Abstract
Within exposure-based trauma treatments for posttraumatic stress disorder (PTSD), imagery vividness during imaginal exposure of the traumatic memory is an understudied but potentially important predictor of treatment outcome. Further, this relationship has only been studied in women to date, and never among individuals with PTSD and substance use disorders which could impact ability to produce vivid mental imagery and its impact. The current study investigated whether imagery vividness ratings during in-session exposure predicted post-treatment PTSD symptom severity in a sample of men and women with comorbid PTSD and substance use disorders, and also examined whether gender moderated this relationship. A sample of 71 participants who received an exposure-based trauma treatment were included in the analyses. PTSD symptom severity was assessed using both the Clinician Administered PTSD Scale (CAPS) and the Impact of Event Scale-Revised (IES-R). Results varied according to method of assessing PTSD symptom severity. Higher imagery vividness was associated with better treatment outcome when assessed by the CAPS, with vividness in later sessions relating more strongly to outcome than vividness in earlier sessions. With the IES-R, higher imagery vividness ratings predicted more favorable treatment outcome for men, but less favorable treatment outcomes for women. Findings are discussed in the context of using imagery vividness to maximize treatment outcomes and future research directions involving scientific replication.
Keywords: Exposure therapy, PTSD, treatment outcome, cognitive behavioral therapy, trauma, substance abuse
Posttraumatic stress disorder (PTSD) is a debilitating psychological disorder that can develop following exposure to traumatic events. PTSD is a prevalent public health concern (i.e., lifetime prevalence of 6.8%; NCS-R; Kessler, Berglund, Demler, Jin, & Merikangas, 2005) that is associated with a variety of problems, including psychological comorbidity (e.g., depression, panic attacks, substance use), occupational impairment, and elevated health care costs (Hofmann, Litz, & Weathers, 2002; Kessler, 2000; Walker et al., 2003). Several effective treatments for PTSD currently exist (see Foa, Keane, Friedman, & Cohen, 2008 for a review), including Prolonged Exposure (Foa, Hembree, & Rothbaum, 2007). Prolonged Exposure consists of several components typical of cognitive-behavioral treatments, including in vivo and imaginal exposure. In imaginal exposure, patients repeatedly recount their most distressing traumatic memory, while in vivo exposure involves gradual and systematic exposure to individualized trauma triggers (e.g., situations, objects).
Prolonged Exposure is a highly effective treatment for PTSD (Powers, Halpern, Ferenschak, Gillihan, & Foa, 2010). This intervention is largely based on Emotional Processing Theory (EPT; Foa & Kozak, 1986; Foa & Riggs, 1993), which proposes that fear structures—specifically, with pathological/erroneous associations between the physiological, behavioral, and cognitive elements of fear—are the initiating and maintaining factors underlying anxiety disorders. With respect to PTSD specifically, EPT posits that exposure to trauma-related stimuli reduces fear because activation of the fear structure during exposure allows for the correction of inaccurate stimulus-stimulus and stimulus-response associations (Foa & Rothbaum, 1998). The fear structure is theorized to be activated when the patient uses imagery to recount the target or index trauma (i.e., during imaginal exposure in and out of session; Foa, Dancu, Hembree, Jaycox, & Street, 1999; Rothbaum, Meadows, Resick, & Foy, 2000), and, according to Foa and Kozak (1986), habituation of fear is one possible mechanism of change. Examination of additional mechanisms underlying the effectiveness of Prolonged Exposure is warranted, however, and would allow for further development of theoretical models of trauma recovery. Further, it is unclear how individual differences may interact with Prolonged Exposure treatment components to predict responsiveness to treatment. Both areas of study are of high importance with respect to maximizing therapeutic outcomes for the most individuals possible.
Imagery Vividness
One potential mechanism of exposure-based treatments that remains less studied is emotional engagement, assessed through imagery vividness, with the index trauma memory during imaginal exposure. In order to increase engagement with the trauma memory, and the fear response conditioned to the memory, patients are instructed to recount the trauma. Patients are encouraged to include the thoughts and feelings experienced during the trauma and to describe them in present (rather than past) tense as vividly as possible. Vividness ratings can be collected throughout the exposure session in conjunction with ratings of distress (i.e., subjective units of distress [SUDS]; Wolpe, 1958) in order to assist the clinician in assessing how intensely the patient is reliving the event. Hackmann, Ehlers, Speckens, & Clark (2004) found that, among individuals with PTSD, both vividness of intrusive memories and distress decreased with treatment (as well as other elements of the memories such as frequency of intrusions). This highlights a potential association between imagery vividness and the emotional salience of the memory, with an important research question becoming whether vividness can be used to directly assist with treatment for PTSD.
Based on the proposition that more vivid trauma memory imagery elicits greater trauma-related fear, Rauch and colleagues (2004) examined whether higher imagery vividness in early sessions would predict better outcomes in women who had experienced sexual or non-sexual assault. They found that (a) vividness ratings collected during imaginal exposure positively correlated with SUDS in early imaginal exposure sessions, (b) this correlation weakened in later sessions, (c) vividness decreased significantly with continued imaginal exposure over the course of Prolonged Exposure sessions, and, perhaps most importantly, (d) vividness was not related to PTSD outcome. The authors attributed this unexpected null finding to a restricted range of vividness ratings (actual ratings were not reported in the published manuscript), potentially reflecting enhanced overall imagery ability with respect to trauma memories (Bryant & Harvey, 1996). They also proposed that patients may only need to reach a threshold of vividness for fear to be elicited, and that higher levels of vividness may not impact, or may even interfere with, outcome (i.e., over engagement).
