Abstract
Diacylglycerol kinase (DGK) isoforms regulate signal transduction and lipid metabolism. DGKδ deficiency leads to hyperglycemia, peripheral insulin resistance, and metabolic inflexibility. Thus, dysregulation of other DGK isoforms may play a role in metabolic dysfunction. We investigated DGK isoform mRNA expression in extensor digitorum longus (EDL) and soleus muscle, liver as well as subcutaneous and epididymal adipose tissue in C57BL/6J mice and obese and insulin-resistant ob/ob mice. All DGK isoforms, except for DGKκ, were detectable, although with varying mRNA expression. Liver DGK expression was generally lowest, with several isoforms undetectable. In soleus muscle, subcutaneous and epididymal adipose tissue, DGKδ was the most abundant isoform. In EDL muscle, DGKα and DGKζ were the most abundant isoforms. In liver, DGKζ was the most abundant isoform. Comparing obese insulin-resistant ob/ob mice to lean C57BL/6J mice, DGKβ, DGKι, and DGKθ were increased and DGKε expression was decreased in EDL muscle, while DGKβ, DGKη and DGKθ were decreased and DGKδ and DGKι were increased in soleus muscle. In liver, DGKδ and DGKζ expression was increased in ob/ob mice. DGKη was increased in subcutaneous fat, while DGKζ was increased and DGKβ, DGKδ, DGKη and DGKε were decreased in epididymal fat from ob/ob mice. In both adipose tissue depots, DGKα and DGKγ were decreased and DGKι was increased in ob/ob mice. In conclusion, DGK mRNA expression is altered in an isoform- and tissue-dependent manner in obese insulin-resistant ob/ob mice. DGK isoforms likely have divergent functional roles in distinct tissues, which may contribute to metabolic dysfunction.
Keywords: Adipose tissue, liver, skeletal muscle
Introduction
Increased accumulation of lipid intermediates, such as triglycerides, diacylglycerol (DAG), ceramides, and long-chain fatty acid coenzyme A, contribute to the development of insulin resistance (Erion and Shulman 2010; Samuel and Shulman 2012; Zhang et al. 2013). Ectopic accumulation of specific lipid metabolites (diacylglycerols and/or ceramides) in liver and skeletal muscle may be caused by increased fatty acid delivery/synthesis when the adipose tissue storage capacity is exceeded and/or decreased mitochondrial fatty acid oxidation (Erion and Shulman 2010; Zhang et al. 2013). Increased intramuscular DAG activates a serine/threonine kinase signaling pathway, including protein kinase C (PKC), which results in serine phosphorylation of insulin receptor substrate (IRS) -1, inhibition of IRS-1 tyrosine phosphorylation, and reduced insulin-stimulated glucose uptake and metabolism (Samuel and Shulman 2012). In liver, increased DAG content reduces insulin-stimulated glycogen synthesis and decreases the suppression gluconeogenesis (Samuel and Shulman 2012). Thus, altered activity and/or abundance of enzymes involved in lipid metabolism influences the accumulation of different lipid intermediates and impinges upon intracellular signaling events.
Diacylglycerol kinases (DGKs) control the level of two important lipid messengers: DAG and phosphatidic acid (PA). DGKs catalyze a reaction that removes DAG, by converting this lipid to PA at the plasma membrane, endoplasmic reticulum and nucleus, and thereby terminates DAG-derived signals (Shulga et al. 2011). Consequently, DGK isoform expression may be altered in obesity and type 2 diabetes. Ten mammalian DGK isoforms have been classified into five subgroups based on different regulatory domains in their primary structure, although all isoforms have a catalytic domain and at least two C1 domains (Shulga et al. 2011; Sakai and Sakane 2012). DGK isoforms are unique, not only structurally, but also in their expression pattern, subcellular localization, regulatory mechanisms and DAG preferences, suggesting isoform-specific functional roles. While DGK isoform expression has been surveyed in various tissues including in mouse and rat reproductive organs (Toya et al. 2005; Shionoya et al. 2015), immune cells during an inflammatory reaction (Yamamoto et al. 2014), rat retina (Hozumi et al. 2013), lung (Katagiri et al. 2005), and regenerating liver (Nakano et al. 2012), and human failing hearts (Bilim et al. 2011), mRNA expression of DGK isoforms in tissues important for metabolic homeostasis in the context of type 2 diabetes and obesity is unknown.
DGK isoforms regulating wide variety of physiological processes including growth, metabolism, proliferation, immunological and neural development (Topham 2006; Krishna and Zhong 2013; Ishisaka and Hara 2014; Shirai and Saito 2014; Yamamoto et al. 2014). DGKδ expression is decreased in skeletal muscle from type 2 diabetic patients and haploinsufficiency (DGKδ+/−) in mice leads to insulin resistance and late-onset obesity (Chibalin et al. 2008). However, the role of other DGK isoforms in the development of metabolic disorders, including insulin resistance and obesity, is largely unknown. Here, we determined the tissue-specific mRNA expression of DGK isoforms in insulin-sensitive tissues including skeletal muscle, liver and two separate adipose tissue depots (subcutaneous and epididymal). We also explore the effect of severe obesity and insulin resistance on DGK isoform mRNA expression by comparing tissue-specific profiles between obese ob/ob (B6.V-LepOb/J) and lean C57BL/6J mice.
Material and Methods
Animals
Male lean C57BL/6J and obese ob/ob (B6.V-LepOb/J) mice were purchased from Charles River Laboratories (Italy). Animals were maintained in a temperature- and light-controlled environment (12-h light, 12-h dark cycle) and had free access to water and food. At 12 weeks of age, fed mice were anesthetized via intraperitoneal injection of 2.5% Avertin (0.02 mL/g body weight). Skeletal muscles (extensor digitorum longus (EDL) and soleus), adipose tissues (subcutaneous and epididymal) and liver were harvested from lean C57BL/6J (body weight 24.4 ± 0.2 g) and obese ob/ob (body weight 47.7 ± 1.2 g) and clamp-frozen in liquid nitrogen and stored for mRNA analysis. The regional animal ethics committee of Northern Stockholm approved all experimental procedures.
