Abstract
Sclerotic skin manifestations of chronic graft-versus-host disease (ScGVHD) lead to significant morbidity, including functional disability from joint range of motion (ROM) restriction. No superior second-line therapy has been established for steroid-refractory disease. Imatinib mesylate is a multi-kinase inhibitor of several signaling pathways implicated in skin fibrosis with in vitro antifibrotic activity. We performed an open label pilot Phase 2 trial of imatinib in children and adults with corticosteroid refractory ScGVHD. Twenty patients were enrolled in a 6 month trial. Eight received a standard dose (adult: 400 mg daily; children: 260 mg/m2 daily). Due to poor tolerability, 12 additional patients underwent a dose escalation regimen (adult: 100 mg daily initial dose up to 200 mg daily maximum; children initial dose 65 mg/m2 daily up to 130 mg/m2 daily). Fourteen patients were evaluable for primary response, improvement in joint range of motion (ROM) deficit, at 6 months. Primary outcome criteria for partial response (PR) was met in 5/14 (36%), stable disease (SD) in 7/14 (50%), and progressive disease (PD) in 2/14 (14%) patients. Eleven (79%) patients, including 5 PR and 6 with SD, demonstrated a positive gain in ROM (range 3–94% improvement in deficit). Of 13 patients with measurable changes at 6 months, the average improvement in ROM deficit was 24.2% (IQR: 15.5% to 30.5%; p=0.011).
This trial is registered at http://clinicaltrials.gov as NCT007020689.
Introduction
Sclerotic-type chronic graft-versus-host disease (ScGVHD) of the skin is characterized by progressive fibrosis of the dermis and subcutaneous tissues. ScGVHD is generally a late manifestation of chronic graft-versus-host disease (cGVHD), typically developing > 1 year after allogeneic hematopoietic cell transplantation.1 It develops in approximately 15% of patients with cGVHD, but poses a disproportionate challenge to management.1,2 In an NIH cGVHD natural history study patients with ScGVHD had been treated with an average of 4.7 prior therapies, compared to 2.8 therapies for patients with non-sclerotic cGVHD.3 ScGVHD may lead to skin pain, ulceration, restricted chest wall expansion, diminished joint range of motion (ROM) and contractures leading to functional disability, and is second only to bronchiolitis obliterans as a cause of severe cGVHD-related morbidity.4
Topical treatments, phototherapy, extracorporeal photopheresis (ECP), and systemic immunosuppressive agents have been employed for treatment of ScGVHD. Topical therapies are limited by poor drug penetration to deep fibrotic tissues. Phototherapy, (ultraviolet [UV]B and psoralen and UV-A [PUVA]), is similarly hampered by lack of UV penetration to deep dermal tissues. UVA-1 phototherapy utilizes long wavelength UVA light that penetrates into the dermis, and has shown efficacy for ScGVHD in several small case series, but is only available at a limited number of medical centers in the United States.5–7 Extracorporeal photopheresis (ECP) is also limited by local availability and cost.8 To date, no single or combination salvage regimen has demonstrated superior efficacy for ScGVHD.
Imatinib mesylate represents a novel targeted approach to the management of ScGVHD through inhibition of specific signaling pathways implicated in skin fibrosis. Imatinib has inhibitory activity against platelet derived growth factor (PDGF) receptor, among other tyrosine kinases. Elevated PDGF and its receptor have been found in the skin and bronchoalveolar lavage fluid in patients with systemic sclerosis.9,10 Stimulatory PDGF-receptor antibodies have been described in patients with systemic sclerosis11 and extensive chronic GVHD12, suggesting a direct mechanistic link to skin fibrosis via the PDGF pathway; however, the pathogenic significance of these antibodies remains unclear.13
Nevertheless, pre-clinical models of fibrosis in the skin and lungs suggest a therapeutic benefit of imatinib on tissue fibrosis.14 In light of these findings, we conducted a pilot phase 2 study to determine its therapeutic activity using a multi-modality assessment approach including strict response criteria that correlate with clinically meaningful functional improvement.
Patients, materials, and methods
Adult and pediatric patients (≥4 years) with a history of ScGVHD, according to NIH Consensus Group Criteria,15 were enrolled at the NIH Clinical Center (clinicaltrials.gov identifier: NCT00331968) between December 2008 and February 2011. The primary objective was clinical improvement in ScGVHD as measured by change in range of motion (ROM) of one or more joints significantly limited by skin fibrosis. Secondary objectives were: imatinib tolerability in patients with ScGVHD, responsiveness and utility of outcome criteria for ScGVHD evaluation using multi-modality assessments (MRI, skin scoring, patient-reported outcomes [PRO] measures), functional assessments and evaluate biomarkers of disease activity, assess steady state levels of imatinib, and evaluate the response of other cGVHD organ manifestations. The research protocol was approved by NCI Institutional Review Board and all participants provided informed written consent. Imatinib was provided to the NCI under a collaborative agreement between Novartis and the Division of Cancer Treatment and Diagnosis, NCI.
The study design was a single arm, open label trial with the primary endpoint measured at 6 months. Eight patients were initially treated with 400 mg (adults) or 260 mg/m2 (children) imatinib daily in cohort 1. However, due to poor tolerability and the need for dose reduction to manage adverse effects in all patients, the protocol was amended and a second cohort of 12 patients were enrolled and treated using intra-patient dose escalation, in which imatinib was initiated at 100 mg (adults) and 65 mg/m2 (children) daily and increased to a maximum of 200 mg and 130 mg/m2 daily after one month as tolerated (cohort 2). All patients (or parents) were required to keep a daily medication log and symptom diary. Inclusion criteria included age ≥ 4, biopsy-proven ScGVHD resulting in ROM restriction ≥ 25% of normal range at one or more joints, disease refractory to systemic corticosteroids (1 mg/kg/day × 14 days) or patients with stable disease but for whom systemic steroids or calcineurin inhibitors could not be tapered without disease flare, Karnofsky/Lansky ≥ 60%, absolute neutrophil count (ANC) ≥ 1,000/μL, platelet count ≥ 50,000/μL, total bilirubin < 3x upper limit of normal (ULN), transaminase < 5x ULN, normal age-adjusted renal function or creatinine clearance ≥ 60/mL/min/1.73m2, and normal cardiac function. Exclusion criteria were clinically significant systemic illness, including active infection, pregnant or breast-feeding females or females unwilling to practice birth control during and for two months after treatment, HIV, active HBV or HCV, persistent malignancy, ongoing chemotherapy, radiation or immunotherapy, prior treatment with imatinib or other tyrosine kinase inhibitor after transplant, hypersensitivity to imatinib, known brain metastases, and concurrent investigational treatment for cGVHD, including ECP. Eligible patients may not have received monoclonal antibody therapy within 6 weeks of enrollment. To minimize drug-drug interactions, patients taking potent inhibitors or inducers of P450 CYP3-A4 were excluded.
