ghrelin production-related genes in the stomach and appetite-related genes in the hypothalamus was analyzed by real-time PCR. Plasma levels of total and active ghrelin, growth hormone and leptin were measured by ELISA. Main results include: (1) Daily diet intake was greater in the LP group than in the CT group in early pregnancy, but substantially lower in late pregnancy; (2) Daily gain in body weight was substantially lower in the LP group in late pregnancy; (3) Expression of ghrelin production-related genes in the stomach and plasma total ghrelin levels were increased in LP group in late pregnancy; (4) Plasma active ghrelin levels were elevated in the LP group at mid-late pregnancy, but growth hormone and leptin levels were uncorrelated with active ghrelin in late pregnancy; and (5) Hypothalamic expression of ghrelin-stimulated genes in LP rats was unassociated with the changes in both plasma ghrelin levels and the diet intake. Taken together, the appetite in LP rats is greater in early pregnancy but reduced at late pregnancy, possibly due to ghrelin insensitivity in appetite regulation.
Keywords: Diet intake, ghrelin, low-protein diet, pregnancy, rat
Introduction
Studies on fetal programming of cardiovascular and metabolic diseases have enormous clinical significance, and various animal models have used nutritional (Goyal et al. 2009, 2010), hormonal (Dodic et al. 2001; Forhead and Fowden 2004; Connors et al. 2010), and surgical (Alexander 2003) manipulations during gestation to understand the mechanisms involved. Among these models, pregnant rats given a low-protein diet have been widely used (Kautzky-Willer and Handisurya 2009). Low-protein diets during pregnancy decrease both placental and fetal weights (Jansson et al. 2006; Gao et al. 2012a,b), and both male and female offspring develop hypertension in adulthood (Langley-Evans et al. 1996, 1999; Gangula et al. 2005; McMullen and Langley-Evans 2005a,b; Sathishkumar et al. 2012). Although it is well established that nutrients including protein and amino acids are critical for fetal growth, to date, there are few studies investigating systematically the diet intake and body weight gain in pregnant rats fed a low-protein diet (Desai et al. 1996; Fernandez-Twinn et al. 2003).
Appetite is regulated by interaction between the central nervous system and peripheral organs. Certain regions in the hypothalamus, including in particular the arcuate nucleus (ARC), integrate various signals such as ghrelin, leptin, and insulin in regulating appetite (Naslund and Hellstrom 2007; Ladyman et al. 2010; Sobrino et al. 2014). Among the known appetite-regulating signals, ghrelin is the only peripherally produced hormone which is known to stimulate appetite and thus, is considered to be a critical regulator of appetite and nutritional status (Fernandez-Fernandez et al. 2006; Angelidis et al. 2012).
Ghrelin is secreted by the stomach in response to hunger and fasting (Toshinai et al. 2001; Cummings et al. 2004). In the stomach, the ghrelin precursor (prepro-ghrelin) is cleaved into proghrelin, which is further cleaved to produce ghrelin, a 28-amino acid peptide termed desacyl ghrelin; desacyl ghrelin is acetylated by the enzyme MBOAT4 [membrane bound O-acyltransferase domain containing 4, also known as GOAT (ghrelin O-acyltransferase)] (Gutierrez et al. 2008; Yang et al. 2008) and becomes ‘active ghrelin’ with a high affinity to GHSR1a (growth hormone secretagogue type 1a receptor) (Kojima et al. 1999). Active ghrelin is inactivated by the enzyme BCHE (butyrylcholinesterase) (De et al. 2004).
Active ghrelin produced in the stomach is released into the blood circulation, and it stimulates appetite in part by its actions on NPY/AgRP (neuropeptide Y/Agouti-related peptide) expressing neurons in the ARC (Kojima and Kangawa 2010). Briefly, active ghrelin binds to GHSR1a and stimulates phosphorylation of AMPK (AMP-activated protein kinase). AMPK phosphorylates ACC (acetyl-CoA carboxylase) which promotes fatty acid metabolism and CPT1 (carnitine palmitoyltransferase) activation. Free radical production in fatty acid metabolism in mitochondria stimulates expression of Ucp2 (uncoupling protein 2) (Andrews et al. 2008), which is followed by increased diet intake. In addition, NPY/AgRP hormones suppress the activity of CART/POMC (cocaine-amphetamine regulated transcript/proopiomelanocortin) expressing neurons in the ARC (Funahashi et al. 2003; Naslund and Hellstrom 2007). In addition, active ghrelin induces release of growth hormone from the pituitary (Kojima and Kangawa 2005), which is initiated by active ghrelin binding to GHSR1a in growth hormone-releasing hormone cells in the ARC (Sun et al. 2004).
To date, little is known about ghrelin-induced appetite stimulation during pregnancy, and specifically in pregnant dams fed a low-protein diet. During pregnancy, increased appetite and food intake are critical for facilitating energy storage for the high metabolic demands of pregnancy and subsequent lactation (Ladyman et al. 2010). It has been reported that ghrelin production is stimulated by dietary protein, and reduced by fat and carbohydrates (Erdmann et al. 2003), thus the reduced protein content in the LP diet may affect ghrelin-regulated diet intake in pregnant dams. To test this, we investigated, in pregnant rats: (1) dietary intake during gestation; (2) daily gain in body weight; (3) plasma levels of total and active ghrelin; (4) expression of ghrelin production-related genes Ghrl, Mboat4, and Bche in the stomach; and (5) expression of appetite stimulation marker genes (Npy, Agrp), appetite inhibition marker genes (Pomc, Cart) and ghrelin signaling pathway-related genes (Gshr, Fasn, Cpt1a, Cpt1c, Ucp2) in the whole hypothalamus as well as in the ARC.