RNA isolation, cDNA synthesis and qPCR
Tissues were homogenized using the TissueLyser (Qiagen, Hilden, Germany). Total RNA in adipose tissue and liver was isolated using RNeasy Lipid Tissue Mini Kit and RNeasy Mini Kit (Qiagen), respectively. A deoxyribonuclease I (QIAGEN) digestion step was included to eliminate DNA contamination. Total RNA in skeletal muscle was isolated using TRIzol reagent (Invitrogen Life Technologies Ltd, Paisley, UK), according to the manufacturer's protocol. RNA concentration and purity were determined with a Nanodrop ND-1000 spectrophotometer (Nano-Drop Technologies, Wilmington, DE, USA) and all samples had a 260/280 and a 260/230 ratio >2.0 indicating pure RNA. RNA integrity was verified with the Experion (Bio-Rad Laboratories, Hercules, CA) and all samples had a RQI value >8 indicating intact RNA. cDNA was synthesized from 1 or 2 μg total RNA in a final reaction volume of 20 μL using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Stockholm, Sweden), according to the manufacturer's protocol.
Real-time qPCR
Real-time qPCR was performed in a StepOnePlus Realtime qPCR System (Applied Biosystems) using Taqman Gene Expression Assays (Table1). Multiplex qPCR was performed in duplicate using 20 ng cDNA mixed with TaqMan Gene Expression Master Mix (Applied Biosystems) in a total volume of 20 μL. Cycling conditions were as follows: 95°C for 10 min, 40 cycles at 95°C for 15 sec and 60°C for 1 min.
Table 1.
Taqman gene expression assays
| Symbol | Name | Taqman Assay ID |
|---|---|---|
| Target genes | ||
| Dgka | Diacylglycerol kinase, alpha (α) | Mm00444048_m1* |
| Dgkb | Diacylglycerol kinase, beta (β) | Mm00618478_m1* |
| Dgkg | Diacylglycerol kinase, gamma (γ) | Mm00446756_m1* |
| Dgkd | Diacylglycerol kinase, delta (δ) | Mm00617404_m1* |
| Dgkh | Diacylglycerol kinase, eta (η) | Mm01312241_m1* |
| Dgkk | Diacylglycerol kinase, kappa (κ) | Mm01340751_m1* |
| Dgke | Diacylglycerol kinase, epsilon (ε) | Mm00444676_m1* |
| Dgkz | Diacylglycerol kinase, zeta (ζ) | Mm00661896_m1* |
| Dgki | Diacylglycerol kinase, iota (ι) | Mm01159464_m1* |
| Dgkq | Diacylglycerol kinase, theta (θ) | Mm01198794_m1* |
| Reference genes | ||
| Rn18S | 18S rRNA | Mm03928990_g1* |
| Actb | Actin, beta | Mm00607939_s1* |
| B2 m | Beta-2-microglobulin | Mm00437762_m1* |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | Mm99999915_g1* |
| Gusb | Beta-glucuronidase | Mm00446953_m1 |
| Hprt1 | Hypoxanthine-guanine phosphoribsyltransferase | Mm00446968_m1* |
| Pgk1 | Phosphoglycerate kinase 1 | Mm00435617_m1* |
| Ppia | Peptidylprolyl isomerase A, cyclophilin A | Mm02342429_g1* |
| Rplp0 | Ribosomal protein, large, P0 | Mm01974474_gH* |
| Tbp | TATA-box-binding protein | Mm00446973_m1* |
*The TaqMan gene expression assay with the best coverage for that specific gene.
Data were analyzed with Step One Software v2.1 (Applied Biosystems). Relative gene expression was calculated with the ΔΔCq method (Livak and Schmittgen 2001) and expressed as fold changes compared with DGKε mRNA in C57BL/6J mice. Ten candidate reference genes (Table1) were validated with NormFinder algorithm incorporated into the GenEx software (MultiD Analyses AB, Gothenburg, Sweden). The relative expression was normalized in EDL against the reference genes Gusb, Hprt1 and Ppia, in soleus against the reference genes 18S, Actb, Hprt1, Ppia, Rplp0 and Tbp, in liver against the reference genes Actb, B2 m, and Ppia, in subcutaneous adipose tissue against the reference genes B2 m, Pgk1, Ppia, and Tbp, and in epididymal adipose tissue against the reference genes Pgk1 and Ppia. Isoforms with a Cq > 35 were considered below the limit of detection.
Statistics
Results are presented as mean ± SEM. Differences in gene expression between C57BL/6J and ob/ob mice were determined by Student's t-test on logarithmic transformed data. Significance was accepted at P < 0.05.
Results
Tissue expression profile in C57BL/6J mice
None of the reference genes were stably expressed across all investigated tissues (Table2). Therefore, data are presented as Cq values (Table3) and normalized against gene expression of each specific DGK isoform in EDL muscle (Fig.1) for the comparison between isoforms in different tissues of C57BL/6J mice.