Skin involvement was assessed by comprehensive dermatologic examination by a dermatologist with expertise in cGVHD (E.W.C) and quantified by separate body surface area (BSA) assessments of epidermal and fibrotic tissue involvement (ScGVHD). Clinical evidence of ScGVHD was determined by the presence of skin thickening, rippling or nodularity of subcutaneous tissues upon deep palpation and ROM limitation. Histologic confirmation of ScGVHD was obtained by 6 mm punch skin biopsy. Joint involvement was determined by a physiatrist with expertise in cGVHD who performed joint ROM measurements, grip strength, and 2 and 6 minute walk tests. Joint ROM was compared to percent-predicted (% predicted) ROM for each joint using values established by the American Academy of Orthopedic Surgeons.16
NIH cGVHD organ severity (range 0–3) was graded by a transplant clinician with expertise in cGVHD (K.B.) using the NIH Consensus Criteria.15 The average score for each patient was calculated by dividing the total score by 7 domains in men (skin, eye, oral, joint, GI, hepatic, pulmonary), or 8 domains (including gynecologic) in women.17 The NIH global score was graded “mild,” “moderate,” or “severe” by consensus at a multidisciplinary meeting.15 Other subspecialty evaluations included oral medicine (Schubert scale), ophthalmology (Schirmer’s scoring, eye examination), occupational therapy and gynecology (female patients). In addition, the NIH Consensus Response Criteria (Form A/Form B) were assessed at each time point and organ responses as per NIH Consensus Response Criteria were calculated at the 6 month time point.18
Additional evaluations included pulmonary function testing, MRI at the site of sclerotic skin involvement, and 10 PRO or performance-based measures of symptoms, functional status, and QoL measures: Pincer strength (PS),19 36 item Manual Ability Measure (MAM-36),20 Jebsen-Taylor Hand Function Test (JTHFT),21 Disabilities of the Arm, Shoulder and Hand (DASH)22, Grooved Pegboard (GP),23 Assessment of Motor and Process Skills (AMPS),24 Human Activity Profile (HAP),25,26 Lee Chronic GVHD Symptom Scale,27 Short Form-36 Health Survey version 2 (SF-36),28,29 and cGVHD Activity Assessment-Patient Self Report.18 Steady state plasma imatinib concentrations were assessed prior to start of treatment and after 1 and 3 months on study in cohort two.
The primary endpoint for response to therapy was assessed at 6 months. Skin scoring and ROM assessment was performed at baseline and every three months thereafter. Up to three ‘target’ joints with ≥ 25% ROM reduction associated with skin fibrosis at baseline were included in the primary outcome; however, if fewer than three joints were involved, a single joint or the average ROM loss from 2 affected joints was used. Joints were prioritized by the most significant functional limitations, excluding joints with confounding reasons for decreased ROM. Similar ROM assessments have been employed to measure clinical response to treatment of radiation-induced skin fibrosis.30,31 The average percentage change in ROM deficit from baseline to 6 months was obtained based on the number of degrees of ROM change (6 months)/total ROM deficit (baseline) at each joint, and response was defined as > 25% or greater improvement in the deficit. Progression was defined as > 25% decline in ROM deficit (confirmed by a repeat evaluation 2–4 weeks later) or > 1 steroid pulse during a three month period. All others who did not meet the above criteria for progression or response (i.e. between 25% gain and 25% loss in ROM) were considered stable disease. Maximal response was no further improvement over two sequential three-month evaluations. Tapering of immunosuppression was allowed for patients stable or improving after 6 weeks of therapy.
Biomarker studies
Immunophenotyping
For analysis of T lineage subsets, thawed PBMC (107 cells/mL) were stimulated with PMA (100 ng/mL) (Sigma) and ionomycin (1 ug/mL) (Sigma) for 6 hours at 37° C, adding Golgistop and Golgiplug (BD Biosciences) after 2 hours. Following stimulation, cells were stained with CD3 and CD4, fixed and permeabilized (eBioscience) for intracellular staining with antibodies against Tbet, IFNγ IL-4 (BD Biosciences), FOXP3, IL-17 (eBioscience), IL-13, IL-22 (Biolegend) and isotope controls, and analyzed on a Gallios flow cytometer (Beckman Coulter).
Plasma TGF-β1 and Phospho-Smad 2
The concentration of total TGF-β1 in plasma was measured using a human TGF-β1 Quantikine ELISA kit (R&D Systems, Minneapolis, MN) following acid activation of samples according to the manufacturer’s instructions. The extent of platelet degranulation in plasma samples was determined using an Imunoclone platelet factor 4 (PF4) ELISA kit from American Diagnostica (Stamford, CT). Phospho-Smad2 localization in skin sections was detected by immunohistochemistry as previously described.32
B cell activation assays
For analysis of phosphorylation of BLNK and Syk, cryopreserved PBMCs were thawed and allowed to rest overnight (5 × 106 cells/mL), as previously described.33 1 × 106 cells were stimulated with 5 μg/mL of the F(ab′)2 fragment of IgM (Jackson Immunoresearch) for 5 min at 37 °C. Following stimulation, cells were immediately fixed (BD cytofix buffer), permeabilized (BD Perm Buffer III), and stained using antibodies from BD Biosciences: BLNK (pY84, clone J117-1278) or Syk (pY348, clone 1120-722), PLCγ (pY759) and BTK (pY223). As only four patients had sufficient numbers of viably frozen PBMCs to evaluate BCR signaling alterations, results for this assay are reported in the supplemental section.