The results of this study call into question the predictive utility of imagery vividness ratings. In fact, while assessing vividness ratings during imaginal exposure was part of the original Prolonged Exposure manual (Foa & Rothbaum, 1998), vividness ratings were not included in the most recent version of the treatment (Foa et al., 2007). Given the theorized importance of emotional engagement with the trauma memory as a mechanism of fear elicitation, and the manual’s prescribed goal to have patients neither under- nor over-engage with the memory, further examination of the role of imagery vividness in Prolonged Exposure outcomes is warranted.
An additional characteristic that may be important when investigating the impact of imagery vividness on PTSD treatment outcome is biological gender. Studies have consistently demonstrated a higher prevalence of PTSD in women than in men (see Olff, Langeland, Draijer, & Gerson, 2007 for a review) and mechanisms for this difference require further investigation. It is also possible that the mechanisms impacting treatment outcome might differ between the genders. For example, there is some evidence for gender-based differences in fear extinction (e.g., Milad et al., 2010; Stark et al., 2006), the primary hypothesized mechanism of change in exposure. Research findings on gender differences in vividness ability among non-clinical samples, however, are mixed. Campos (2014) found no gender differences with regard to general vividness in a sample of college students, for example, while Richardson (1995) demonstrated that women had higher scores than men on self-reported vividness when utilizing the Vividness of Visual Imagery Questionnaire (Marks, 1973). Meanwhile, Karatzias and colleagues (2009) found no difference between men and women on vividness in a sample of individuals with PTSD. Given that previous research has not been extended to investigating potential gender differences in the relationship between vividness ratings and PTSD treatment outcome, such inquiry is warranted.
Investigating the role of imagery vividness in imaginal exposure may also be particularly relevant to individuals with comorbid PTSD and substance use disorders. PTSD is highly prevalent in samples of individuals with substance use disorders (i.e., 36–50%), and individuals in treatment for substance use disorders with comorbid PTSD have poorer prognoses than individuals without the diagnosis (Brady, Back, & Coffey, 2004). Cognitive impairments among individuals with substance use disorders may also impact the ability to generate vivid images. Individuals with substance use disorders are at risk for numerous cognitive deficits resulting from excessive substance use, including poorer memory functioning (Glass, et al., 2009; Javanovski, Erb, & Zakzanis, 2005; London et al., 2005; Scott, Woods, Matt, Meyer, Heaton, & Atkinson, 2007; Solowij & Battisti, 2008) and poorer visual spatial memory than non-substance users (Ersche, Clark, London, Robbins, & Sahakian, 2006). Thus, vividness ability may also be compromised in this population, and the impact of vividness on PTSD treatment outcome might be different among individuals with comorbid PTSD and substance use disorders than in those with PTSD only.
To our knowledge, the study by Rauch and colleagues (2004) is the only report to date examining the impact of imagery vividness on treatment outcome following participation in an exposure-based trauma therapy for PTSD. Given the unexpected null findings of this study, it is important to address methodological limitations that may have impacted study outcomes. First, their investigation was limited to women, such that the effect of gender could not be examined. Second, Rauch et al. (2004) used only one assessment of PTSD, whereas replicating and extending findings using multiple modes of assessment could improve the reliability of findings regarding the potential usefulness of assessing vividness during PTSD. Finally, the study by Rauch and colleagues was restricted to participants who had experienced sexual or non-sexual assault as opposed to a wider spectrum of traumatic event exposure.
The present study aimed to replicate and extend Rauch and colleagues’ (2004) study in a sample of individuals of men and women with comorbid PTSD and substance use disorders. Hypotheses for this study were as follows: (1) Consistent with the study by Rauch and colleagues (2004), there will be a stronger, positive relationship between imagery vividness ratings and SUDS during earlier imaginal exposure sessions than during later sessions; and (2) Higher overall vividness and SUDS ratings will be negatively associated with PTSD symptom severity post-treatment.
We also aimed to answer the following exploratory questions: Does the relationship between imagery vividness and PTSD severity at outcome vary across sessions? And second, are vividness and post-treatment PTSD outcomes differentially related in women versus in men?
Method
Participants
Participants included 71 individuals with comorbid PTSD and substance use disorders enrolled in residential substance use treatment. Individuals who were psychotic, actively manic, had suicidal intent, or were taking a benzodiazepine were excluded from the study. Exclusion criteria also included having medical conditions that would impair cognitive functioning, PTSD linked to combat-related trauma, and non-English speaking individuals. All participants received an assessment two to five days prior to beginning treatment and immediately following treatment. While the larger treatment study included additional measures, only those relevant to the present aims are discussed in this paper. Table 1 describes the sociodemographic profiles of men and women in the sample and mean PTSD symptom levels at baseline. The sample had been exposed to a range of different traumas, including, but not limited to, physical assault (80.3%) sexual trauma (60.6%), accidents (56.3%), and disasters (40.8%). There were no differences between men and women with regard to number of traumas (t[69] = −1.31, p = .19). From the traumatic events assessed, women had a higher overall rate of exposure to sexual trauma (χ2[1] = 17.10, p < .001), while men showed a higher prevalence of “weapon” (χ2[1] = 4.01, p = 0.045, “being seriously injured” (χ2[1] = 4.78, p = .029, and “witness” (χ2[1] = 13.99, p < .001. No other gender differences in trauma types were observed. The majority of participants were currently taking psychotropic medications (60.6%; e.g., Trazodone, Seroquel, Prozac), had been hospitalized for a psychiatric problem (36.6%) in the past, and had engaged in outpatient treatment for a mental health problem (38%).