Table 2.
mRNA expression of reference genes in insulin-sensitive tissues from C57BL/6J mice
| Gene | EDL | Soleus | Liver | Subcutaneous | Epididymal |
|---|---|---|---|---|---|
| Rn18S | NA | 9.66 ± 0.06 | NA | 8.83 ± 0.12 | NA |
| Actb | NA | 23.84 ± 0.07 | 21.95 ± 0.10 | 18.18 ± 0.23 | NA |
| B2m | 24.65 ± 0.12 | 24.61 ± 0.08 | 20.25 ± 0.08 | 19.86 ± 0.14 | NA |
| Gapdh | NA | 19.79 ± 0.05 | NA | 20.56 ± 0.15 | 19.47 ± 0.14 |
| Gusb | 29.36 ± 0.12 | 29.17 ± 0.09 | NA | 26.49 ± 0.12 | NA |
| Hprt1 | 27.58 ± 0.07 | 27.86 ± 0.07 | NA | 25.45 ± 0.12 | 24.93 ± 0.10 |
| Pgk1 | 22.88 ± 0.09 | 25.14 ± 0.08 | 25.09 ± 0.07 | 25.51 ± 0.11 | 26.20 ± 0.11 |
| Ppia | 24.93 ± 0.08 | 25.61 ± 0.06 | 21.44 ± 0.09 | 21.93 ± 0.12 | 20.92 ± 0.11 |
| Rplp0 | 22.35 ± 0.06 | 21.98 ± 0.10 | NA | 20.25 ± 0.20 | NA |
| Tbp | NA | 31.56 ± 0.10 | NA | 30.85 ± 0.15 | NA |
Data are presented as Cq values and expressed as mean ± SEM. NA, not applicable.
Table 3.
DGK isoform mRNA expression in insulin-sensitive tissues from C57BL/6J mice
| Gene | EDL | Soleus | Liver | Subcutaneous | Epididymal |
|---|---|---|---|---|---|
| DGKα | 27.6 ± 0.1 | ND | 30.8 ± 0.1 | 32.3 ± 0.5 | 33.8 ± 0.3 |
| DGKβ | 32.0 ± 0.1 | 32.7 ± 0.2 | ND | 33.8 ± 0.5 | 31.9 ± 0.2 |
| DGKγ | 33.2 ± 0.1 | 32.4 ± 0.1 | ND | 33.2 ± 0.2 | 31.9 ± 0.3 |
| DGKδ | 29.2 ± 0.1 | 26.8 ± 0.1 | 34.4 ± 0.4 | 25.4 ± 0.2 | 24.8 ± 0.1 |
| DGKη | 31.8 ± 0.1 | 31.6 ± 0.1 | 33.7 ± 0.2 | 30.6 ± 0.1 | 29.9 ± 0.3 |
| DGKκ | ND | ND | ND | ND | ND |
| DGKε | 29.7 ± 0.1 | 28.9 ± 0.1 | 33.0 ± 0.1 | 28.5 ± 0.1 | 28.6 ± 0.1 |
| DGKζ | 27.4 ± 0.1 | 27.8 ± 0.1 | 28.5 ± 0.1 | 25.9 ± 0.3 | 28.1 ± 0.1 |
| DGKι | 34.7 ± 0.2 | 32.9 ± 0.1 | ND | 32.7 ± 0.2 | 34.0 ± 0.6 |
| DGKθ | 33.2 ± 0.1 | 32.8 ± 0.1 | 33.5 ± 0.2 | 30.6 ± 0.1 | 32.7 ± 0.1 |
Data are presented as Cq values and expressed as mean ± SEM. ND, not detected.
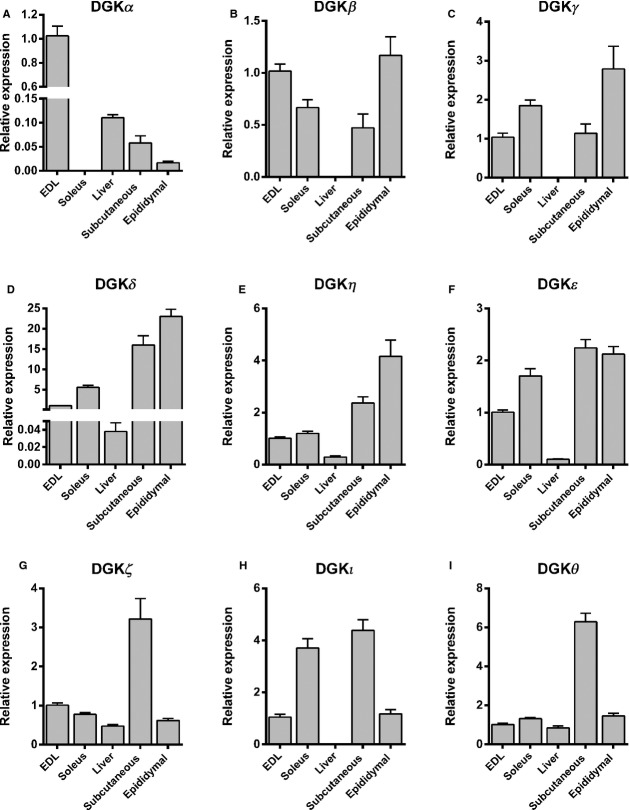
Figure 1.
DGK isoform expression profile. mRNA expression of (A) DGKα, (B) DGKβ, (C) DGKγ, (D) DGKδ, (E) DGKη, (F) DGKε, (G) DGKζ, (H) DGKι and (I) DGKθ in EDL muscle, soleus muscle, liver, subcutaneous adipose tissue, and epididymal adipose tissue from C57BL/6J mice. Data are presented relative to respective mRNA in EDL muscle. Results are mean ± SEM. n = 8–10.
The DGKα gene was highly expressed in EDL muscle, but not expressed in soleus muscle and lowly expressed in liver and adipose tissue (Fig.1A). DGKβ and DGKγ were not expressed in liver, while levels were similar between skeletal muscle and adipose tissues (Fig.1B and C). DGKδ and DGKη were expressed at higher levels in adipose tissues compared to skeletal muscle, with very low levels in liver (Fig.1D and E). A similar expression pattern was observed DGKε (Fig.1F). The expression profile for DGKζ and DGKθ between tissues was similar (Fig.1G and I); however a threefold and sixfold higher expression, respectively, was observed in subcutaneous adipose tissue, and similar levels were observed between skeletal muscle, liver and epididymal adipose tissue. Overall, mRNA expression of DGKζ was higher than DGKθ (Table2). DGKι was 4-fold higher in soleus and subcutaneous adipose tissue compared to EDL and epididymal adipose tissue, while not detected in liver (Fig.1H).