Statistical analysis
The initial goal of this pilot study was to enroll 10 evaluable patients in order to assess the change in ROM from baseline to 6 months. With this as the one planned primary endpoint, 10 patients would have provided 80% power for a two-tailed 0.05 alpha level test to detect 1.0 SD change in ROM from baseline to 6 months. A paired t-test was to be used to evaluate the change if the data were normally distributed. If the data were not normally distributed (p<0.05 by a Shapiro-Wilks test), then a Wilcoxon signed rank test was to be used instead. To allow for patient ‘drop out’ due to disease progression, recurrent malignancy, compliance issues, unacceptable toxicity, or the need for additional systemic therapy for cGVHD prior to the 6 month evaluation, enrollment of up to 13 patients was allowed. As this was a small, one-armed pilot trial, there was no control for natural history or regression to the mean, but ScGVHD is typically static or progressive and does not remit or improve spontaneously.
The initial statistical design was revised because none of the initial 8 patients tolerated the 400 mg dose. Efficacy in the 200 mg escalation cohort was thus evaluated, in accordance with the original power calculations, in a second cohort of up to 10 evaluable patients enrolled. With 8 patients from the higher dose level and up to 13 total patients from the lower dose level, the final sample size was amended to include up to 21 patients, to allow for a small number of non-evaluable patients.
Secondary measures included toxicity, lung manifestations, biomarkers and PRO and performance-based endpoints. All secondary endpoints were considered exploratory and thus adjustment of p values to control family-wise error rates was not performed. Differences in secondary outcomes from baseline to 6 months were determined by a paired t-test after confirming normality of the differences.
Results
Demographics and clinical characteristics
The general demographics and transplant history of the study population is shown in Table 1. The majority were Caucasian (n= 17) and male (n=14), the median age was 51.5 years (range 7–60), median time from transplant was 55 months (range 12.7–121), and median time from cGVHD diagnosis was 40 months (range 4.8–112.9). Eight patients were enrolled in cohort 1 and twelve in cohort 2. cGVHD-specific characteristics of the study population are shown in Table 2. Patients were using a median of two immunosuppressive agents (range 0–2) and 13 were on steroids at the time of enrollment.
Table 1.
Patient Demographics
| Subject characteristics | N=20 |
|---|---|
| Age, median years (range) | 51.5 (7–60) |
| Gender | 30% female/70 % male |
| Months from Transplant, median (range) | 55.4 (12.7 – 121) |
| Months from cGVHD Dx, median (range) | 39.85 (4.8–112) |
| Myeloablative regimen | 55 % |
| Donor match (6/6) | 90 % |
| Donor source | |
| BM | 10 % |
| PB | 90 % |
| Cord | 0 % |
| Ethnicity | |
| Caucasian | 90 % |
| African American | 5 % |
| Hispanic | 5 % |
| cGVHD category | |
| Overlap | 5 % |
| Classic | 95 % |
| Late acute | 0 % |
| cGVHD presentation | |
| De novo | 30 % |
| Quiescent | 20 % |
| Progressive | 50 % |
| Global NIH cGVHD score | |
| Mild (1) | 0 % |
| Moderate (2) | 0 % |
| Severe (3) | 100 % |
Table 2.
Baseline cGVHD Characteristics
| Pt # | Age | Sex | Average NIH cGVHD Score at baseline | cGVHD affected organs at baseline | Evaluable joints at baseline | Concomitant ISM | % BSA moveable sclerosis (baseline) | % BSA nonmoveable sclerosis (baseline) | Baseline ROM % (of predicted) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | M | 1.28 | 5 | 3 | Pred/tacro | 57.15 | 7.56 | 37% |
| 2 | 60 | M | 1.14 | 4 | 3 | Pred | 2.7 | 56.7 | 56% |
| 3 | 10 | F | 0.75 | 3 | 2 | MPred/MTX | 6.3 | 36.9 | 73% |
| 4 | 52 | F | 1.0 | 4 | 3 | Pred/siro | 0.18 | 19.98 | 7% |
| 5 | 30 | M | 1.48 | 6 | 3 | Pred/siro/tacro | 8.1 | 77.94 | 34% |
| 6 | 51 | M | 1.86 | 7 | 3 | MPred/tacro/siro/MMF | 59.4 | 0 | 47% |
| 7 | 55 | F | 1.90 | 7 | 3 | Pred/siro/MMF | 29.7 | 32.4 | 61% |
| 8 | 58 | M | 1.57 | 6 | 3 | Tacro | 15.3 | 36.9 | 35% |
| 9 | 60 | M | 1.71 | 7 | 3 | Pred/tacro/siro | 3.33 | 9 | 42% |
| 10 | 53 | M | 1.43 | 5 | 3 | Tacro/MMF | 0 | 8.28 | −7% |
| 11 | 28 | F | 1.75 | 7 | 3 | Pred/siro/MMF | 82.08 | 0 | 56% |
| 12 | 56 | M | 1.43 | 6 | 3 | Tacro/MMF | 12.6 | 8.64 | 37% |
| 13 | 34 | F | 1.38 | 6 | 3* | Pred/tacro | 1.8 | 71.1 | 27% |
| 14 | 46 | M | 1.29 | 5 | 3 | Pred/MMF | 5.4 | 31.5 | 32% |
| 15 | 55 | M | 1.43 | 6 | 3 | Pred/siro | 49.77 | 10.8 | 22% |
| 16 | 55 | M | 0.86 | 2 | 1 | Siro/MMF | 0 | 9.54 | 71% |
| 17 | 7 | F | 1.13 | 4 | 3 | MPred/tacro/MMF | 84.24 | 0 | 64% |
| 18 | 21 | M | 1.57 | 4 | 3 | Siro | 15.84 | 4.5 | 54% |
| 19 | 48 | M | 1.14 | 4 | 3 | Tacro/MMF | 9.45 | 11.7 | 33% |
| 20 | 18 | M | 2.00 | 7 | 3 | None | 0 | 23.4 | −5% |
ISM = immunosuppressive medication, Pred = prednisone, MPred = methylprednisolone, MTX = methotrexate, siro = sirolimus, tacro = tacrolimus, MMF = mycophenolate mofetil.
Note: Pt #13 started with 3 measureable joints at enrollment on study. She experienced an episode of acute shoulder pain while on study and evaluation revealed avascular necrosis of that shoulder. Therefore, only 2 joints were used in the final analysis.
Almost all patients (n = 18) had three affected joints with >25% ROM deficit for composite ROM analysis. One patient had two affected joints and one had a single joint used for ROM assessment. Ankle dorsiflexion was the most commonly affected joint (n=18), followed by shoulder abduction (n=15), shoulder flexion (n = 8), wrist extension (n = 6), wrist flexion (n = 6), ankle plantar flexion (n=2), hip internal rotation (n =1), and knee flexion (n = 1). The median of the average NIH cGVHD score was 1.41 (range 0.75–2.00), with a median of 5.5 cGVHD-affected organs, (range 2–7).