Materials and Methods
Animal and diets
All procedures were approved by the Animal Care and Use Committee at Baylor College of Medicine and were in accordance with those published by the US National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011). Timed pregnant Sprague–Dawley rats weighing between 175 and 225 g were purchased from Harlan (Houston, TX). At Day 3 of pregnancy (Day 1 was determined by the presence of sperm in the virginal smear after breeding), rats were randomly divided into two dietary groups, housed individually and fed a control (CT, 20% casein) or low-protein (LP, 6% casein) diet until they were killed on Days 10, 14, 18, 19, 21, or 22 of pregnancy (n = 8–10 rats/diet/day of pregnancy). Both diet intake and body weights were recorded at 24-h intervals, and assessed daily between 7.00AM and 8.00AM. The isocaloric low-protein and normal-protein diets were purchased from Harlan Teklad (Cat. TD.90016 and TD.91352, respectively; Madison, WI). The contents of these diets were described in a previous study and isocaloric value of the diet was maintained by the increased sucrose content (Vehaskari et al. 2001). The contents of other nutrients except methionine are also similar between the LP diet and normal CT diet. The content of methionine was reduced in the LP diet proportionately. In pregnant rats fed with the LP diet, plasma levels of total nonessential amino acids were increased and total essential amino acids were reduced, whereas no changes were observed in total free amino acids (Gao et al. 2012a). In contrast, in fetal plasma all amino acids except threonine were unchanged in late pregnancy (Rees et al. 1999). In addition, our previous studies showed that the LP diet did not affect the litter size or delivery time (Gangula et al. 2005; Jansson et al. 2006), but reduced both placental and fetal weights (Gao et al. 2012a,b) and neonatal survival rate (Gangula et al. 2005). All offspring were hypertensive as they became adults with more severe and earlier onset in males (3-month old), compared to females (6-month old) (Gangula et al. 2005; Sathishkumar et al. 2012).
Tissue collection
Rats were killed by CO2 inhalation between 8.00 am and 10.00 am on Days 10, 14, 18, 19, 21, or 22 of pregnancy. Whole blood was collected by left ventricle puncture using a 10 mL syringe and an 18G needle and injected into BD Vacutainer blood collection tube containing K2-EDTA (Cat. 36643, BD, Franklin Lakes, NJ). The blood was then mixed with protease inhibitor, Pefabloc SC (Cat. 76307; Sigma-Aldrich, St. Louis, MO) to a final concentration of 1 mg/mL and centrifuged at 3000 g for 15 min at 4°C. The supernatant was aliquotted, snap frozen in liquid nitrogen and stored at −80°C until analysis.
The stomach was trimmed free of fat and pancreatic tissues, slit open and the contents were removed by wiping with Kimwipe tissues. The cleaned stomach was snap frozen in liquid nitrogen and stored at −80°C until analysis.
The hypothalamus and ARC were collected using the dissection procedures described in a previous report (Heffner and Seiden 1980). Briefly, the brain was quickly removed and oriented properly in the precooled rat brain matrix (coronal sections; Cat. RBM-4000C; ASI Instruments, Warren, MI). Square blades were placed into the given positions in brain matrix according to a rat brain atlas (Paxinos and Watson 1998). To dissect the hypothalamus, blades were placed at the middle of optic chiasm and the end of the hypothalamus (3.5–8 mm; Interaural), and the thalamus was trimmed off by cutting longitudinally at the tip of Corpus Callosum. To dissect the ARC region, blades were placed at 2 and 5 mm back from the middle of optic chiasm (6.88–4.48 mm; Interaural). The section was trimmed to the thickness of 3 mm from the caudal surface of the hypothalamus (−2.12 to −4.52 mm; Bregma). The sections of the hypothalamus and ARC were snap frozen in liquid nitrogen and stored at −80°C until analysis.
RNA extraction and RT-PCR
Total RNA was extracted from the stomach, the hypothalamus, and ARC (n = 8–10 rats per diet per day of pregnancy) by Trizol reagent (Cat. 15596-018; Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. The possible genomic DNA in total RNAs was digested with RNA free DNase I (Cat. 79254; Qiagen Inc., Valencia, CA), followed by clean-up procedures using a Qiagen RNeasy Minikit (Cat. 74104; Qiagen). In all these procedures the manufacturer's instructions were followed. Complementary DNA (cDNA) was synthesized from 1 μg of total RNA by reverse transcription in a total volume of 20 μL by using a MyCycler Thermal Cycler (Cat. 170-9703, Bio-Rad Laboratories, Hercules, CA) under the following conditions: One cycle at 28°C for 15 min, 42°C for 50 min, and 95°C for 5 min.
Quantitative real-time PCR
Real-time PCR detection was performed on a CFX96 Real Time PCR Detection System (Cat. 184-5096; Bio-Rad). Primers were designed using Primer 3 Version 4 and are shown in Table1. Syber Green Supermix (Cat. 170-8882; Bio-Rad) was used for amplification of Ghrl, Mboat4, and Bche (ghrelin production-related genes) in the stomach, Npy and Agrp (marker genes for appetite stimulation), Pomc and Cart (marker genes for appetite inhibition), and Gshr, Ucp2, Fasn, Cpt1a, and Cpt1c (ghrelin signaling-related genes) in the hypothalamus, and Npy and Agrp, Pomc and Cart in the ARC region of the hypothalamus. Actb served as internal control to normalize target gene expressions in these organ/tissues. The reaction mixture was incubated at 95°C for 10 min and cycled according to the following parameters: 95°C for 30 sec and 60°C for 1 min for 40 cycles. Amplification of a single product was confirmed by melting curve analysis. A negative control without cDNA was performed to test primer specificity. The relative gene expression was calculated by use of the threshold cycle (CT) Actb/CT target gene.
Table 1.
Quantitative real-time PCR primers.
| Gene | Forward primer (5′→3′) | Reverse primer (5′→3′) | GenBank accession No. | Product size (bp) |
|---|---|---|---|---|
| Ghrl | AGCCCAGCAGAGAAAGGAAT | GTGGCTGCAGTTTAGCTGGT | NM_021669.2 | 50 |
| Mboat4 | CCAGGAGCAGGAGTTCTTTG | AGTGCAGGGAAAAAGAGCAA | NM_001107317.2 | 79 |
| Bche | AGTGGGCGTTAACAAGGATG | AAACCAGGAGCACCGTACAC | NM_022942.1 | 57 |
| Npy | TACTCCGCTCTGCGACACTA | TGTCTCAGGGCTGGATCTCT | NM_012614.2 | 72 |
| Agrp | AAGCTTTGGCAGAGGTGCTA | GACTCGTGCAGCCTTACACA | NM_033650.1 | 76 |
| Cartpt | CCCTACTGCTGCTGCTACCT | CACGGCAGAGTAGATGTCCA | NM_017110.1 | 89 |
| Pomc | GCTTCATGACCTCCGAGAAG | TCTTGATGATGGCGTTCTTG | NM_139326.2 | 66 |
| Ghsr | TCCACGTGGGAAGATACCTC | CAGGTTGCAGTACTGGCTGA | NM_032075.3 | 83 |
| Fasn | GGCATCATTGGGCACTCCTT | GCTGCAAGCACAGCCTCTCT | NM_017332.1 | 83 |
| Cpt1a | CAGCTCGCACATTACAAGGA | TGCACAAAGTTGCAGGACTC | NM_031559.2 | 124 |
| Cpt1c | CACACCTGTTCGATGTCCAC | TTGATTGCTTGCTGGAGATG | NM_001034925.2 | 144 |
ELISA on plasma total and active ghrelin
Total ghrelin in plasma from pregnant rats was measured by Enzyme-Linked Immuno Sorbent Assay (ELISA) kit (Cat. EZRGRT-91K; Millipore, Billerica MA), according to the manufacturer's instructions. Per manufacturer, the lowest detectable level of total ghrelin is 0.04 ng/mL; the specificity for active and des-octanoyl ghrelin is 85% and 100%, respectively; the intraassay CV is 2.0–3.2%; the interassay CV is 1.1–1.7%.