We next compared the relative isoform expression within each tissue. In EDL muscle, DGKα and DGKζ had the highest expression level (Table3). While DGKα was not expressed in soleus muscle, DGKδ was the highest isoform expressed (Table3 and Fig.1). In general the expression level for all DGK isoforms was low in liver compared to other tissues, with DGKβ, DGKγ, DGKκ and DGKι were under the detection limit of the assay (Table3 and Fig.1). The expression level of the DGK isoforms also differed between the two adipose tissue depots analyzed; DGKβ, DGKγ, DGKδ and DGKη were more highly expressed in epididymal adipose tissue as compared to subcutaneous adipose tissue with the inverse noted for DGKα, DGKζ, DGKι and DGKθ (Table3 and Fig.1). DGKδ had the highest expression in both adipose depots (Table3). DGKζ was the only isoform with a Cq < 30 in all investigated tissues (Table3). DGKβ, DGKγ, DGKη, DGKι and DGKθ were expressed at low levels in all tissues (Cq > 30; Table3) and DGKκ was not detected in any of the investigated tissues.
Tissue expression profile in C57BL/6J and ob/ob mice
Several DGK isoforms were differentially expressed between ob/ob and C57BL/6J mice. In EDL muscle, DGKζ was the predominant isoform, followed by DGKα, DGKδ and DGKε (Fig.2A). Moreover, expression of DGKβ, DGKι and DGKθ was increased, whereas DGKε was decreased in EDL muscle from ob/ob compared to C57BL/6J mice (Fig.2A). In soleus muscle, DGKδ was the predominant isoform followed by DGKζ and DGKε (Fig.2B). Expression of DGKδ and DGKι was increased, whereas DGKβ, DGKη, and DGKθ were decreased in soleus of ob/ob compared to C57BL/6J mice (Fig.2B). In liver, DGKζ was the predominant isoform followed by DGKα (Fig.3). Liver DGKδ and DGKζ expression was increased in ob/ob compared to C57BL/6J mice (Fig.3). In subcutaneous adipose tissue, DGKδ was the predominant isoform followed by DGKζ (Fig.4A). The DGKη and DGKι expressions were increased and the DGKα and DGKγ expressions were decreased in subcutaneous adipose tissue of ob/ob compared to C57BL/6J mice (Fig.4A). In epididymal adipose tissue, DGKδ was the predominant isoform followed by DGKζ (Fig.4B). DGKζ and DGKι expression was increased and DGKα, DGKβ, DGKγ, DGKδ, DGKη, and DGKε expression was decreased in epididymal adipose tissue of ob/ob compared to C57BL/6J mice (Fig.4B).
Figure 2.

mRNA expression of DGK isoforms in skeletal muscle from C57BL/6J mice (white bars) and ob/ob mice (black bars). (A) Highly expressed (left panel) and lowly expressed (right panel) DGK isoforms in EDL muscle; (B) highly expressed (left panel) and lowly expressed (right panel) DGK isoforms in soleus muscle; Data are normalized to reference genes (see Material and Methods) and presented relative to DGKε mRNA in tissues from C57BL/6J mice. Results are mean ± SEM. n = 8–10. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 3.
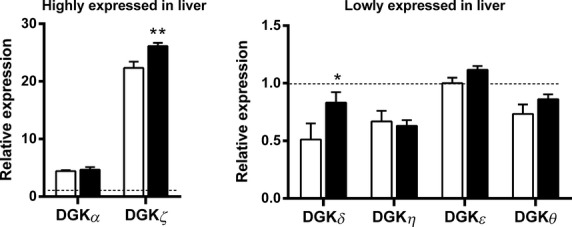
mRNA expression of DGK isoforms in liver from C57BL/6J mice (white bars) and ob/ob mice (black bars). Highly expressed (left panel) and lowly expressed (right panel) DGK isoforms in liver. Data are normalized to reference genes (see Material and Methods) and presented relative to DGKε mRNA in tissues from C57BL/6J mice. Results are mean ± SEM. n = 8–10. *P < 0.05, **P < 0.01.
Figure 4.
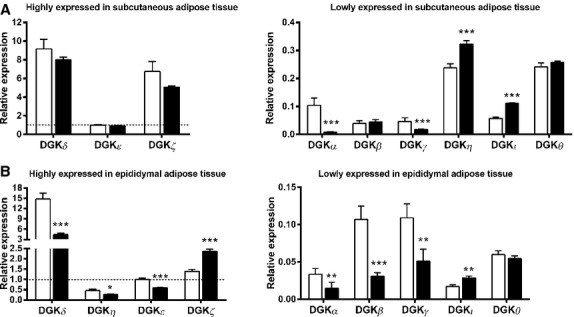
mRNA expression of DGK isoforms in subcutaneous and epididymal adipose tissue from C57BL/6J mice (white bars) and ob/ob mice (black bars). (A) Highly expressed (left panel) and lowly expressed (right panel) DGK isoforms in subcutaneous adipose tissue; (B) Highly expressed (left panel) and lowly expressed (right panel) DGK isoforms in epididymal adipose tissue. Data are normalized to reference genes (see Material and Methods) and presented relative DGKε mRNA in tissues from C57BL/6J mice. Results are mean ± SEM. n = 8–10. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
We first surveyed the DGK isoform expression profile in skeletal muscle, adipose tissue and liver from lean mice. Overall we found the DGK isoform expression profile was similar between glycolytic EDL and oxidative soleus muscle, except in the case of DGKα where mRNA expression was undetectable in soleus muscle. In liver, DGKζ was the predominant isoform, with comparable levels of DGKθ and DGKδ, confirming an earlier report (Shulga et al. 2013). Of note, expression of DGKβ, DGKγ, DGKκ and DGKι in this tissue was minimal or not detected. In subcutaneous and epididymal adipose tissue, isoform-specific DGK expression patterns were also observed. While DGKζ was the predominant isoform in liver, DGKδ was the predominant isoform in subcutaneous and epididymal adipose tissue, consistent with earlier reports (Lowe et al. 2013; Shulga et al. 2013). Likewise, DGKδ was highly expressed in skeletal muscle, consistent with human RNA-seq expression data (Krupp et al. 2012). Isoforms in the same subfamily did not necessarily share same expression profile and isoforms from different subfamilies were expressed at similar magnitude, further highlighting the tissue-specific roles of DGK isoforms.