Primary outcome: change in range of motion
Of the 20 participants enrolled, 14 (70%) were evaluable for primary endpoint response at 6 months. Of the six patients that did not make it to the 6 month primary endpoint analysis, four patients voluntarily withdrew prior to 6 months, one was removed for toxicity and one was removed for leukemic relapse. All patients were included in the toxicity analysis. Two patients (#3 and #17) experienced progressive disease (PD), one at the 3 month time point (Table 3). Five patients demonstrated a partial response (PR) with > 25% improvement in ROM deficit; and 7 patients had stable disease. Six of the 7 stable patients had a positive gain in ROM deficit (range 3–22%) but did not meet the 25% threshold for partial response. Of 13 patients with measurable ROM changes at 6 months, the mean increase in ROM was 24.2% of the previous deficit (IQR: 15.5% to 30.5%; p=0.011 by paired t-test). Among the 11 patients overall that demonstrated ROM improvement, the average gain in ROM was 31% of the previous ROM deficit.
Table 3.
Primary Endpoint Measures
| Pt. | Target Joints | Total Baseline ROM deficit (degrees) | Baseline ROM (% Pred) | Response* 3 month | Response* 6 month | Total 6 month ROM deficit (degrees) | Response | Final Dose |
|---|---|---|---|---|---|---|---|---|
| 2 | Wrist FL (2), ankle PF | 71 | 56% | 69% | 94% | 4 | PR | 300 mg |
| 3 | Ankle DF (2) | 11 | 73% | −93% | Off-study Progression | Off-study-Progression | PD | 300 mg |
| 7 | Wrist EX, wrist FL(2) | 80 | 61% | 45% | 35% | 50 | PR | 200 mg |
| 8 | Shoulder AB, shoulder FL, ankle DF | 169 | 35% | 13% | 16% | 150 | SD^ | 200 mg |
| 10 | Ankle DF (2), Ankle PF | 83 | −7% | 11% | 21% | 66 | SD^ | 200 mg |
| 12 | Shoulder AB, shoulder FL, ankle DF | 123 | 37% | 11% | 16% | 106 | SD^ | 100 mg |
| 13 | Hip IR, ankle DF | 38 | 27% | 65% | 61% | 17 | PR | 200 mg |
| 14 | Shoulder AB, shoulder FL, ankle DF | 150 | 32% | 17% | 27% | 108 | PR | 200 mg |
| 15 | Shoulder AB (2), ankle DF | 191 | 22% | 24% | 22% | 158 | SD^ | 200 mg |
| 16 | Knee FL | 39 | 71% | 13% | 3% | 38 | SD | 200 mg |
| 17 | Shoulder AB (2), shoulder FL | 193 | 64% | 12% | −25% | 241 | PD | 100 mg |
| 18 | Wrist EX (2) | 60 | 54% | 6% | −2% | 59 | SD | 200 mg |
| 19 | Shoulder FL, shoulder AB, ankle DF | 128 | 33% | 22% | 31% | 90 | PR | 200 mg |
| 20 | Shoulder AB, wrist EX, ankle DF | 205 | −5% | 6% | 15% | 174 | SD^ | 200 mg |
AB: abduction, DF: dorsiflexion, Ex: extension, FL: flexion, IR: internal rotation, PL: plantarflexion
(2) indicates bilateral joints assessed
Based on % improvement in each joint deficit compared to baseline/# joints
Did not reach 25% improvement threshold for PR, but patient experienced ROM improvement with functional gains.
Patients were unable to reach ‘neutral’ position at one or more target joints and, therefore, had negative ROM at baseline.
Secondary outcome measures
Of the 14 subjects included in the primary endpoint analysis, 13 were evaluable for secondary endpoint assessments (1 patient progressed prior to the 6 month evaluation and was taken off study). There was no significant change in average NIH cGVHD score (p=0.73 by paired t-test), NIH cGVHD Provider Global Rating Score (p=0.47), Lung Function Score (p=0.29), or other cGVHD organ manifestations as per the NIH cGVHD organ response criteria, including skin score, at 6 months (Table 4); however, several patients showed a visible change in skin texture and anecdotally reported skin softening and improved flexibility despite a lack of significant change in ScGVHD BSA (Figure 1). ROM was not significantly associated with any functional or patient reported outcomes, including grip strength, walk times, the HAP,25,26 Lee Chronic GVHD Symptom Scale,27 SF-36,28,29 and cGVHD Activity Assessment-Patient Self Report.18 Overall, 8 patients were able to reduce immunosuppression within the first 6 months, 5 patients with stable disease had no change in immunosuppression, and 1 patient with progression required an increase in systemic therapy (Table 4).
Table 4.
Select cGVHD Response Criteria Measures
| Pt. # | Imatinib ROM Response (6 mo.) | Total Skin Score (Baseline) | Total Skin Score (6 mo.) | NIH cGVHD Provider Global Rating Score (Baseline) | NIH cGVHD Provider Global Rating Score (6 mo.) | LFS (Baseline) | LFS (6 mo.) | Change in Immunosuppression |
|---|---|---|---|---|---|---|---|---|
| 2 | PR | 66.6 | 54 | 5 | 4 | 2 | 2 | ↓ Pred 20mg QD to 5mg QOD |
| 3 | PD | 43.38 | n/a | 3 | n/a | 9 | n/a | ↓ MPred 16mg QOD to 4mg QOD |
| 7 | PR | 66.24 | 55.53 | 6 | 8 | 3 | 8 | ↓Pred: 24mg QD to 20mg QD |
| 8 | SD^ | 53.1 | 55.26 | 6 | 7 | 5 | 6 | No change |
| 10 | SD^ | 10.8 | 6.12 | 5 | 4 | 3 | 3 | No change |
| 12 | SD^ | 21.24 | 30.06 | 6 | 7 | 4 | 5 | ↓Tacro 2mg QAM 1.5mg QPM to 0.5mg BID |
| 13 | PR | 79.2 | 62.28 | 6 | 4 | 4 | 4 | Pred↓ 25 mg QD to 15mg QD; Tacro ↓ 2mg BID to 1mg BID |
| 14 | PR | 39.96 | 40.5 | 7 | 6 | 5 | 5 | Pred↓ 2.5mg QD to 2.5mg QOD |
| 15 | SD^ | 71.46 | 61.83 | 7 | 6 | 7 | 6 | ↓Siro: 2mg QD to 1mg QD |
| 16 | SD | 9.54 | 8.1 | 5 | 4 | 3 | 2 | No change |
| 17 | PD | 84.96 | 85.11 | 6 | 8 | 5 | 6 | Pred wean then ↑ to 12.5 mg BID; Tacro↑1.0 BID to 1.5mg BID |
| 18 | SD | 26.64 | 24.3 | 8 | 8 | 8 | 8 | No change |
| 19 | PR | 21.15 | 37.8 | 8 | 5 | 9 | 9 | MMF ↓ 1gm/BID to d/c′d |
| 20 | SD^ | 23.4 | 25.2 | 8 | 8 | 2 | 2 | No change |
LFS = lung function score, Pred = prednisone, MPred = methylprednisolone, MTX = methotrexate, siro = sirolimus, tacro = tacrolimus, MMF = mycophenolate mofetil, d/c′ed = discontinued.