Active ghrelin in plasma from pregnant rats was measured by ELISA kit (Cat. EZRGRA-90K; Millipore), according to the manufacturer's instructions. Per manufacturer, the lowest detectable level of rat active ghrelin is 8 pg/mL; the specificity for active ghrelin is 100%; the intraassay CV is 1.0–6.0%; the interassay CV is 1.0–5.0%.
ELISA on plasma growth hormone
Growth hormone in plasma from pregnant rats was measured by ELISA kit (Cat. KRC5311, Life Technologies, Grand Island, NY), according to the manufacturer's instructions. Per manufacturer, the lowest detectable level of rat growth hormone is 0.5 ng/mL; the specificity for growth hormone is 100%; the intraassay CV is 3.3–6.1%; the interassay CV is 3.1–6.0%.
ELISA on plasma leptin
Leptin in plasma from pregnant rats was measured by ELISA kit (Cat. EZRL-83K; Millipore, Billerica MA), according to the manufacturer's instructions. In this assay, the lowest detectable level of rat leptin is 0.08 ng/mL; the specificity for leptin is 100%; the intraassay CV is 1.9–2.5%; the interassay CV is 3.0–3.9%.
Statistical analysis
All quantitative data were subjected to least-squares analysis of variance (ANOVA) using the general linear models procedures of the Statistical Analysis System (Version 9.3, SAS Institute, Cary, NC). Daily diet intake was presented as the ratio of diet intake (gram) to the body weight (gram) measured at the time of diet given to rats every morning. Daily gain in body weight was presented as the difference in body weight between a given day and 1 day before. Accumulative gain in body weight was presented as the difference in body weight between a given day and Day 3 of pregnancy. Data on daily diet intake, daily gain in body weight, accumulative gain in body weight, gene expression, and plasma hormone levels were analyzed for effects of day of pregnancy, diet treatment, and their interaction. Log transformation of variables was performed when the variance of data were not homogenous among treatment groups, as assessed by the Levene's test. A P-value ≤ 0.05 was considered significant; a P-value > 0.05 and ≤0.10 was considered a trend toward significance. Data were presented as least-squares means (LSMs) with overall standard errors (SE).
Results
Daily diet intake
During Days 4–11 and 13–14 of pregnancy, the ratio of daily diet intake to body weight was 4.3–29.8% higher in the LP group than in the CT group (P < 0.05). In contrast, during Days 17–20 of gestation, the ratio of daily diet intake to body weight was 18.2–48.9% lower in the LP group than in the CT group ((P < 0.05; Fig.1).
Figure 1.
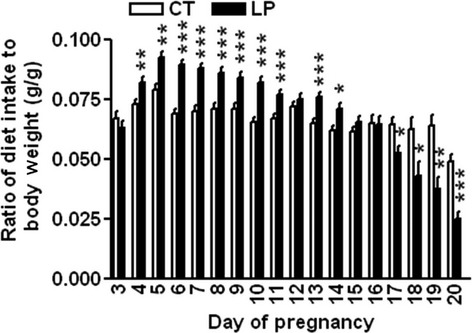
Daily diet intake. CT: control; LP, low-protein diet. The bar represents the mean ± SEM expressed as the ratio of diet intake (gram) to body weight (gram) of pregnant rats on each day of pregnancy (n = 8–10). *P < 0.05; **P < 0.01; ***P < 0.001.
Maternal body weight changes
Daily gain in body weight was similar between LP and CT groups during gestational Days 3–12, and lower (P < 0.05) in the LP group on Days 13, 15, 17–20. On Days 17–20 of gestation, the gain in body weight in LP rats was (P < 0.001) reduced to 53.1%, 31.5%, 9.7%, and 0.7% of that in CT rats, respectively (Fig.2A). The cumulative gain in body weight was unchanged in LP rats on Day 10, but was reduced to 88.7% (P < 0.05), 80.0% (P < 0.01), and 55.4% (P < 0.001) of that in CT rats on Days 14, 18, and 21 of gestation, respectively (Fig.2B).
Figure 2.
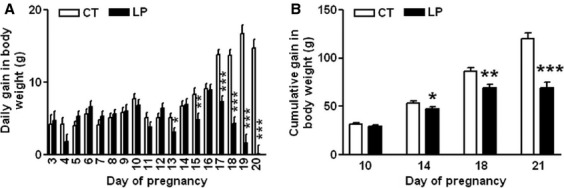
Daily gain in body weight during Day 3–20 of gestation (A) and cumulative gain in body weight on Days 10, 14, 18, and 21 of gestation (B). CT: control; LP, low-protein diet. The bar represents the mean ± SEM (n = 8–10). *P < 0.05; **P < 0.01; ***P < 0.001.
Expression of ghrelin production and activation-related genes in the stomach
The mRNA levels of Ghrl in the stomach were unchanged in LP rats compared to CT rats on Day 10 of gestation, but were increased by 1.55-fold (P < 0.01), 1.38-fold (P < 0.05), and 1.55-fold (P < 0.001) in LP rats on Days 14, 18, and 21 of gestation, respectively (Fig.3A). The mRNA levels of Mboat4 in the stomach were also increased by 1.53-fold (P < 0.05), 2.36-fold (P < 0.05), 2.48-fold (P < 0.05), and 2.40-fold (P < 0.01) in LP rats on Days 10, 14, 18, and 21, respectively (Fig.3B). However, the mRNA levels of Bche in the stomach were unchanged in LP rats compared to CT rats on Days 10, 14, 18, and 21 of gestation (Fig.3C).
Figure 3.