Several genetically modified DGK mouse models have been generated (Rodriguez de Turco et al. 2001; Zhong et al. 2003; Regier et al. 2005; Crotty et al. 2006; Olenchock et al. 2006; Chibalin et al. 2008; Shirai et al. 2010), each demonstrating distinct phenotypic traits, further implying functional diversity in DGK isoform-specific signaling (Shirai and Saito 2014). Given that both the substrate (DAG) and the product (PA) of the reaction catalyzed by DGK serve as metabolic intermediates and potent signaling molecules, an imbalance in these substrates may have an impact on cell growth and metabolism. Ectopic intracellular accumulation of DAG is associated with insulin resistance in skeletal muscle and liver (Erion and Shulman 2010; Samuel and Shulman 2012; Zhang et al. 2013; Zachariah Tom et al. 2014). Consequently, an imbalance in expression of DGKs may have a role in the pathogenesis of various metabolic disorders including type 2 diabetes, obesity, and nonalcoholic fatty liver disease. Thus, we next determined the expression of DGK isoforms in obese insulin-resistant ob/ob mice.
DGKα, DGKβ and DGKγ belong to the type I subgroup of DGKs, which have a calcium-binding EF hand motif (Shulga et al. 2011). DGKα is abundant in T cells and has a role in T-cell activation and T-cell anergy (Olenchock et al. 2006). In healthy humans, DGKα mRNA levels are comparable between adipose tissue, liver and skeletal muscle, DGKγ mRNA is increased in skeletal muscle compared to liver and adipose tissue, whereas DGKβ is minimally expressed in these tissues (Krupp et al. 2012). DGKα and DGKγ are highly expressed in mouse pancreatic β-cells and diminished levels of these isoforms attenuates insulin secretion (Kurohane Kaneko et al. 2013), implying a role in metabolic disease. Indeed, mRNA of these isoforms was reduced in subcutaneous and epididymal adipose tissue of ob/ob mice, but the functional significance is unknown. Genetic studies have linked DGKβ to diabetes risk. A meta-analysis of 21 genome-wide association studies found an association between the loci containing DGKβ (DGKB-TMEM195) and type 2 diabetes (Dupuis et al. 2010). Even though this isoform was lowly expressed, DGKβ mRNA was increased EDL muscle, and concomitantly decreased in soleus muscle and epididymal adipose tissue from ob/ob mice. Although DGKβ may be involved in type 2 diabetes, knockout mice show attention-deficit behavior and a hyperactive phenotype, implicating a predominant role in neurological, rather than metabolic disorders (Ishisaka et al. 2012).
The members of type II DGKs, DGKδ, DGKη, and DGKκ, have pleckstrin homology domains and sterile α motifs (Sakai and Sakane 2012). We found that DGKδ and DGKη mRNA was readily detected in all insulin-sensitive tissues, whereas DGKκ was undetected. RNA-Seq data show DGKκ is lowly expressed in most tissues, with the highest expression noted in testes and brain (Krupp et al. 2012). In contrast, DGKδ is widely expressed in insulin-sensitive tissue (Chibalin et al. 2008; Krupp et al. 2012) and plays a major role in regulating the synthesis of a broad range of lipid species (Shulga et al. 2013). DGKδ also increases lipid synthesis by promoting de novo fatty acid synthesis (Shulga et al. 2013). DGKδ+/− mice have increased DAG content in skeletal muscle, leading to impaired peripheral insulin sensitivity and age-dependent obesity (Chibalin et al. 2008). Here we report DGKδ is highly expression in skeletal muscle and adipose tissue, and to a lower extent in liver, consistent with a previous study (Lowe et al. 2013). Total DGK activity is reduced in skeletal muscle and adipose tissue, but not liver from DGKδ+/− mice (Chibalin et al. 2008), indicating DGKδ plays a major role in peripheral tissues and a minor role in liver. In ob/ob mice, DGKδ mRNA was increased in liver and soleus muscle, decreased in epididymal adipose tissue and unaltered in subcutaneous adipose; further highlighting the impact of metabolic disease on DGKδ expression profiles. Hyperglycemia, a cardinal feature of type 2 diabetes, regulates DGKδ abundance and subcellular localization (Miele et al. 2007; Chibalin et al. 2008; Takeuchi et al. 2012), as well as DGKδ enzyme activity against palmitic acid-containing DAG species (Sakai et al. 2014). Interestingly saturated fatty acids increase and monounsaturated fatty acids attenuated DGKδ mRNA expression in C2C12 cells (Sakiyama et al. 2014). Consequently, the level of glycemia or different lipid species in type 2 diabetes or obesity may impact the abundance and activity of DGKδ. DGKη regulates a wide variety of physiological events including cell growth and proliferation. DGKη is linked to bipolar disorder (Moya et al. 2010), lung cancer (Nakano et al. 2014), and heart failure (Bilim et al. 2011), but its functional role in metabolic disorder is unclear. In ob/ob mice, DGKη was slightly reduced in soleus muscle and epididymal adipose tissue, while increased in subcutaneous adipose tissue. While DGKδ plays a role in metabolic disease, DGKη regulates other fundamental processes including proliferation and differentiation via Ras/B-Raf/C-Raf/MEK/ERK signaling (Yasuda et al. 2009). Dissecting the role of DGKδ and DGKη along diverse metabolic and growth-promoting pathways may further elucidate the diverse pathogenesis of chronic disorders including insulin resistance, heart failure, sarcopenia, and cancer.