Did not reach 25% improvement threshold for PR, but patient experience ROM improvement with functional gain
Figure 1. Improvement in skin tightness following imatinib therapy.

(A) Baseline. (B) After 6 months of treatment, there is increased pigmentation but marked reduction in rippled appearance of the skin (dose 200 mg daily). (C) End of active treatment (9 months), there is persistent pigmentation but overall marked improvement in ROM. (D) One year after ending active treatment, patient continues to have increased softening of skin fibrosis and reduction in hyperpigmentation (partial response, pt. 7).
Baseline and follow-up MRI studies were obtained on 10 patients (Supplemental Table 1). The majority of patients exhibited abnormalities in the skin, subcutis, fascia, or muscle at baseline. Comparison studies at 6 month demonstrated persistent/stable MRI findings in most patients, including 3 of 4 patients who met criteria for partial response.
Adverse events
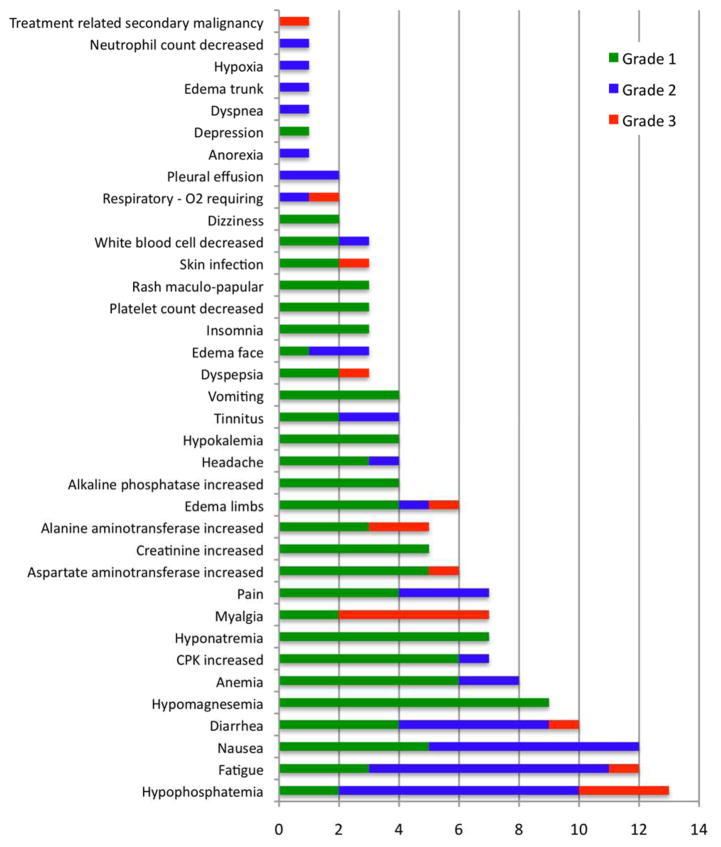
All 20 patients enrolled were included in the analysis for adverse events. Imatinib was generally poorly tolerated at the 400 mg dose and following dose reduction to 300 mg. Hypophosphatemia was most frequently observed, occurring in 13/20 patients (65%) and requiring oral supplementation in most individuals (Figure 2). Other adverse reactions experienced by at least 50% of participants included fatigue (60%), nausea (60%), and diarrhea (50%). The most clinically significant adverse event was disrupted fluid homeostasis in 60% of patients (12/20). Six patients developed limb edema (1 Grade III), 3 developed facial edema, 1 had trunk edema, and 2 patients developed pleural effusions, one of whom required hospitalization and supplemental oxygen. Edema also exacerbated pre-existing pain in sclerotic areas and appeared to preferentially collect centrally, presumably due to hidebound skin that restricted peripheral fluid collection. Notably, several patients complained of worsening muscle symptoms, particularly pain/cramping (7), myalgias (7) and CPK elevations (5 Grade 1, 1 Grade 2). Another clinically significant AE was tinnitus (n=4), which has been previously reported with imatinib therapy.34 Of note, AEs appeared to be dose related and patients re-challenged at lower doses generally tolerated treatment better. All of the patients that experienced significant grade 2–3 edema or fluid disturbance that required discontinuation of treatment were receiving the 400 mg daily dose of imatinib. In addition, in the second cohort of subjects who received 100 mg for 1 month, then increased to 200 mg daily, several AEs increased in either severity or frequency with the increase in dose. For example, many patients experienced low grade hypophosphatemia during the first month, which increased in severity with the dose increase. Gastrointestinal side effects (nausea, vomiting, diarrhea), rarely reported at the 100 mg dose level, were common after the increase to 200 mg. In contrast, AEs such as fatigue and muscle cramping frequently started at the lower dose of 100 mg daily, but appeared to subside over time.
Figure 2. Adverse events.
Waterfall plot reveals adverse reactions (AEs) to imatinib in patients with ScGVHD (n =20). Green bars represent grade 1 AEs, blue bars represent grade 2 AEs, and orange bars represent grade 3 AEs.