Table 1.
Demographic Characteristics of the Sample and Symptoms of PTSD at Baseline
| Males | Females | |
|---|---|---|
| n (%) | n (%) | |
| 41 (57.7) | 30 (42.3) | |
| Age | ||
| 18–30 | 13 (31.7) | 12 (40.0) |
| 31–50 | 23 (56.1) | 16 (53.3) |
| 51+ | 5 (12.2) | 2 (6.7) |
| Race/Ethnicity | ||
| White/Caucasian | 29 (70.7) | 25 (83.3) |
| Black/African American | 10 (24.4) | 5 (16.7) |
| Other | 2 (4.8) | 0 (0.0) |
| Mean (SD) | Mean (SD) | |
| Income | $32,414.63 ($26,562.17) | $33,856.67 ($40,958.72) |
| CAPS Total Score | 77.66 (16.15) | 78.20 (17.24) |
| IES-R Total | 52.78 (16.21) | 48.67 (16.43) |
| Number of Traumatic Events | 8.90 (5.56) | 10.57 (4.84) |
Note. IES-R = Impact of Event Scale-Revised. CAPS = Clinician Administered PTSD Scale.
PTSD (for non-combat trauma) and substance dependence diagnoses were determined using the Clinician Administered PTSD Scale (CAPS; Blake et al., 1995) and the Computerized Diagnostic Interview Schedule (CDIS; Robins et al., 2000). Following the initial assessment, participants were enrolled in trauma-focused exposure therapy (EXP), which consisted of 9 – 12, 60-minute sessions. Sixty-minute sessions were utilized so that the intervention would better fit standard clinical practice and, thus, potentially speed implementation and dissemination of study findings. Initial sessions focused on psychoeducation about trauma and breathing retraining, and the remainder of the sessions consisted of both imaginal and in vivo exposures (see [masked for review]) for additional details regarding the treatment protocol). Given that this protocol was developed for a comorbid substance use sample, information regarding the relation between trauma symptoms and substance use was presented and discussed with patients throughout the entire treatment as needed.
Measures
Clinician Administered PTSD Scale (CAPS; Blake et al., 1995)
The CAPS is a semi-structured interview and is considered a gold-standard for assessing and diagnosing PTSD using DSM-IV criteria. It provides a symptom severity rating, which is calculated based on the frequency and intensity of each symptom assessed (0–136). The CAPS has high reliability and high convergent validity with other PTSD assessments (Weathers, Keane, & Davidson, 2001). For this study, the CAPS was used to diagnose PTSD, as well as to assess PTSD severity ratings. Inter-rater Kappa reliability for CAPS administration was .94, and Cronbach’s alpha for the CAPS in the current sample was .84.
Impact of Event Scale-Revised (IES-R; Weiss & Marmar, 1997)
The IES-R is a 22-item self-report measure used to assess participants’ subjective response (i.e., intrusions, avoidance, and hyperarousal) to their identified most traumatic life event (which was the focus of imaginal exposure throughout treatment). This scale has demonstrated good predictive and discriminative validity (Hyer & Brown, 2008; Weiss, 2004). Participants’ total IES-R score at post-treatment was utilized in the present study as a primary outcome measure of PTSD symptom severity. Cronbach’s alpha for the IES-R was .92 in the current sample. A decision was made to examine the impact of vividness on both the CAPS and the IES-R because, while the CAPS is a gold standard diagnostic tool for PTSD, the IES-R is often used as a more feasible method of tracking progress over the course of treatment.
Subjective Units of Distress ratings (SUDS; Wolpe, 1958)
SUDS ratings represent a subjective distress scale for participants. They are individualized for each participant using anchors reflecting past events or hypothetical situations eliciting differing degrees of SUDs for the specific individual. Throughout the imaginal exposure they are elicited from participants approximately every five minutes to assess their distress throughout the exposure. SUDS ranged from 0 – 100, with 0 indicating absolutely no distress and 100 being the most extreme distress. In the current study, the SUDS rating paired with the highest vividness rating within each session (i.e., reported by the participant at the same time as their highest vividness reporting) was used in order to examine the research question of whether SUDS and vividness co-vary across imaginal exposure sessions. For all other research questions pertaining to treatment outcome, the highest SUDS ratings reported in each session were used consistent with the study by Rauch and colleagues (2004).
Imagery vividness ratings
Participants were asked how vividly they were experiencing their memory during imaginal exposure. Participants were asked to provide SUDS ratings approximately every 5 min. Vividness ratings were collected along with the SUDS ratings approximately every 10 min. These ratings also ranged from 0 – 100, with 0 representing the absence of an image and 100 representing an extremely vivid image. In line with the study by Rauch et al. (2004), the highest vividness rating reported in each completed imaginal exposure session was used.