DGKε is the only member of the type III DGKs and is distinguished by a unique structure and substrate specificity toward arachidonate-containing DAG (Shulga et al. 2011). DGKε knockout mice have a higher resistance to seizures induced by electroconvulsive shock (Rodriguez de Turco et al. 2001), while transgenic mice overexpressing DGKε are protected from experimental cardiac hypertrophy (Niizeki et al. 2008). DGKε is expressed in various immune cells, with increased levels observed in response to inflammatory stimuli (Yamamoto et al. 2014). In humans, DGKε is highly expressed in brain and spleen, with lower, but comparable levels in liver, skeletal muscle and adipose tissue (Krupp et al. 2012). We observed DGKε expression was marginally reduced in EDL muscle and appreciably reduced in epididymal adipose tissue from ob/ob mice. This was unexpected given that overexpression of DGKɛ in muscle cells leads to defects in insulin signaling (Cazzolli et al. 2007).
The subfamily of type IV isoforms includes DGKζ and DGKι, which contain ankyrin repeats, a C-terminal nuclear localization signal and a PDZ-binding motif, as well as MARCKS homology region (Shulga et al. 2011). Analogous to DGKα, DGKζ plays a critical role in immune cells (Zhong et al. 2003). However, DGKζ is ubiquitously expressed in most tissues and most predominant in the brain (Krupp et al. 2012), suggesting a role in several organ systems. We found DGKζ to be highly expressed in all insulin-sensitive tissues. DGKζ expression was increased in epididymal adipose tissue and liver from ob/ob mice, while similar levels were noted in subcutaneous adipose tissue, soleus muscle and EDL muscle compared to C57BL/6J mice. The second member in the type IV DGK group, DGKι, is predominately expressed in brain, but lowly expressed in insulin sensitivity tissues (Krupp et al. 2012). We found DGKι mRNA expression was increased in soleus and EDL muscle, as well as subcutaneous and epididymal adipose tissue from ob/ob mice. The expression of DGKι decreases in 3T3-L1 cells during adipocyte differentiation (Shulga et al. 2013). Interestingly, the National Heart, Lung, and Blood Institute Family Heart Study (FHS) genome-wide linkage scan identified DGKι is a candidate gene for influencing BMI (Laramie et al. 2009), but secondary validation and functional studies are required to confirm this association.
DGKθ, the single member of the type V DGK, has three C1 domains, a Gly/Pro-rich domain and a pleckstrin homology domain (Shulga et al. 2011). Overexpression of DGKθ in mouse hepatocytes increases PA and decreases DAG content, while concomitantly impairing insulin signaling (Zhang et al. 2014). Consistent with other DGK isoforms, DGKθ is highly expressed in different regions of the brain in rats (Houssa et al. 1997). We observed that DGKθ mRNA was increased in EDL muscle, decreased in soleus muscle and unaltered in liver and adipose tissue depots in ob/ob versus lean mice. Nevertheless, DGKθ mRNA was lowly expressed compared to other isoforms, similar to RNA-Seq data from human tissues (Krupp et al. 2012). DGKθ has emerged as a relevant target for metabolic regulation given evidence that this isoforms acts as a key mediator of bile-acid-stimulated modulation PA-dependent mTOR and Akt signaling and glucose homoeostasis in HepG2 cells and primary human hepatocytes (Cai and Sewer 2013).
The identification of reference genes that are consistently expressed across several tissues is a frequent limitation, given the fact that even references genes often have a variable tissue-specific mRNA expression profile. To deal with this limitation, we profiled several reference genes expressed in the various metabolic tissues under study. Based on the results of this analysis, we were unable to identify a reference gene that was stable in all the tissues studied. Therefore, we present the Cq values and have normalized the data for the analysis comparing the expression of different DGK isoforms across the various tissues against the expression level in EDL muscle. Because we were unable to the normalize mRNA expression of the individual DGK isoforms between the various tissues to a common set of reference genes, we cannot exclude that this approach might have induced potential artifacts. When comparing mRNA expression profile of DGK isoforms within each tissue of C57BL/6J and ob/ob mice, the isoforms were normalized to the reference genes relevant for each tissue and then to a calibrator gene.
In summary, we provide evidence for tissue-specific expression profiles of DGK isoforms insulin-sensitive tissue from lean C57BL/6J mice. DGKδ is the most abundant isoform in soleus muscle, subcutaneous and epididymal adipose tissue. DGKα and DGKζ are the predominant isoforms in EDL muscle. Finally, in liver, DGKζ is the predominant isoform, with comparable levels of DGKθ and DGKδ noted. Overall, mRNA expression of DGK isoforms was generally lower in liver compared to skeletal muscle and adipose tissue. In conclusion, DGK expression was altered in an isoform- and tissue-specific manner in obese insulin-resistant ob/ob mice, suggesting DGKs play unique roles in each tissue and may play a role in metabolic disorders. Further studies are warranted to elucidate whether the altered DGK isoform expression profile in observed in obese insulin-resistant ob/ob mice has deleterious impact on tissue levels of PA and DAG as well as total DGK activity. Several DGK isoforms likely work in concert to modulate growth and metabolism in insulin sensitivity tissues.
Conflict of Interest
None declared.