Steady state serum levels of imatinib therapy in ScGVHD
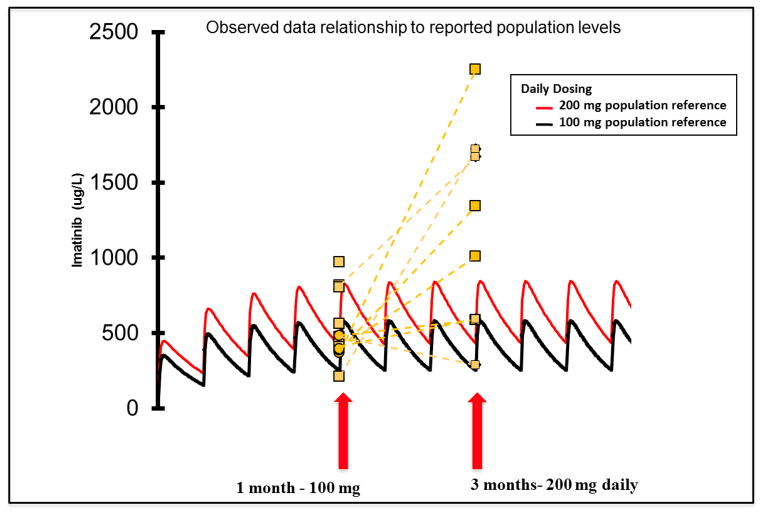
Because of the poor tolerability at the 400 mg daily dose level, imatinib serum levels were measured in the second cohort to determine whether higher than anticipated drug levels were associated with the increased toxicity observed in cohort 1. Serum samples were drawn at 1 month (100 mg) and 2 months (200 mg) on treatment. Steady state serum imatinib concentrations from 8 patients at the 200 mg daily dose ranged from 592–2255 ng/mL (mean 1157 ng/mL), which is within the inhibitory range of the drug on PDGFR (IC50 range ~0.1–0.3μM).
Interestingly, 6/8 samples at the 200 mg dose exceeded the population reference drug level for the 200 mg dose (Figure 3). In three patients, the observed drug level was > 2 fold higher than expected by the population reference. One of these patients was a 7 year old subject receiving 100 mg (~150 mg/m2). Although she had the highest measured imatinib serum level (2255 ug/L), she reported only grade 1–2 AEs (mostly gastrointestinal), with the exception of grade 3 transaminitis possibly related to imatinib. Drugs known to interfere with imatinib metabolism were not permissible while on study, suggesting that polypharmacy or decreased hepatic drug metabolism may increase the imatinib in the cGVHD setting. One patient (#18) suspected of poor drug compliance had non-detectable levels at both time points.
Figure 3. Pharmacokinetics of imatinib treatment in patients with ScGVHD.
Data for steady state serum levels in 8 patients receiving imatinib treatment in cohort 2. Levels are superimposed on 100 mg and 200 mg daily dose population controls, show in black and red, respectively. Patients with ScGVHD show much higher than expected levels at the 200 mg dosing level. 1 patient had no detectable levels at either time point (not shown).
TGF-β studies
Levels of TGF-β1 in plasma were normalized to PF4 levels to account for TGF-β1 derived from platelet degranulation during sample preparation. The normalized levels showed no obvious correlation to outcomes, although levels in most patients were slightly decreased at 3 months and rose again at 6 months (Supplemental Figure 1). IHC staining of skin for phospho-Smad2, a marker for activation of the TGF-β signaling pathway, showed no appreciable change pre- and post- treatment (Supplemental Table 2).
Immunophenotyping
Although the number of patient samples was small, no discernable pattern or change in frequency or absolute numbers of the Treg, Th1, Th17 or Th2 lymphocyte populations was noted (Supplemental Figure 2).
Discussion
To our knowledge, this is the first prospective clinical trial of imatinib specifically for treatment of sclerotic skin cGVHD, although several studies have evaluated the use of imatinib in the steroid refractory cGVHD setting.35–37 Using ROM improvement as a surrogate marker of disease response obviates many of the limitations associated with skin scoring of cGVHD identified in previous studies, which rely on clinician scoring or BSA measurement in which numerical improvement may not be clinically meaningful. In fact, several patients in the current study reported subjective improvement in skin softening but failed to demonstrate a decrease in overall body surface area involvement, consistent with a qualitative rather quantitative response that is difficult to capture using currently recommended skin scoring systems.
To date, there is conflicting evidence regarding the efficacy and tolerability of imatinib for steroid-refractory ScGVHD. Two early European case series reported response rates of 50%38 and 79%35, respectively, and more recently, improved overall survival in patients responding to imatinib therapy.36 In contrast, a larger recent retrospective review of 39 cases of treatment-refractory ScGVHD treated with imatinib showed limited responses and poor tolerability.39 The authors found a 30% overall response rate and only one complete response. Additionally, they found high rates of fluid retention, similar to the present study. Poor tolerability and limited responses were also reported in a small pilot for pulmonary cGVHD40 and a Phase 1 dose escalation study for steroid refractory cGVHD.37 Responses were seen in 40% of patients, including 4 of 6 patients with ScGVHD. As in these prior studies, our study, which required sclerotic skin involvement, showed imatinib to be poorly tolerated. Many of the AEs encountered manifested as worsening of pre-existing baseline symptoms such as muscle cramping, tinnitus and edema. Acral edema was particularly uncomfortable in patients with hidebound skin and several patients also experienced central fluid shifts (trunk, pulmonary, pleural).
A major goal of the current trial was to use an outcome measure that would be sensitive to change and represent meaningful clinical improvement. For this reason, joint ROM at a markedly restricted joint, measured by an experienced physiatrist, was chosen, along with a battery of functional and QoL measures designed to fully characterize disease burden and response. Given that the NIH referral population is enriched for patients with refractory and long-standing skin disease, we believe that the ROM improvements shown represent meaningful benefit in a subset of patients. It is possible that the drug would show greater efficacy if initiated closer to the time of onset of skin fibrosis, rather than after a prolonged period of joint restriction. Furthermore, the trajectory of improvement in skin fibrosis is slow and, therefore, subtle improvements in skin softening may be difficult to accurately quantify. It is unclear why the various PRO and performance scales did not reflect changes in ROM. It is conceivable that in a resilient population with long-standing ROM restriction, that even if patients could perform tasks of daily living more easily after treatment, these changes may not be adequately captured on the scales employed.