Analytic Plan
Participants were included in the present study if they completed at least 1 session of imaginal exposure. The mean number of imaginal sessions completed was 5.92 for males (Range: 1–9) and 5.66 for females (Range: 1–9).
We examined several research questions in the current analysis. We used a multilevel modeling approach in order to evaluate session-by-session and pre- to post-treatment change in SUDS, imagery vividness, and treatment outcome (the highest vividness rating within each session was identified as the primary independent variable). Multilevel modeling confers the advantage of providing robust statistical analyses with missing data (Field, 2009). In analyses that included random effects, an autoregressive error covariance matrix was defined, as residual error is likely to be correlated within individuals across time (Hox, 2010). While we included slope and intercept within participants as random effects in appropriate models, our primary focus was on the fixed effects of predictor variables. For treatment outcome variables, findings reflect multiple linear regression models that controlled for baseline levels of PTSD symptoms. Predictor variables were centered prior to analyses. We also examined the skewness of potential mediators and dependent variables prior to analyses. Vividness demonstrated a negative skew, which was corrected with a square root transformation.
Results
Correlations between IES-R, CAPS, and global averages of highest SUDS and imagery vividness ratings across all imaginal sessions completed can be found in Table 2.
Table 2.
Correlational matrix, means, and standard deviations (SD) of primary variables.
| 1. | 2. | 3. | 4. | 5. | 6. | Mean | SD | |
|---|---|---|---|---|---|---|---|---|
| 1. IES-R Baseline | 1 | .38** | .41** | .32* | −.02 | .03 | 51.53 | 16.31 |
| 2. IES-R Follow-up | .19 | .66** | .42** | .03 | 16.82 | 16.19 | ||
| 3. CAPS Baseline | .45** | −.02 | .05 | 79.51 | 17.43 | |||
| 4. CAPS Follow-up | .27 | −.10 | 33.87 | 21.71 | ||||
| 5. Average overall SUDS | .47** | 63.50 | 18.15 | |||||
| 6. Average overall imagery vividness | 1 | 81.09 | 15.02 |
Note.
significant at the .01 level;
significant at the .05 level.
IES-R = Impact of Event Scale-Revised. CAPS = Clinician Administered PTSD Scale. SUDS = Subjective Units of Distress Scale.
Imagery Vividness Scores
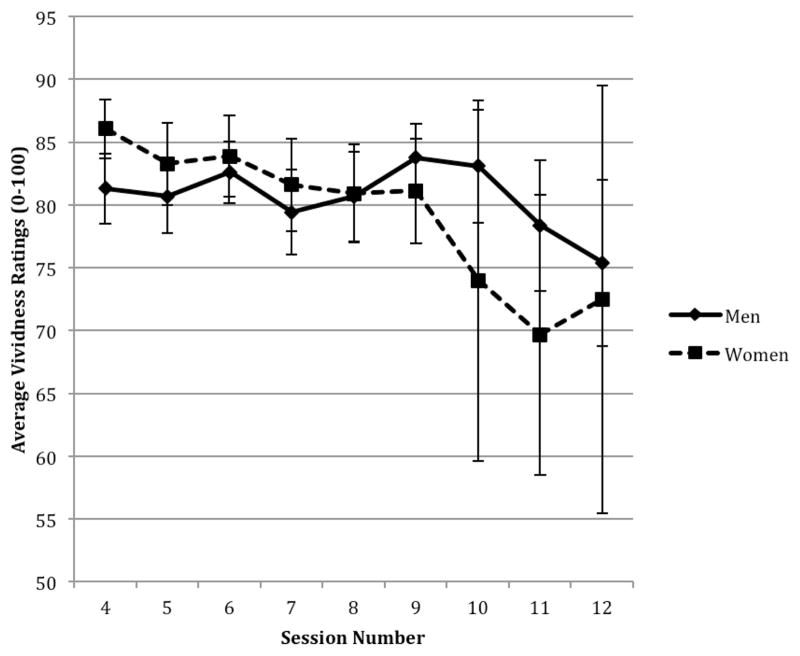
To examine whether imagery vividness scores changed across sessions and whether gender impacted vividness ratings over time, we evaluated main effects of Gender and Session and a Gender x Session interaction. In this model, men reported lower overall vividness than women and vividness remained more stable across sessions for men relative to women. Women reported higher levels of initial vividness, with vividness decreasing more quickly over time for women (see Table 3 and Figure 1).
Table 3.
Main effects and interactions of predictor variables on SUDS and imagery vividness scores.
| Fixed Effects | Random Effects | Model AIC | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Effect | B | SE b | 95% CI | Slope | Intercept | ||
| Vividness Ratings | .06** | 2.98** | 1451.63 | ||||
| Session | .002 | .06 | −.12, .12 | ||||
| Gender | −.08 | .44 | −.96, .80 | ||||
| Gender x Session | −.21 | .09 | −.39, −.03* | ||||
| Paired SUDS scores | 19.31** | 252.44** | 3479.07 | ||||
| Session | −8.26 | .71 | −9.68, −6.85** | ||||
| Vividness | 2.35 | .63 | 1.10, 3.59** | ||||
| Vividness x Session | −.84 | .25 | −1.33, −.34** | ||||
Note.
significant at the .01 level;
significant at the .05 level.