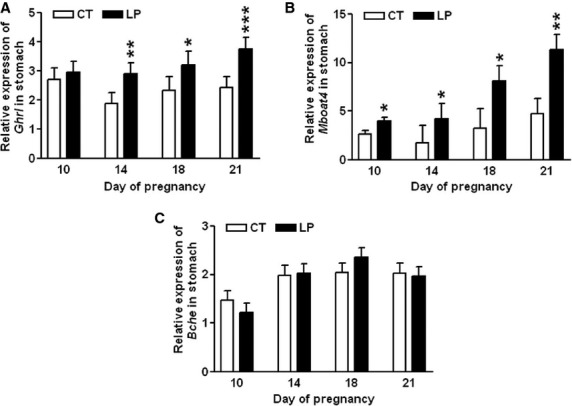
Quantitative real-time PCR analysis of Ghrl (A), Mboat4 (B), and Bche (C) in the stomach on Days 10, 14, 18, and 21 of gestation. CT: control; LP, low-protein diet. The bar represents the mean ± SEM expressed as relative units of mRNA standardized against Actb (n = 8–10). *P < 0.05; **P < 0.01; ***P < 0.001.
Appetite regulation marker and ghrelin signaling gene expressions in the hypothalamus
Expression of Npy, Agrp, and Pomc in the hypothalamus, was unaltered in LP rats on Day 10 of gestation compared to that in CT rats, whereas expression of Cart was increased (P < 0.05) in LP rats. On Day 21, mRNA expression of Npy, Agrp, Pomc, and Cart was not affected in LP rats (Figs.4A–D).
Figure 4.
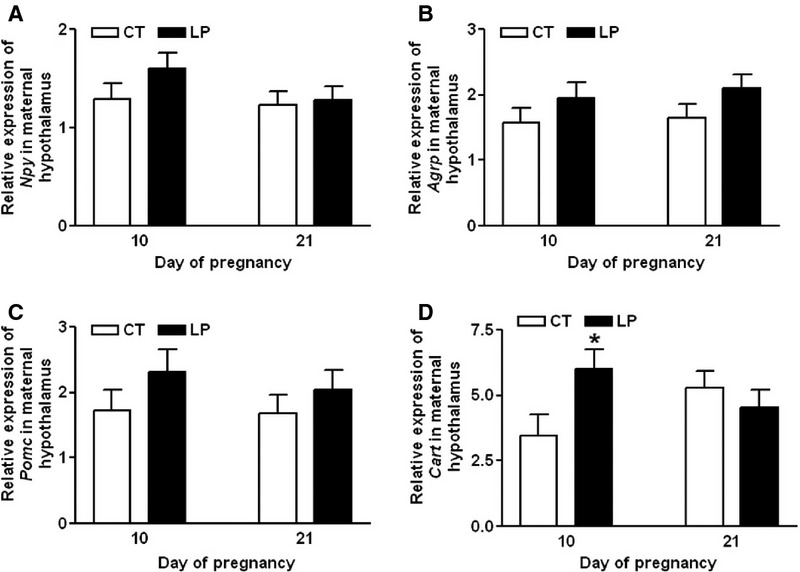
Quantitative real-time PCR analysis of Npy (A), Agrp (B), Pomc (C), and Cart (D) in the hypothalamus on Days 10 and 21 of gestation. CT: control; LP, low-protein diet. The bar represents the mean ± SEM expressed as relative units of mRNA standardized against Actb (n = 8–10). *P < 0.05.
Expression of Ghsr (Fig.5A), Fasn (Fig.5B), Cpt1a (Fig.5C), and Cpt1c (Fig.5D) in the hypothalamus, was unaltered in LP rats on Days 10 and 21 of gestation compared to that in CT rats. Expression of Fasn in both CT and LP groups was reduced by 1.65-fold (P < 0.01) and 1.32-fold (P < 0.05), respectively, on Day 21 compared to Day 10 of gestation. Expression of Ucp2 (Fig.5E) in LP group was decreased (P < 0.05) on Day 10 and increased (P < 0.01) on Day 21 of gestation; in CT rats, expression of Ucp2 was decreased (P < 0.05) by 1.92-fold on Day 21 compared to that on Day 10.
Figure 5.
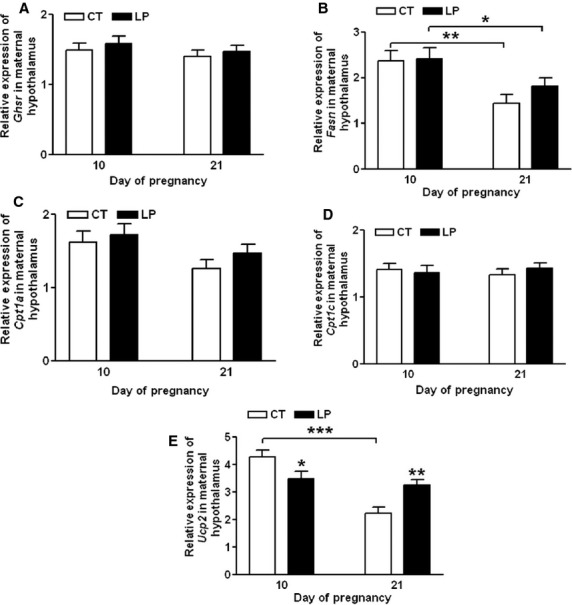
Quantitative real-time PCR analysis of Ghsr (A), Fasn (B), Cpt1a (C), Cpt1c (D), and Ucp2 (E) in the hypothalamus on Days 10 and 21 of gestation. CT: control; LP, low-protein diet. The bar represents the mean ± SEM expressed as relative units of mRNA standardized against Actb (n = 8–10). *P < 0.05; **P < 0.01; ***P < 0.001.
Appetite regulation marker gene expressions in the ARC region of the hypothalamus
Expression of Npy, Agrp, Pomc, and Cart in the ARC region of the hypothalamus was unaltered in LP rats compared to that in CT rats on Day 19 of gestation, whereas expression of Agrp was increased (P < 0.05) and Cart was reduced (P < 0.05) in LP rats on Day 22 (Fig.6A–D).
Figure 6.
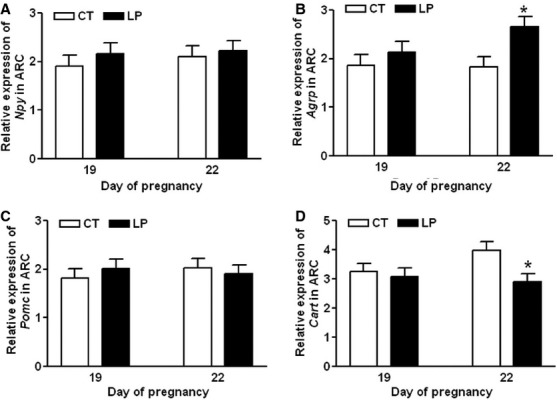
Quantitative real-time PCR analysis of Npy (A), Agrp (B), Pomc (C), and Cart (D) in arcuate nucleus region of the hypothalamus on Days 19 and 22 of gestation. CT: control; LP, low-protein diet; ARC, arcuate nucleus. The bar represents the mean ± SEM expressed as relative units of mRNA standardized against Actb (n = 8–10). *P < 0.05.