ScGVHD is characterized by variable areas of skin involvement at different tissue depths, posing a challenge for accurate assessment in clinical trials based on skin pliability alone. In addition, edema is a frequent finding in GVHD, particularly in patients with active fibrosis, which can be difficult to differentiate from skin fibrosis, and which can be further complicated by fluid shifts caused by imatinib treatment. For example, patient 7 had a partial ROM response, but had little change in affected BSA. Nevertheless, the patient described significant improvement and clinical photographs showed an appreciable change in the rippled appearance of her skin over time (Figure 1). MRI has been proposed as a tool to assess deep-seated sclerotic changes.41 The majority of patients who underwent MR imaging demonstrated abnormalities in the skin, subcutaneous tissue or fascia. However, these findings remained stable at 6 months, even in patients with significant ROM improvement, suggesting that MR imaging may not be sufficiently sensitive to change for use as response tool in the clinical trial setting.
The inclusion of steady state imatinib serum concentrations in this study provides the first insight into the poor tolerability of the drug described in several reports in the cGVHD setting.37,39 In some instances, serum levels at the 200 mg dose level were several fold higher than expected. Therefore, we conclude that dosing at the 400 mg dose could lead to toxic levels in these patients. This patient population is invariably on multiple medications, which cumulatively may significantly inhibit imatinib metabolism.
In our study, evaluation of TGF-β1 plasma levels by ELISA did not correlate with outcomes and IHC staining of skin tissue samples for phospho-SMAD2 similarly showed no appreciable change over time. Consistent with a role of B lymphocytes in the induction of fibrosis in dermal fibroblasts in systemic sclerosis,42 our B-cell activation studies showed preliminary evidence that decreased B-cell signaling may correlate with disease response. However, given the small number of patients, additional testing is required to confirm these findings.
Because of the pilot nature of this study, primary responses were determined at 6 months and long-term efficacy requires further study. However, all patients were contacted 1 year after study completion for follow up. The outcomes and duration of responses were quite variable. Several subjects that had improvement in ROM continued to do well, with no reported loss of ROM after discontinuation. Two patients that worsened within 2 months after imatinib discontinuation restarted drug and again experienced improvement in skin softness and range of motion. Of note, no patients died while on-study, but 2 patients died within 1 year of coming off-treatment (1 subject with PD; 1 with a PR). In conclusion, our findings suggest that treatment with imatinib may lead to a functionally meaningful improvement in joint range of motion in a subset of patients with treatment-refractory disease. Given the lack of a superior salvage therapy for ScGVHD, we believe that low-dose imatinib, given as part of a multi-therapy approach and given earlier in the course of disease, warrants further exploration in a larger, randomized study which incorporates the NIH joint/fascia scale, joint ROM, the Photographic ROM (P-ROM),43 and long term benefit as assessed by failure free survival.44
Supplementary Material
Supplementary Figure 1. Plasma TGF-β1 levels normalized to PF4 during treatment with imatinib.
Patients 2,5,7,8 (cohort 1) were initially treated with 400mg daily and all required dose reduction due to adverse events. Patients 8–20 began treatment at 100mg daily and increased to 200mg daily at one month.
Supplementary Figure 2. Lymphocyte immunophenotyping.
(A) Absolute and relative percentage (B) of Treg, Th1, Th17 and Th2 cells before imatinib therapy and after 6 months of treatment based on non-responders, partial responders, and progressive disease. (C) Cytokine assays failed to show a consistent pattern of expression or change during treatment with imatinib (patients 14 and 19, partial responders).
Supplementary Figure 3. B cell activation before and after imatinib.
A significant increase in B cell activation and B Cell Receptor (BCR) signaling via spleen tyrosine kinase (Syk) has been associated with active cGVHD. As imatinib is known to bind to Syk35 and attenuate BTK signaling in CML, we used a phosphoflow assay to study four patients with sufficient numbers of viably frozen PBMCs to determine if BCR signaling was altered in treated patients. Intracellular staining of Syk, BLNK, PLCγ and BTK, molecules is known to be stimulated sequentially after BCR engagement. Samples were taken before, three, and/or nine months after start of imatinib therapy. Total PBMC were thawed and rested before stimulation with anti-μ for 5 minutes. The parent gate was CD19+ B cells and the positive staining gate for each phosphoantibody was set based on the unstimulated controls (not shown). The frequency of Syk, BLNK, PLCγ, or BTK phosphorylation is marked by the rectangular gates.
The proportion of B cells with phosphorylation of Syk and 3 downstream molecules (BLNK, PLCg and BTK), tended to decrease in 2/3 patients tested who achieved a PR. Also consistent with findings in CML patients, BTK activation was decreased by 23–41% in patients 19 and 13 after imatinib administration. In 2/3 patients with a PR, BCR signaling decreased, despite decreasing immunosuppression in these patients. Together, these findings suggest that imatinib may affect Syk-mediated BCR signaling in ScGVHD B cells.
Supplemental Table 1. MRI Imaging of ScGVHD.
Supplemental Table 2. Phospho-Smad2 staining of ScGVHD skin
Highlights.
We prospectively studied imatinib mesylate in steroid-refractory sclerotic-type chronic GVHD.
Primary endpoint was improvement in joint range-of-motion (ROM) at 6 months.
ROM improved in 11/14 evaluable patients (range 3–94% improvement).
Imatinib was poorly tolerated at the 400mg dose.