SUDS = Subjective Units of Distress Scale. SE = standard error. CI = confidence interval. AIC = Akaike’s Information Criterion. CIs that do not include zero indicate significant predictors. Vividness ratings are square root transformed and, therefore, produce Bs with signs in the opposite direction from what is indicated. To ease interpretation, signs of Bs and the 95% confidence intervals have been reversed to be more intuitive.
Figure 1.
Average imagery vividness across sessions for men and women.
Bars = standard errors
SUDS Scores
We examined whether imagery vividness predicted SUDS and whether the predicted relationship between vividness and SUDS changed over time (specifically utilizing ‘paired’ ratings, i.e., by taking the SUDS scores that were paired with the highest vividness ratings per session). As expected, as sessions progressed over the course of treatment, SUDS scores for participants decreased. Overall, vividness was positively associated with SUDS scores (see Table 3). Further, Hypothesis 1 was supported in that a significant interaction was observed between Vividness and Session such that vividness was less likely to predict SUDS scores as individuals progressed through treatment.
PTSD Outcomes
Interview measure: CAPS
In a model that included individual data points representing the highest imagery vividness and highest SUDS ratings for each session, Hypothesis 2 was partially supported in that Vividness was negatively related to CAPS score. However, contrary to Hypothesis 2, SUDS were positively related to CAPS score. When a Vividness x Session interaction term was introduced to the model in order to evaluate whether vividness differentially predicted outcome across sessions, this term was statistically significant. Higher levels of vividness in later, but not earlier sessions, predicted more favorable post-treatment CAPS scores. In a model that included main effects for Vividness, SUDS, and Gender, along with a Gender x Vividness interaction term, Gender significantly predicted post-treatment CAPS score, with men evidencing worse (higher) post-treatment CAPS scores adjusted for pretreatment scores as compared to women. However, the Gender X Vividness interaction was not statistically significant in this model (see Table 4).
Table 4.
Main effects and interactions of predictor variables on overall CAPS scores.
| Fixed Effects | Model AIC | |||
|---|---|---|---|---|
|
|
||||
| Effect | B | SE b | 95% CI | |
| 2917.52 | ||||
| CAPS Baseline | .48 | .05 | .37, .59** | |
| Vividness | −1.82 | .52 | −2.85, −.79** | |
| Highest SUDS | .15 | .04 | .08, .22** | |
| 2914.56 | ||||
| CAPS Baseline | .47 | .06 | .35, .58** | |
| Vividness | −1.62 | .52 | −2.66, −.58** | |
| Highest SUDS | .14 | .04 | .07, .21** | |
| Vividness x Session | −.46 | .20 | −.86, −.06* | |
| 2915.89 | ||||
| CAPS Baseline | .49 | .06 | .38, .61** | |
| Vividness | −1.79 | .73 | −3.34, −.34* | |
| Highest SUDS | .16 | .04 | .09, .23** | |
| Gender | −4.72 | 1.98 | −8.63, −.82* | |
| Gender x Vividness | −.03 | 1.01 | −1.95, 2.01 | |
Note.
significant at the .01 level;
significant at the .05 level.
IES-R = Impact of Event Scale-Revised. CAPS = Clinician Administered PTSD Scale. SUDS = Subjective Units of Distress Scale. SE = standard error. CI = confidence interval. AIC = Akaike’s Information Criterion. CIs that do not include zero indicate significant predictors. Vividness ratings are square root transformed and, therefore, produce Bs with signs in the opposite direction from what is indicated. To ease interpretation, signs of Bs and the 95% confidence intervals have been reversed to be more intuitive.
Self-report measure: IES-R
We replicated analyses from the CAPS with a self-report measure of PTSD symptom severity, the IES-R. Contrary to Hypothesis 2, there was no main effect of Vividness on IES-R score, however, SUDS was a significant predictor as hypothesized. When the Vividness x Session interaction was included in the model, this interaction did not reach significance. SUDS rating remained a significant predictor of post-treatment IES-R score, however. When Gender and the Vividness x Gender interaction were introduced into the model, both SUDS and Vividness emerged as significant predictors (although gender did not). A significant Vividness x Gender interaction also emerged, and follow-up on this interaction indicated that for men, Vividness was negatively related to IES-R symptoms at post-treatment (r = −.17, p = .02). Meanwhile, for women, Vividness was positively related to symptoms as self-reported by the IES-R post-treatment (r = .18, p = .03; see Table 5).
Table 5.