Plasma levels of total and active ghrelin
Total ghrelin levels in maternal plasma were similar in LP and CT rats on Day 10 of gestation, but were elevated (P < 0.001) by 2.25-fold, 3.33-fold, and 2.74-fold in LP rats on Days 14, 18, and 21 of gestation, respectively (Fig.7A). The active ghrelin levels in maternal plasma were similar in LP and CT rats on Day 10 and 21, but were elevated (P < 0.01) by 1.73-fold, and 1.96-fold i n LP rats compared to CT rats on Days 14 and 18, respectively (Fig.7B).
Figure 7.
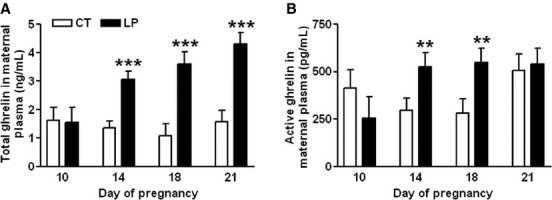
ELISA assays on plasma total (A) and active (B) ghrelin on Days 10, 14, 18, and 21 of gestation. CT: control; LP, low-protein diet; the error bar represents the mean ± SEM (n = 8–10). **P < 0.01; ***P < 0.001.
Plasma levels of growth hormone
Besides regulating the food intake, ghrelin signaling in the hypothalamus has been shown to alter the release of growth hormone from the pituitary (Sun et al. 2004; Kojima and Kangawa 2005). Because the majority of circulating growth hormone in pregnant rats is of pituitary origin (Carlsson et al. 1990), we investigated whether growth hormone levels are affected in LP rats. Growth hormone levels in maternal plasma in the LP group compared to CT group were increased (P < 0.05) by 1.76-fold on Day 14, unchanged on Day 18, and slightly decreased (P = 0.08) on Day 21 of gestation (Fig.8).
Figure 8.
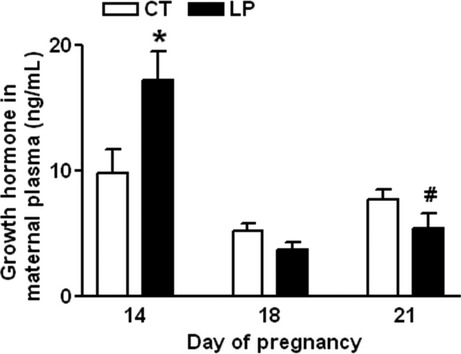
ELISA assay on plasma growth hormone on Days 14, 18, and 21 of gestation. CT: control; LP, low-protein diet. The bar represents the mean ± SEM (n = 8–10). *P < 0.05; #P = 0.08.
Plasma levels of leptin
Lepin is a potent anorexigenic factor, thus it can offset the stimulatory effect of ghrelin in regulating food intake (Tena-Sempere 2007). We investigated whether changes in plasma leptin levels contribute to the reduced diet intake in LP rats in late pregnancy. Plasma leptin levels were similar in LP and CT rats on Days 14 and 19, but decreased (P < 0.001) by threefold in the LP group compared to the CT group on Day 22 of gestation (Fig.9).
Figure 9.
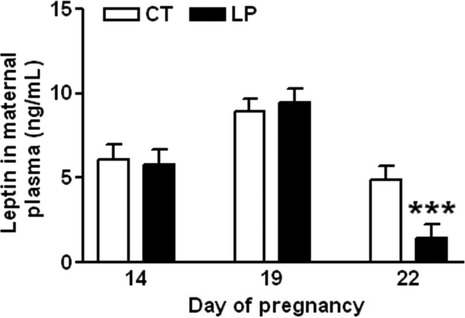
ELISA assay on plasma leptin on Days 14, 19, and 22 of gestation. CT: control; LP, low-protein diet. The bar represents the mean ± SEM (n = 8–10). ***P < 0.001.
Discussion
This study reports, for the first time, that a low-protein diet affects the diet consumption in pregnant rats. However, the variations in diet intake during pregnancy in the LP group are not associated with changes in the only known peripheral appetite stimulatory hormone, ghrelin. In particular, the elevated plasma ghrelin levels in LP rats were not associated with increased diet intake in late pregnancy, and therefore protein insufficiency in LP rats was exacerbated in late pregnancy. Thus, perhaps it is the enhanced catabolism of nutrient reservoir in LP pregnant dams, demonstrated by reduced body weight gain in this study (Fig.2B), which contributes largely to nutritional homeostasis in late pregnancy in both pregnant dams (Gao et al. 2012a) and fetuses (Rees et al. 1999). Findings in this study provide new information on the diet intake pattern in pregnant rats fed a low-protein diet and that diet intake is variable throughout gestation.
The diet intake in LP rats was biphasic, with increased intake during early pregnancy and reduced intake during mid- and late pregnancy (Fig.1). This differs from a previous report (Desai et al. 1996), in which similar diet consumptions were observed in both CT and LP groups. The discrepancy may be due to the differences in measurement methods; in the previous report (Desai et al. 1996) diet intake was expressed as cumulative weekly intake, whereas we measured daily intake, and thus, our study describes the dynamic changes in diet intake and body weight gain in pregnancy more rigorously in a species with relatively short gestation period.
Protein metabolism in the normal rat pregnancy includes anabolic and catabolic phases (Naismith and Morgan 1976), with Day 14 of pregnancy being the demarcation between these two phases (Naismith 1969). In the first 2 weeks of pregnancy, the ‘anabolic phase’, pregnant dams build up a reserve of nutrients, which are mobilized in the catabolic phase, to support the rapid growth of the feto-placental unit in late pregnancy (Naismith and Morgan 1976). In our study, the diet intake in LP rats was higher than in the control group during Days 3–12. The protein content is 20% in the CT diet and 6% in the LP diet. The increased diet intake in LP rats may partly compensate for the reduced amount of protein in the diet, but cannot make up for the significantly lower levels of protein in the LP diet. Thus, protein insufficiency could be a major factor in this rat model fed a low-protein diet in early and mid-pregnancy. In late pregnancy, the diet intake in LP rats was only 18.2–48.9% of that in CT rats during Day 17–20 of gestation, and thus the insufficiencies in both protein and energy could be exacerbated in LP rats. As a consequence, the gain in body weight was markedly reduced in late pregnancy (Fig.2B), and more of the nutrients accumulated during early pregnancy may be mobilized during late pregnancy. More importantly, the reduced diet intake in late pregnancy in LP rats results in decreases in other components of the diet. Among those diet components, taurine (Scabora et al. 2015) and folic acid (Torrens et al. 2006) supplemented to LP dams have been shown to rescue the adult offspring from developing hypertension. Added sucrose to the diet has been shown to influence the food intake, however, we suggest that the modest differences in the sucrose content of the LP vs. CT diet (57 vs. 43%), would not fully account for the differences in their diet intake. Thus, our study suggests the possible involvement of energy, macro- and micronutrients besides protein insufficiency in the process of fetal programming in LP rats.