References
- Bilim O, Shishido T, Toyama S, Suzuki S, Sasaki T, Kitahara T, et al. Differential regulation of diacylglycerol kinase isoform in human failing hearts. J. Cardiothorac. Surg. 2011;6:65. doi: 10.1186/1749-8090-6-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai K. Sewer MB. Diacylglycerol kinase theta couples farnesoid X receptor-dependent bile acid signalling to Akt activation and glucose homoeostasis in hepatocytes. Biochem. J. 2013;454:267–274. doi: 10.1042/BJ20130609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzolli R, Mitchell TW, Burchfield JG, Pedersen DJ, Turner N, Biden TJ, et al. Dilinoleoyl-phosphatidic acid mediates reduced IRS-1 tyrosine phosphorylation in rat skeletal muscle cells and mouse muscle. Diabetologia. 2007;50:1732–1742. doi: 10.1007/s00125-007-0709-x. [DOI] [PubMed] [Google Scholar]
- Chibalin AV, Leng Y, Vieira E, Krook A, Bjornholm M, Long YC, et al. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell. 2008;132:375–386. doi: 10.1016/j.cell.2007.12.035. [DOI] [PubMed] [Google Scholar]
- Crotty T, Cai J, Sakane F, Taketomi A, Prescott SM. Topham MK. Diacylglycerol kinase delta regulates protein kinase C and epidermal growth factor receptor signaling. Proc. Natl Acad. Sci. USA. 2006;103:15485–15490. doi: 10.1073/pnas.0604104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erion DM. Shulman GI. Diacylglycerol-mediated insulin resistance. Nat. Med. 2010;16:400–402. doi: 10.1038/nm0410-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssa B, Schaap D, van der Wal J, Goto K, Kondo H, Yamakawa A, et al. Cloning of a novel human diacylglycerol kinase (DGKθ) containing three cysteine-rich domains, a proline-rich region, and a pleckstrin homology domain with an overlapping Ras-associating domain. J. Biol. Chem. 1997;272:10422–10428. doi: 10.1074/jbc.272.16.10422. [DOI] [PubMed] [Google Scholar]
- Hozumi Y, Matsui H, Sakane F, Watanabe M. Goto K. Distinct expression and localization of diacylglycerol kinase isozymes in rat retina. J. Histochem. Cytochem. 2013;61:462–476. doi: 10.1369/0022155413483574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishisaka M. Hara H. The roles of diacylglycerol kinases in the central nervous system: review of genetic studies in mice. J. Pharmacol. Sci. 2014;124:336–343. doi: 10.1254/jphs.13r07cr. [DOI] [PubMed] [Google Scholar]
- Ishisaka M, Kakefuda K, Oyagi A, Ono Y, Tsuruma K, Shimazawa M, et al. Diacylglycerol kinase beta knockout mice exhibit attention-deficit behavior and an abnormal response on methylphenidate-induced hyperactivity. PLoS One. 2012;7:e37058. doi: 10.1371/journal.pone.0037058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri Y, Ito T, Saino-Saito S, Hozumi Y, Suwabe A, Otake K, et al. Expression and localization of diacylglycerol kinase isozymes and enzymatic features in rat lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L1171–L1178. doi: 10.1152/ajplung.00237.2004. [DOI] [PubMed] [Google Scholar]
- Krishna S. Zhong XP. Regulation of lipid signaling by diacylglycerol kinases during T cell development and function. Front Immunol. 2013;4:178. doi: 10.3389/fimmu.2013.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp M, Marquardt JU, Sahin U, Galle PR, Castle J. Teufel A. RNA-Seq Atlas—a reference database for gene expression profiling in normal tissue by next-generation sequencing. Bioinformatics. 2012;28:1184–1185. doi: 10.1093/bioinformatics/bts084. [DOI] [PubMed] [Google Scholar]
- Kurohane Kaneko Y, Kobayashi Y, Motoki K, Nakata K, Miyagawa S, Yamamoto M, et al. Depression of type I diacylglycerol kinases in pancreatic beta-cells from male mice results in impaired insulin secretion. Endocrinology. 2013;154:4089–4098. doi: 10.1210/en.2013-1356. [DOI] [PubMed] [Google Scholar]
- Laramie JM, Wilk JB, Williamson SL, Nagle MW, Latourelle JC, Tobin JE, et al. Multiple genes influence BMI on chromosome 7q31-34: the NHLBI Family Heart Study. Obesity (Silver Spring) 2009;17:2182–2189. doi: 10.1038/oby.2009.141. [DOI] [PubMed] [Google Scholar]
- Livak KJ. Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lowe CE, Zhang Q, Dennis RJ, Aubry EM, O'Rahilly S, Wakelam MJ, et al. Knockdown of diacylglycerol kinase delta inhibits adipocyte differentiation and alters lipid synthesis. Obesity (Silver Spring) 2013;21:1823–1829. doi: 10.1002/oby.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele C, Paturzo F, Teperino R, Sakane F, Fiory F, Oriente F, et al. Glucose regulates diacylglycerol intracellular levels and protein kinase C activity by modulating diacylglycerol kinase subcellular localization. J. Biol. Chem. 2007;282:31835–31843. doi: 10.1074/jbc.M702481200. [DOI] [PubMed] [Google Scholar]
- Moya PR, Murphy DL, McMahon FJ. Wendland JR. Increased gene expression of diacylglycerol kinase eta in bipolar disorder. Int. J. Neuropsychopharmacol. 2010;13:1127–1128. doi: 10.1017/S1461145710000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Hozumi Y, Iwazaki K, Okumoto K, Iseki K, Saito T, et al. Altered expression of diacylglycerol kinase isozymes in regenerating liver. J. Histochem. Cytochem. 2012;60:130–138. doi: 10.1369/0022155411429154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Iravani A, Kim M, Hozumi Y, Lohse M, Reichert E, et al. Diacylglycerol kinase eta modulates oncogenic properties of lung cancer cells. Clin. Transl. Oncol. 2014;16:29–35. doi: 10.1007/s12094-013-1036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niizeki T, Takeishi Y, Kitahara T, Arimoto T, Ishino M, Bilim O, et al. Diacylglycerol kinase-epsilon restores cardiac dysfunction under chronic pressure overload: a new specific regulator of Galpha(q) signaling cascade. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H245–H255. doi: 10.1152/ajpheart.00066.2008. [DOI] [PubMed] [Google Scholar]
- Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, et al. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat. Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- Regier DS, Higbee J, Lund KM, Sakane F, Prescott SM. Topham MK. Diacylglycerol kinase iota regulates Ras guanyl-releasing protein 3 and inhibits Rap1 signaling. Proc. Natl Acad. Sci. USA. 2005;102:7595–7600. doi: 10.1073/pnas.0500663102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Turco EB, Tang W, Topham MK, Sakane F, Marcheselli VL, Chen C, et al. Diacylglycerol kinase epsilon regulates seizure susceptibility and long-term potentiation through arachidonoyl- inositol lipid signaling. Proc. Natl Acad. Sci. USA. 2001;98:4740–4745. doi: 10.1073/pnas.081536298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H. Sakane F. Recent progress on type II diacylglycerol kinases: the physiological functions of diacylglycerol kinase delta, eta and kappa and their involvement in disease. J. Biochem. 2012;152:397–406. doi: 10.1093/jb/mvs104. [DOI] [PubMed] [Google Scholar]
- Sakai H, Kado S, Taketomi A. Sakane F. Diacylglycerol kinase delta phosphorylates phosphatidylcholine-specific phospholipase C-dependent, palmitic acid-containing diacylglycerol species in response to high glucose levels. J. Biol. Chem. 2014;289:26607–26617. doi: 10.1074/jbc.M114.590950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakiyama S, Usuki T, Sakai H. Sakane F. Regulation of diacylglycerol kinase delta2 expression in C2C12 skeletal muscle cells by free fatty acids. Lipids. 2014;49:633–640. doi: 10.1007/s11745-014-3912-9. [DOI] [PubMed] [Google Scholar]
- Samuel VT. Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionoya T, Usuki T, Komenoi S, Isozaki T, Sakai H. Sakane F. Distinct expression and localization of the type II diacylglycerol kinase isozymes inverted question mark, inverted question mark and inverted question mark in the mouse reproductive organs. BMC Dev. Biol. 2015;15:6. doi: 10.1186/s12861-015-0055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai Y. Saito N. Diacylglycerol kinase as a possible therapeutic target for neuronal diseases. J. Biomed. Sci. 2014;21:28. doi: 10.1186/1423-0127-21-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai Y, Kouzuki T, Kakefuda K, Moriguchi S, Oyagi A, Horie K, et al. Essential role of neuron-enriched diacylglycerol kinase (DGK), DGKbeta in neurite spine formation, contributing to cognitive function. PLoS One. 2010;5:e11602. doi: 10.1371/journal.pone.0011602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulga YV, Topham MK. Epand RM. Regulation and functions of diacylglycerol kinases. Chem. Rev. 2011;111:6186–6208. doi: 10.1021/cr1004106. [DOI] [PubMed] [Google Scholar]
- Shulga YV, Loukov D, Ivanova PT, Milne SB, Myers DS, Hatch GM, et al. Diacylglycerol kinase delta promotes lipogenesis. Biochemistry. 2013;52:7766–7776. doi: 10.1021/bi401178y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M, Sakiyama S, Usuki T, Sakai H. Sakane F. Diacylglycerol kinase delta1 transiently translocates to the plasma membrane in response to high glucose. Biochim. Biophys. Acta. 2012;1823:2210–2216. doi: 10.1016/j.bbamcr.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Topham MK. Signaling roles of diacylglycerol kinases. J. Cell. Biochem. 2006;97:474–484. doi: 10.1002/jcb.20704. [DOI] [PubMed] [Google Scholar]
- Toya M, Hozumi Y, Ito T, Takeda M, Sakane F, Kanoh H, et al. Gene expression, cellular localization, and enzymatic activity of diacylglycerol kinase isozymes in rat ovary and placenta. Cell Tissue Res. 2005;320:525–533. doi: 10.1007/s00441-005-1089-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Tanaka T, Hozumi Y, Saino-Saito S, Nakano T, Tajima K, et al. Expression of mRNAs for the diacylglycerol kinase family in immune cells during an inflammatory reaction. Biomed. Res. 2014;35:61–68. doi: 10.2220/biomedres.35.61. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Kai M, Imai S, Takeishi K, Taketomi A, Toyota M, et al. Diacylglycerol kinase eta augments C-Raf activity and B-Raf/C-Raf heterodimerization. J. Biol. Chem. 2009;284:29559–29570. doi: 10.1074/jbc.M109.043604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariah Tom R, Garcia-Roves PM, Sjögren RJO, Jiang LQ, Holmström MH, Deshmukh AS, et al. Effects of AMPK Activation on Insulin Sensitivity and Metabolism in Leptin-Deficient ob/ob Mice. Diabetes. 2014;63:1560–1571. doi: 10.2337/db13-0670. [DOI] [PubMed] [Google Scholar]
- Zhang C, Klett EL. Coleman RA. Lipid signals and insulin resistance. Clin. Lipidol. 2013;8:659–667. doi: 10.2217/clp.13.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Hwarng G, Cooper DE, Grevengoed TJ, Eaton JM, Natarajan V, et al. Inhibited insulin signaling in mouse hepatocytes is associated with increased phosphatidic acid but not diacylglycerol. J. Biol. Chem. 2014;290:3519–3528. doi: 10.1074/jbc.M114.602789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, et al. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat. Immunol. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]