Main effects and interactions of predictor variables on overall IES-R scores.
| Fixed Effects | Model AIC | |||
|---|---|---|---|---|
|
|
||||
| Effect | B | SE b | 95% CI | |
| 2820.86 | ||||
| IES Baseline | .28 | .04 | .21, .36 | |
| Vividness | −.20 | .33 | −.86, .45 | |
| Highest SUDS | .11 | .02 | .06, .16 | |
| 2820.64 | ||||
| IES Baseline | .28 | .03 | .20, .35 | |
| Vividness | −.11 | .33 | −.77, .55 | |
| Highest SUDS | .10 | .02 | .06, .15 | |
| Vividness x Session | −.19 | .12 | −.44, .06 | |
| 2814.79 | ||||
| IES Baseline | .29 | .02 | .22, .37 | |
| Vividness | −1.18 | .45 | −2.07, −.28 | |
| Highest SUDS | .10 | .02 | .05, .15 | |
| Gender | 1.41 | 1.30 | −1.15, 3.98 | |
| Vividness x Gender | 1.94 | .63 | .69, 3.20 | |
Note. SE = standard error. CI = confidence interval. AIC = Akaike’s Information Criterion. IES-R = Impact of Event Scale-Revised. CAPS = Clinician Administered PTSD Scale. SUDS = Subjective Units of Distress Scale. CIs that do not include zero indicate significant predictors. Vividness ratings are square root transformed and, therefore, produce Bs with signs in the opposite direction from what is indicated.
Discussion
This is the first known study to examine the impact of imagery vividness on outcomes of trauma-focused exposure therapy for PTSD in a sample of both men and women with comorbid substance use disorders. This is a unique sample in which to investigate the impact of vividness given the high comorbidity between PTSD and substance use disorders as well as the potential impact that comorbid substance use disorders could have on vividness ability and on the vividness-outcome association. A number of noteworthy findings emerged from this study.
First, there were associations between imagery vividness ratings and paired SUDS ratings, and this relation changed over the course of treatment. Specifically, in support of Hypothesis 1, vividness ratings were less likely to predict SUDS further into treatment. This finding is consistent with Rauch and colleagues (2004), who understood the finding in the context of SUDS improving over time during treatment while vividness remained high. Second, although session (i.e., point in course of treatment) was not significantly related to vividness, a session by gender interaction indicated that vividness decreased for women over sessions more so than for men. This is consistent with prior studies showing that individuals diagnosed with PTSD and social phobia reported decreased vividness following rescripting techniques of distressing memories (Hackmann et al., 2004; Wild, Hackmann, & Clark, 2007). Although an overall decrease in vividness was not found in this sample, vividness ratings in the Rauch et al. (2004) study also remained high over treatment. With regard to the finding that women showed greater decreases in vividness over sessions than men, Niedzwienska (2003) did not find gender differences in vividness among individuals in a non-clinical sample who were asked to write about their three most vivid memories. However, the author did find that women included more detail as well as more emotional content than men in their memories. Thus, it is possible that as the reduction in emotional salience of the memory occurs over the course of PTSD treatment, women’s ability or willingness to produce vivid images, compared to men, may be impacted. However, such a result requires further scientific inquiry.
Third, in support of Hypothesis 2, imagery vividness was negatively associated with post-treatment total CAPS symptom severity above and beyond the impact of SUDS. Moreover, vividness later in treatment was more strongly associated with outcome than vividness ratings earlier on in treatment. Within the context of Emotional Processing Theory, this finding might suggest that greater vividness ratings reflect greater engagement with the trauma memory, which in turn, leads to greater symptom reduction through greater activation and correction of the fear structure and restructuring of the fear structure (Foa, Dancu, Hembree, Jaycox, & Street, 1999; Rothbaum, Meadows, Resick, & Foy, 2000). However, as discussed below, there may be important gender differences in this effect, as well as a session effect for when achieving high vividness may be most beneficial. With regards to the relationship between vividness and the assessment of total PTSD symptoms by the IES-R, vividness did not predict outcome in the initial analytic model, contrary to Hypothesis 2. There are several available outcome measures for assessing PTSD symptomatology and treatment responsiveness. The CAPS is known to provide a gold standard clinician-based assessment of PTSD while the IES-R is a self-report measure of trauma-related symptoms. In the current study, findings that were statistically significant using one measure of PTSD and not significant while using the other require replication and should be interpreted with caution. However, since the CAPS is a clinician-based diagnostic interview and considered to be more reliable for this reason, current findings outlining associations between vividness and PTSD outcome using the CAPS can likely be considered to be stronger evidence.
Fourth, gender played a role in the relationship between imagery vividness and PTSD outcome, but this was also dependent on what assessment of PTSD outcome was used. Gender was related to total CAPS symptom severity post-treatment such that men showed slightly higher symptom severity than did women. However, the relationship between vividness and PTSD treatment outcomes did not differ by gender. When the IES-R was examined, vividness only became a statistically significant predictor of outcome when the gender by vividness interaction was introduced into the model, and this interaction was also significant. Specifically, vividness was associated with fewer symptoms on the IES-R at follow-up for men, while for women it was related to greater symptoms. In sum, findings by gender related to treatment outcome and vividness were mixed, and a number of possibilities might explain the discrepancies (e.g., variations in PTSD symptom profiles, type of index trauma, assessment measures used). For example, the IES-R (DSM-IV) does not map on exactly to symptoms of PTSD and thus, may also capture non-specific psychological distress that may interact differently with vividness in men and women.