Both total and active ghrelin are produced via complicated posttranslational processing (Kojima and Kangawa 2005), the positive correlation between the enhanced gene expression of Ghrl and Mboat4 in the stomach and elevated plasma ghrelin levels has been reported in both normal-cycling and pregnant rats (Gualillo et al. 2002; Gonzalez et al. 2008; Reimer et al. 2010). In this study, enhanced expression of Ghrl in the stomach in LP rats was found during mid-late pregnancy (Fig.3), thus total ghrelin production in the stomach was also elevated in LP rats in mid-late pregnancy, and may contribute to the increased plasma levels of total ghrelin (Fig.7A). In contrast, the placenta may not significantly contribute to the increased levels of plasma ghrelin, because the expression of Ghrl is low and restricted to the placental labyrinth zone, and primarily occurs during late gestation (Gualillo et al. 2001). Moreover, expression of Ghrl in rat placenta was unchanged in LP rats (data not shown). Similar to total ghrelin, active ghrelin levels in plasma were increased in LP rats during mid-late pregnancy (Fig7B), coincident with the upregulation of Mboat4 expression in the stomach (Fig.3B). Ghrelin-induced appetite stimulation has been shown by many paradigms (Kojima and Kangawa 2005; Naslund and Hellstrom 2007; Sobrino et al. 2014), thus, the elevated plasma levels of both total and active ghrelin may be a response to the insufficiency of both energy and nutrients (Langley-Evans et al. 1999). It is noteworthy that, on Day 21 of pregnancy, active ghrelin levels in the LP rats were similar to those in CT rats (Fig.7B), although the expression of Mboat4 in the stomach was higher in LP rats than in CT rats (Fig.3B). This inconsistency may reflect reduced MBOAT4 activity in LP rats near term, which already experienced a prolonged fasting status, a situation similar to chronic undernutrition (Liu et al. 2008).
This study shows that changes in plasma levels of ghrelin were not associated with diet intake in pregnant rats fed a low-protein diet in either early or late pregnancy. During early pregnancy, when LP rats consumed a relatively larger amount of diet, the formation of active ghrelin (Fig.3B) as well as the expression of ghrelin-regulated genes in the hypothalamus (Fig.5) was not enhanced. This indicates that factors other than ghrelin may regulate appetite in early pregnancy by increasing expression of Cart (Fig.4D) and reducing expression of Ucp2 in the hypothalamus (Fig.5E). It is known that ghrelin acts as a short-term appetite stimulator before diet intake (Kojima and Kangawa 2005), but after diet intake its orexigenic effect is quenched by many other hormones including insulin, glucagon-like protein 1, peptide YY, cholecystokinin, and oxyntomodulin (Naslund and Hellstrom 2007; Suzuki et al. 2011). In contrast, when LP rats consumed much lower amount of diet in late gestation (Fig.1), appetite-related gene expression in the hypothalamus (Figs.5) as well as in the ARC (Fig.6) was not increased, despite elevated total (Fig.7A) and active (Fig.7B) ghrelin levels in plasma. Therefore, some of the steps in ghrelin signaling in the hypothalamus may be blunted in the LP rats. It is noteworthy that on Day 22, the increased expression of Agrp (Fig.6B) and the decreased expression of Cart (Fig.6D) were coincident in the ARC of the LP rats, which were not found in the hypothalamus on Day 21 (Fig.4). The reduced expression of Cart may be due to the prolonged fasting-like status (Li et al. 2002) and together with the increased expression of Agrp, may represent an adaptation to long-term fasting. To explore whether the ghrelin insensitivity in food intake regulation is specific, we investigated the effect of the LP dieton plasma levels of growth hormone in pregnant rats, as the other known physiological function of ghrelin in the central nervous system is to stimulate growth hormone release from pituitary (Kojima et al. 1999) and pituitary is the primary source of circulating growth hormone in rats even during pregnancy (Carlsson et al. 1990). Plasma levels of growth hormone were not elevated on Day 18 and 21 of pregnancy (Fig.8) when plasma ghrelin levels were increased in LP rats (Fig.7). This suggests that not only ghrelin signaling for appetite stimulation, but also the ghrelin-induced growth hormone release may be blunted in LP rats It is noteworthy that although bolus i.v. injection of ghrelin to pregnant rats transiently elevates plasma levels of growth hormone (El-Kasti et al. 2008), the effect of sustained high levels of endogenous ghrelin on growth hormone remains unknown.
The stimulatory effect of ghrelin in diet intake in late pregnancy may be inhibited by other factors in LP rats. In this study, we ruled out interference by leptin on ghrelin-induced diet intake in LP rats. Leptin is a potent anorexigenic hormone, and acts as a regulator of appetite, energy homeostasis and reproduction (Tena-Sempere 2007). Plasma levels of leptin were not associated with the changes in diet intake in LP rats in late pregnancy, and indeed were decreased in LP rats when the diet intake was also substantially lower (Fig.9). Similarly, in a study using 4% protein diet fed pregnant rats, plasma leptin levels were also reduced (Jansson et al. 2006), which may upregulate circulating ghrelin levels (Barazzoni et al. 2003). Expressions of other anorexigenic hormones such as Glp1, Glp2, Pyy, Ccka, and Cckb in the small intestine were all unchanged in LP rats in late pregnancy (data not shown). Moreover, angiotensin II is known to inhibit appetite and ghrelin signaling pathway in the hypothalamus (Yoshida et al. 2012). Thus, significantly elevated angiotensin II levels during late pregnancy in LP rats (Gao et al. 2012b, 2013), could play a role in reducing diet intake. Therefore, the inhibitory effects of angiotensin II may offset the stimulatory effects of ghrelin on diet intake in LP rats.
In this study, we found that in pregnant rats fed a low-protein diet, changes in food intake over the course of pregnancy were not correlated with changes in ghrelin. During early pregnancy the production of total ghrelin and formation of active ghrelin in the stomach are not affected by the LP diet. During mid- and late pregnancy, the production of total ghrelin and formation of active ghrelin in the stomach are elevated by the LP diet. However, despite this, diet intake in LP rats is not increased during late pregnancy and is insufficient to support the rapid fetal growth.