Fifth, given that participants were in early abstinence from alcohol and illicit drugs, it is possible that imagery vividness might be negatively impacted in this sample. The average vividness rating of 81.1/100 in the current study compares favorably with vividness ratings across six sessions of imaginal exposure reported in Rauch et al. (2004). In Rauch et al., mean vividness ratings ranged from 88.5 in the first session to 78.5 in the sixth session of imaginal exposure. The similarity in vividness ratings between these two samples suggest that populations with both PTSD and substance use disorders can also elicit vivid images during treatment. It is noteworthy that Rauch et al. (2004) posited that their null findings between vividness ratings and treatment outcome may have been due to a restricted range of vividness ratings among their participants. However, the average vividness rating was also high in the current study despite significant findings between vividness and outcome. This demonstrates the need for future investigation of the association between imagery vividness and PTSD treatment outcome in a variety of samples, situations, and types of trauma exposures in order to elucidate when collecting these ratings might be most helpful versus neutral.
Clinical Implications
The current findings have several potential clinical implications. This study showed that vividness ratings predicted treatment outcome (according to the CAPS) above and beyond the impact of SUDS. Thus, monitoring vividness ratings throughout treatment may be helpful such that clinicians can work with patients to enhance imagery vividness, particularly in later sessions of imaginal exposure. Further, assessing vividness ratings might be especially helpful with patients who are not as emotive or expressive in session. It is also recommended that clinicians be particularly aware of the gender differences in the relationship between vividness and outcome. Specifically, on the IES-R, a negative relationship was found among men between vividness and post-treatment symptom severity, whereas a positive relationship between these constructs was identified for women. While noteworthy, this finding requires scientific replication.
Additionally, clinicians may expect patients to report SUDS and imagery vividness ratings that are more highly correlated in earlier sessions as compared to later sessions. Given that vividness ratings predicted PTSD outcome according to the CAPS (but not the IES-R), clinicians might consider being mindful of vividness scores when developing and assessing their treatment plans and outcome tracking. For example, clinicians with patients who are having difficulty eliciting vivid images during treatment might review the guidelines by Foa et al. (2007) on how to increase engagement with the patient and/or practice improving mental clarity of the trauma memory before proceeding. Finally, concern about the impact of substance abuse on cognitive functioning, specifically clients’ ability to produce vivid images of their trauma, is largely mitigated by data from the current study that suggests patients with co-occurring PTSD and substance dependence can produce trauma images of similar vividness to PTSD patients without a substance use disorder.
Limitations
The current study has some limitations that need to be considered when interpreting the results. The sample consisted of individuals diagnosed with comorbid PTSD and substance use disorders. Thus, while additional research is required with this sample due to high comorbidity rates, generalizing these findings to a PTSD-only diagnosed sample should be done so with caution. It is unclear if any of the results can be attributed to factors specific to substance use disorder. Finally, the vast majority of the sample had not been exposed to combat-related traumas and none of the participants listed a combat-related trauma as their worst traumatic event, which should be considered when generalizing the findings to combat service members and veterans.
Conclusions and Future Directions
The present study’s findings highlight the importance of gathering vividness ratings throughout the course of imaginal exposure. From this, a number of future research directions are also suggested. First, the sample used in this study was unique in that all participants had a comorbid substance use disorder in addition to PTSD. Scientific replication is required among different populations of individuals with PTSD (e.g., combat service members and veterans). Second, additional empirical questions remain to be answered. It is necessary, for example, to examine whether the relationship between vividness and PTSD outcome is in fact linear, or whether there is an optimal level of vividness whereby therapeutic benefit weakens when vividness ratings are too low or too high (Rauch et al., 2004). If vividness continues to show a link to treatment outcome, future research needs to examine how it can be increased or reduced depending on the patient’s needs. Overall, continuing to examine the processes within exposure-based treatments that predict greater treatment outcome for PTSD is needed, with emotional engagement of the trauma memory, as assessed by imagery vividness, being one of these potential mechanisms of change.
Highlights.
Imagery vividness was lower in men than in women and maintained greater stability over treatment in men.
Imagery vividness was a weaker predictor of SUDS as treatment progressed.
Imagery vividness was negatively related to CAPS scores post-treatment, particularly in later sessions.
Imagery vividness was related to fewer symptoms on the IES-R for men and higher symptoms for women.
Acknowledgments
This research was supported in part by a grant from the National Institute on Alcohol Abuse and Alcoholism (R01AA016816, PI: S. Coffey).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD Scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Brady KT, Back SE, Coffey SF. Substance abuse and posttraumatic stress disorder. Current Directions in Psychological Science. 2004;3:206–209. [Google Scholar]
- Bryant RA, Harvey AG. Visual imagery in posttraumatic stress disorder. Journal of Traumatic Stress. 2006;9:613–619. doi: 10.1007/BF02103670. [DOI] [PubMed] [Google Scholar]
- Campos A. Gender differences in imagery. Personality and Individual Differences. 2014;59:107–111. [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. Discovering statistics using SPSS. Sage Publications Limited; 2009. [Google Scholar]
- Foa EB, Dancu CV, Hembree EA, Jaycox LH, Meadows EA, Street GP. A comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. Journal of Consulting and Clinical Psychology. 1999;67:194–200. doi: 10.1037//0022-006x.67.2.194. [DOI] [PubMed] [Google Scholar]
- Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional processing of traumatic experiences. New York: Oxford University Press; 2007. [Google Scholar]
- Foa EB, Keane TM, Friedman MJ, Cohen J. Effective treatments for PTSD: Practice guidelines from the International Society for Traumatic Stress Studies. 2. New York: The Guilford Press; 2008. [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychological Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- Foa EB, Riggs DS. Post-traumatic stress disorder in rape victims. In: Oldham J, Riba MB, Tasman A, editors. American Psychiatric Press Review of Psychiatry. Vol. 12. Washington, DC: American Psychiatric Press; 1993. pp. 273–303. [Google Scholar]
- Foa EB, Rothbaum BO. Treating the trauma of rape. Cognitive-behavior therapy for PTSD. Guilford Press; New York: 1998. [Google Scholar]
- Glass JM, Buu A, Adams KM, Nigg JT, Puttler LI, Jester JM, Zucker RA. Effects of alcoholism severity and smoking on executive neurocognitive function. Addiction. 2009;104:38–48. doi: 10.1111/j.1360-0443.2008.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackmann A, Ehlers A, Speckens A, Clark DM. Characteristics and content of intrusive memories in PTSD and their changes with treatment. Journal of Traumatic Stress. 2004;17:231–240. doi: 10.1023/B:JOTS.0000029266.88369.fd. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Litz BT, Weathers FW. Social anxiety, depression, and PTSD in Vietnam veterans. Journal of Anxiety Disorders. 2002;17:573–582. doi: 10.1016/s0887-6185(02)00227-x. [DOI] [PubMed] [Google Scholar]
- Hox JJ. Multilevel analysis: Techniques and applications. Taylor & Francis; 2010. [Google Scholar]
- Hyer K, Brown LM. The Impact of Event Scale—Revised. American Journal of Nursing. 2008;108:60–67. doi: 10.1097/01.NAJ.0000339101.39986.85. [DOI] [PubMed] [Google Scholar]
- Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: A quantitative review of the evidence. Journal of Clinical and Experimental Neuropsychology. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- Karatzias T, Power K, Brown K, McGoldrick T. Vividness of mental imagery in posttraumatic stress disorder (PTSD): The role of depression. Journal of Behavior Therapy and Experimental Psychiatry. 2009;40:352–358. doi: 10.1016/j.jbtep.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder: The burden to the individual and to society. Journal of Clinical Psychiatry. 2000;61:4–12. [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, Thompson PM, Brody AL, Geaga JA, Hong MS, Hayashi KM, Rawson RA, Ling W. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biological Psychiatry. 2005;58:770–778. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Marks DF. Visual imagery differences in the recall of pictures. British Journal of Psychology. 1973;64:17–24. doi: 10.1111/j.2044-8295.1973.tb01322.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Kilbanski A, Rauch SL, et al. The influence of gonadal hormones on conditioned fear extinction in health humans. Neuroscience. 2010;168(3):652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwienska A. Gender differences in vivid memories. Sex Roles. 49(7/8):321–331. [Google Scholar]
- Olff M, Langeland W, Draijer N, Gersons BPR. Gender differences in posttraumatic stress disorder. Psychological Bulletin. 2007;133(2):183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa EB. A meta-analytic review of Prolonged Exposure for posttraumatic stress disorder. Clinical Psychology Review. 2010;30(6):635–641. doi: 10.1016/j.cpr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Rauch SAM, Foa EB, Furr JM, Filip JC. Imagery vividness and perceived anxious arousal in Prolonged Exposure treatment for PTSD. Journal of Traumatic Stress. 2004;17:461–465. doi: 10.1007/s10960-004-5794-8. [DOI] [PubMed] [Google Scholar]
- Richardson JTE. Gender differences in the Vividness of Visual Imagery Questionnaire: A meta-analysis. Journal of Mental Imagery. 1995;19:177–187. [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM. Diagnostic Interview Schedule for the DSM-IV (DIS-IV) St. Louis, MO: Washington University; 2000. [Google Scholar]
- Rothbaum Meadows. 2000 [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychology Review. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: A review. Current Drug Abuse Reviews. 2008;1:81–98. doi: 10.2174/1874473710801010081. [DOI] [PubMed] [Google Scholar]
- Stark R, Wolf OT, Tabbert K, Kagerer S, Zimmerman M, Kirsch P, et al. Influence of the stress hormone cortisol on fear conditioning in humans: Evidence for sex differences in the response of the prefrontal cortex. NeuroImage. 2006;32(3):1290–1298. doi: 10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Walker EA, Katon W, Russo J, Ciechanowski P, Newman E, Wagner AW. Health care costs associated with posttraumatic stress disorder symptoms in women. Archives of General Psychiatry. 2003;60:369–374. doi: 10.1001/archpsyc.60.4.369. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JRT. Clinician-Administered PTSD scale: a review of the first ten years of research. Depression and Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Weiss DS, Marmar CR. The Impact of Event Scale—Revised. In: Wilson J, Keane T, editors. Assessing psychological trauma and PTSD. New York, NY US: Guilford Press; 1997. pp. 399–411. [Google Scholar]
- Weiss DS. The impact of events scale: Revised. Cross-Cultural Assessment of psychological Trauma and PTSD: International and Cultural Psychology Series. 2004:219–238. [Google Scholar]
- Wild J, Hackmann A, Clark DM. When the present visits the past: Updating trauma memories in social phobia. Journal of Behavior Therapy and Experimental Psychiatry. 2007;38:386–401. doi: 10.1016/j.jbtep.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe J. Psychotherapy by reciprocal inhibition. Oxford England: Stanford University Press; 1958. [Google Scholar]