Acknowledgments
The authors thank Dr. Guangchen Ji, Department of Neuroscience and Cell Biology, the University of Texas Medical Branch at Galveston for technical support for dissecting hypothalamus and arcuate nucleus region. The authors thank Mrs. Sandra Garcia Dale for editorial work on this manuscript and administrative support.
Conflict of Interest
The authors have no conflicts of interest.
References
- Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41:457–462. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, et al. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelidis G, Dafopoulos K, Messini CI, Valotassiou V, Georgoulias P. Messinis IE. Ghrelin: new insights into female reproductive system-associated disorders and pregnancy. Reprod. Sci. 2012;19:903–910. doi: 10.1177/1933719112443880. [DOI] [PubMed] [Google Scholar]
- Barazzoni R, Zanetti M, Stebel M, Biolo G, Cattin L. Guarnieri G. Hyperleptinemia prevents increased plasma ghrelin concentration during short-term moderate caloric restriction in rats. Gastroenterology. 2003;124:1188–1192. doi: 10.1016/s0016-5085(03)00281-6. [DOI] [PubMed] [Google Scholar]
- Carlsson L, Eden S. Jansson JO. The plasma pattern of growth hormone in conscious rats during late pregnancy. J. Endocrinol. 1990;124:191–198. doi: 10.1677/joe.0.1240191. [DOI] [PubMed] [Google Scholar]
- Connors N, Valego NK, Carey LC, Figueroa JP. Rose JC. Fetal and postnatal renin secretion in female sheep exposed to prenatal betamethasone. Reprod. Sci. 2010;17:239–246. doi: 10.1177/1933719109351752. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Frayo RS, Marmonier C, Aubert R. Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am. J. Physiol. Endocrinol. Metab. 2004;287:E297–E304. doi: 10.1152/ajpendo.00582.2003. [DOI] [PubMed] [Google Scholar]
- De VC, Gregoire F, Lema-Kisoka R, Waelbroeck M, Robberecht P. Delporte C. Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology. 2004;145:4997–5005. doi: 10.1210/en.2004-0569. [DOI] [PubMed] [Google Scholar]
- Desai M, Crowther NJ, Lucas A. Hales CN. Organ-selective growth in the offspring of protein-restricted mothers. Br. J. Nutr. 1996;76:591–603. doi: 10.1079/bjn19960065. [DOI] [PubMed] [Google Scholar]
- Dodic M, Baird R, Hantzis V, Koukoulas I, Moritz K, Peers A, et al. Organs/systems potentially involved in one model of programmed hypertension in sheep. Clin. Exp. Pharmacol. Physiol. 2001;28:952–956. doi: 10.1046/j.1440-1681.2001.03556.x. [DOI] [PubMed] [Google Scholar]
- El-Kasti MM, Christian HC, Huerta-Ocampo I, Stolbrink M, Gill S, Houston PA, et al. The pregnancy-induced increase in baseline circulating growth hormone in rats is not induced by ghrelin. J. Neuroendocrinol. 2008;20:309–322. doi: 10.1111/j.1365-2826.2008.01650.x. [DOI] [PubMed] [Google Scholar]
- Erdmann J, Lippl F. Schusdziarra V. Differential effect of protein and fat on plasma ghrelin levels in man. Regul. Pept. 2003;116:101–107. doi: 10.1016/s0167-0115(03)00195-2. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez R, Martini AC, Navarro VM, Castellano JM, Dieguez C, Aguilar E, et al. Novel signals for the integration of energy balance and reproduction. Mol. Cell. Endocrinol. 2006;254–255:127–132. doi: 10.1016/j.mce.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Ozanne SE, Ekizoglou S, Doherty C, James L, Gusterson B, et al. The maternal endocrine environment in the low-protein model of intra-uterine growth restriction. Br. J. Nutr. 2003;90:815–822. doi: 10.1079/bjn2003967. [DOI] [PubMed] [Google Scholar]
- Forhead AJ. Fowden AL. Role of angiotensin II in the pressor response to cortisol in fetal sheep during late gestation. Exp. Physiol. 2004;89:323–329. doi: 10.1113/expphysiol.2004.027185. [DOI] [PubMed] [Google Scholar]
- Funahashi H, Takenoya F, Guan JL, Kageyama H, Yada T. Shioda S. Hypothalamic neuronal networks and feeding-related peptides involved in the regulation of feeding. Anat. Sci. Int. 2003;78:123–138. doi: 10.1046/j.0022-7722.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- Gangula PR, Reed L. Yallampalli C. Antihypertensive effects of flutamide in rats that are exposed to a low-protein diet in utero. Am. J. Obstet. Gynecol. 2005;192:952–960. doi: 10.1016/j.ajog.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Gao H, Sathishkumar KR, Yallampalli U, Balakrishnan M, Li X, Wu G, et al. Maternal protein restriction regulates IGF2 system in placental labyrinth. Front Biosci. 2012a;4:1434–1450. doi: 10.2741/472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Yallampalli U. Yallampalli C. Protein restriction to pregnant rats increases the plasma levels of angiotensin II and expression of angiotensin II receptors in uterine arteries. Biol. Reprod. 2012b;86:68. doi: 10.1095/biolreprod.111.095844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Yallampalli U. Yallampalli C. Gestational protein restriction increases angiotensin II production in rat lung. Biol. Reprod. 2013;88:64. doi: 10.1095/biolreprod.112.103770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez CR, Vazquez MJ, Lopez M. Dieguez C. Influence of chronic undernutrition and leptin on GOAT mRNA levels in rat stomach mucosa. J. Mol. Endocrinol. 2008;41:415–421. doi: 10.1677/JME-08-0102. [DOI] [PubMed] [Google Scholar]
- Goyal R, Galffy A, Field SA, Gheorghe CP, Mittal A. Longo LD. Maternal protein deprivation: changes in systemic renin-angiotensin system of the mouse fetus. Reprod. Sci. 2009;16:894–904. doi: 10.1177/1933719109337260. [DOI] [PubMed] [Google Scholar]
- Goyal R, Goyal D, Leitzke A, Gheorghe CP. Longo LD. Brain renin-angiotensin system: fetal epigenetic programming by maternal protein restriction during pregnancy. Reprod. Sci. 2010;17:227–238. doi: 10.1177/1933719109351935. [DOI] [PubMed] [Google Scholar]
- Gualillo O, Caminos J, Blanco M, Garcia-Caballero T, Kojima M, Kangawa K, et al. Ghrelin, a novel placental-derived hormone. Endocrinology. 2001;142:788–794. doi: 10.1210/endo.142.2.7987. [DOI] [PubMed] [Google Scholar]
- Gualillo O, Caminos JE, Nogueiras R, Seoane LM, Arvat E, Ghigo E, et al. Effect of food restriction on ghrelin in normal-cycling female rats and in pregnancy. Obes. Res. 2002;10:682–687. doi: 10.1038/oby.2002.92. [DOI] [PubMed] [Google Scholar]
- Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc. Natl Acad. Sci. USA. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner TG. Seiden LS. Synthesis of catecholamines from [3H]tyrosine in brain during the performance of operant behavior. Brain Res. 1980;183:403–419. doi: 10.1016/0006-8993(80)90475-8. [DOI] [PubMed] [Google Scholar]
- Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, et al. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J. Physiol. 2006;576(Pt 3):935–946. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautzky-Willer A. Handisurya A. Metabolic diseases and associated complications: sex and gender matter! Eur. J. Clin. Invest. 2009;39:631–648. doi: 10.1111/j.1365-2362.2009.02161.x. [DOI] [PubMed] [Google Scholar]
- Kojima M. Kangawa K. Ghrelin: structure and function. Physiol. Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- Kojima M. Kangawa K. Ghrelin: from gene to physiological function. Results Probl. Cell Differ. 2010;50:185–205. doi: 10.1007/400_2009_28. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H. Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Ladyman SR, Augustine RA. Grattan DR. Hormone interactions regulating energy balance during pregnancy. J. Neuroendocrinol. 2010;22:805–817. doi: 10.1111/j.1365-2826.2010.02017.x. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Gardner DS. Jackson AA. Maternal protein restriction influences the programming of the rat hypothalamic-pituitary-adrenal axis. J. Nutr. 1996;126:1578–1585. doi: 10.1093/jn/126.6.1578. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Welham SJ. Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 1999;64:965–974. doi: 10.1016/s0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- Li HY, Hwang HW. Hu YH. Functional characterizations of cocaine- and amphetamine-regulated transcript mRNA expression in rat hypothalamus. Neurosci. Lett. 2002;323:203–206. doi: 10.1016/s0304-3940(02)00151-9. [DOI] [PubMed] [Google Scholar]
- Liu J, Prudom CE, Nass R, Pezzoli SS, Oliveri MC, Johnson ML, et al. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J. Clin. Endocrinol. Metab. 2008;93:1980–1987. doi: 10.1210/jc.2007-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen S. Langley-Evans SC. Maternal low-protein diet in rat pregnancy programs blood pressure through sex-specific mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005a;288:R85–R90. doi: 10.1152/ajpregu.00435.2004. [DOI] [PubMed] [Google Scholar]
- McMullen S. Langley-Evans SC. Sex-specific effects of prenatal low-protein and carbenoxolone exposure on renal angiotensin receptor expression in rats. Hypertension. 2005b;46:1374–1380. doi: 10.1161/01.HYP.0000188702.96256.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith DJ. The foetus as a parasite. Proc. Nutr. Soc. 1969;28:25–31. [PubMed] [Google Scholar]
- Naismith DJ. Morgan BL. The biphasic nature of protein metabolism during pregnancy in the rat. Br. J. Nutr. 1976;36:563–566. doi: 10.1079/bjn19760109. [DOI] [PubMed] [Google Scholar]
- Naslund E. Hellstrom PM. Appetite signaling: from gut peptides and enteric nerves to brain. Physiol. Behav. 2007;92:256–262. doi: 10.1016/j.physbeh.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Paxinos G. Watson C. The rat brain in stereotaxic coordinates. Fourth ed. San Diego: Academic Press; 1998. [Google Scholar]
- Rees WD, Hay SM, Buchan V, Antipatis C. Palmer RM. The effects of maternal protein restriction on the growth of the rat fetus and its amino acid supply. Br. J. Nutr. 1999;81:243–250. [PubMed] [Google Scholar]
- Reimer RA, Maurer AD, Lau DC. Auer RN. Long-term dietary restriction influences plasma ghrelin and GOAT mRNA level in rats. Physiol. Behav. 2010;99:605–610. doi: 10.1016/j.physbeh.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar K, Balakrishnan M, Chinnathambi V, Gao H. Yallampalli C. Temporal alterations in vascular angiotensin receptors and vasomotor responses in offspring of protein-restricted rat dams. Am. J. Obstet. Gynecol. 2012;206:507–510. doi: 10.1016/j.ajog.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scabora JE, de Lima MC, Lopes A, de Lima IP, Mesquita FF, Braz TD, et al. Impact of taurine supplementation on blood pressure in gestational protein-restricted offspring: effect on the medial solitary tract nucleus cell numbers, angiotensin receptors, and renal sodium handling. J. Renin. Angiotensin. Aldosterone. Syst. 2015;16:47–58. doi: 10.1177/1470320313481255. [DOI] [PubMed] [Google Scholar]
- Sobrino CC, Perianes CA, Puebla JL, Barrios V. Arilla FE. Peptides and Food Intake. Front. Endocrinol. 2014;5:58. doi: 10.3389/fendo.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wang P, Zheng H. Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc. Natl Acad. Sci. USA. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Jayasena CN. Bloom SR. The gut hormones in appetite regulation. J. Obes. 2011;2011:528401. doi: 10.1155/2011/528401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tena-Sempere M. Roles of ghrelin and leptin in the control of reproductive function. Neuroendocrinology. 2007;86:229–241. doi: 10.1159/000108410. [DOI] [PubMed] [Google Scholar]
- Torrens C, Brawley L, Anthony FW, Dance CS, Dunn R, Jackson AA, et al. Folate supplementation during pregnancy improves offspring cardiovascular dysfunction induced by protein restriction. Hypertension. 2006;47:982–987. doi: 10.1161/01.HYP.0000215580.43711.d1. [DOI] [PubMed] [Google Scholar]
- Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, et al. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem. Biophys. Res. Commun. 2001;281:1220–1225. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- Vehaskari VM, Aviles DH. Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int. 2001;59:238–245. doi: 10.1046/j.1523-1755.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Brown MS, Liang G, Grishin NV. Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Semprun-Prieto L, Wainford RD, Sukhanov S, Kapusta DR. Delafontaine P. Angiotensin II reduces food intake by altering orexigenic neuropeptide expression in the mouse hypothalamus. Endocrinology. 2012;153:1411–1420. doi: 10.1210/en.2011-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]