Imatinib serum levels were higher than expected at the 200mg dose.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institutes of Health, Center for Cancer Research, National Cancer Institute and the Cancer Therapy Evaluation Program. Special thanks to the NIH Chronic GVHD Multidisciplinary Team and Christine Booher, who assisted in the data analysis of the occupational therapy-related outcomes. We also thank Dr. Jessica Allen for performing the B cell phosphoflow assay.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Penas PF, Jones-Caballero M, Aragues M, Fernandez-Herrera J, Fraga J, Garcia-Diez A. Sclerodermatous graft-vs-host disease: clinical and pathological study of 17 patients. Archives of dermatology. 2002;138(7):924–934. doi: 10.1001/archderm.138.7.924. [DOI] [PubMed] [Google Scholar]
- 2.Skert C, Patriarca F, Sperotto A, et al. Sclerodermatous chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: incidence, predictors and outcome. Haematologica. 2006;91(2):258–261. [PubMed] [Google Scholar]
- 3.Martires KJ, Baird K, Steinberg SM, et al. Sclerotic-type chronic GVHD of the skin: clinical risk factors, laboratory markers, and burden of disease. Blood. 2011;118(15):4250–4257. doi: 10.1182/blood-2011-04-350249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flowers ME, Parker PM, Johnston LJ, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100(2):415–419. doi: 10.1182/blood-2002-01-0011. [DOI] [PubMed] [Google Scholar]
- 5.Stander H, Schiller M, Schwarz T. UVA1 therapy for sclerodermic graft-versus-host disease of the skin. Journal of the American Academy of Dermatology. 2002;46(5):799–800. doi: 10.1067/mjd.2002.121352. [DOI] [PubMed] [Google Scholar]
- 6.Wetzig T, Sticherling M, Simon JC, Hegenbart U, Niederwieser D, Al-Ali HK. Medium dose long-wavelength ultraviolet A (UVA1) phototherapy for the treatment of acute and chronic graft-versus-host disease of the skin. Bone marrow transplantation. 2005;35(5):515–519. doi: 10.1038/sj.bmt.1704804. [DOI] [PubMed] [Google Scholar]
- 7.Grundmann-Kollmann M, Behrens S, Gruss C, Gottlober P, Peter RU, Kerscher M. Chronic sclerodermic graft-versus-host disease refractory to immunosuppressive treatment responds to UVA1 phototherapy. Journal of the American Academy of Dermatology. 2000;42(1 Pt 1):134–136. doi: 10.1016/s0190-9622(00)90023-9. [DOI] [PubMed] [Google Scholar]
- 8.Greinix HT, Worel N, Just U, Knobler R. Extracorporeal photopheresis in acute and chronic graft-versus-host disease. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2014 doi: 10.1016/j.transci.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Ludwicka A, Ohba T, Trojanowska M, et al. Elevated levels of platelet derived growth factor and transforming growth factor-beta 1 in bronchoalveolar lavage fluid from patients with scleroderma. J Rheumatol. 1995;22(10):1876–1883. [PubMed] [Google Scholar]
- 10.Klareskog L, Gustafsson R, Scheynius A, Hallgren R. Increased expression of platelet-derived growth factor type B receptors in the skin of patients with systemic sclerosis. Arthritis Rheum. 1990;33(10):1534–1541. doi: 10.1002/art.1780331011. [DOI] [PubMed] [Google Scholar]
- 11.Baroni SS, Santillo M, Bevilacqua F, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354(25):2667–2676. doi: 10.1056/NEJMoa052955. [DOI] [PubMed] [Google Scholar]
- 12.Svegliati S, Olivieri A, Campelli N, et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood. 2007;110(1):237–241. doi: 10.1182/blood-2007-01-071043. [DOI] [PubMed] [Google Scholar]
- 13.Dragun D, Distler JH, Riemekasten G, Distler O. Stimulatory autoantibodies to platelet-derived growth factor receptors in systemic sclerosis: what functional autoimmunity could learn from receptor biology. Arthritis Rheum. 2009;60(4):907–911. doi: 10.1002/art.24364. [DOI] [PubMed] [Google Scholar]
- 14.Gordon J, Spiera R. Imatinib and the treatment of fibrosis: recent trials and tribulations. Current rheumatology reports. 2011;13(1):51–58. doi: 10.1007/s11926-010-0146-6. [DOI] [PubMed] [Google Scholar]
- 15.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Burrows H American Academy of Orthopedic Surgeons. Joint Motion: Method of Measuring and Recording. Chicago, IL: American Academy of Orthopedic Surgeons; 1965. [Google Scholar]
- 17.Baird K, Steinberg SM, Grkovic L, et al. National Institutes of Health chronic graft-versus-host disease staging in severely affected patients: organ and global scoring correlate with established indicators of disease severity and prognosis. Biol Blood Marrow Transplant. 2013;19(4):632–639. doi: 10.1016/j.bbmt.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant. 2006;12(3):252–266. doi: 10.1016/j.bbmt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. The Journal of hand surgery. 1984;9(2):222–226. doi: 10.1016/s0363-5023(84)80146-x. [DOI] [PubMed] [Google Scholar]
- 20.Chen CC, Bode RK. Psychometric validation of the Manual Ability Measure-36 (MAM-36) in patients with neurologic and musculoskeletal disorders. Archives of physical medicine and rehabilitation. 2010;91(3):414–420. doi: 10.1016/j.apmr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Sears ED, Chung KC. Validity and responsiveness of the Jebsen-Taylor Hand Function Test. The Journal of hand surgery. 2010;35(1):30–37. doi: 10.1016/j.jhsa.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixon D, Johnston M, McQueen M, Court-Brown C. The Disabilities of the Arm, Shoulder and Hand Questionnaire (DASH) can measure the impairment, activity limitations and participation restriction constructs from the International Classification of Functioning, Disability and Health (ICF) BMC musculoskeletal disorders. 2008;9:114. doi: 10.1186/1471-2474-9-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YC, Magasi SR, Bohannon RW, et al. Assessing dexterity function: a comparison of two alternatives for the NIH Toolbox. Journal of hand therapy: official journal of the American Society of Hand Therapists. 2011;24(4):313–320. doi: 10.1016/j.jht.2011.05.001. quiz 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merritt BK. Validity of using the Assessment of Motor and Process Skills to determine the need for assistance. The American journal of occupational therapy: official publication of the American Occupational Therapy Association. 2011;65(6):643–650. doi: 10.5014/ajot.2011.000547. [DOI] [PubMed] [Google Scholar]
- 25.Herzberg PY, Heussner P, Mumm FH, et al. Validation of the human activity profile questionnaire in patients after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16(12):1707–1717. doi: 10.1016/j.bbmt.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Daughton DM, Fix AJ, Kass I, Bell CW, Patil KD. Maximum oxygen consumption and the ADAPT quality-of-life scale. Archives of physical medicine and rehabilitation. 1982;63(12):620–622. [PubMed] [Google Scholar]
- 27.Lee S, Cook EF, Soiffer R, Antin JH. Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8(8):444–452. doi: 10.1053/bbmt.2002.v8.pm12234170. [DOI] [PubMed] [Google Scholar]
- 28.Bevans MF, Mitchell SA, Barrett AJ, et al. Function, adjustment, quality of life and symptoms (FAQS) in allogeneic hematopoietic stem cell transplantation (HSCT) survivors: a study protocol. Health and quality of life outcomes. 2011;9:24. doi: 10.1186/1477-7525-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 30.Okunieff P, Augustine E, Hicks JE, et al. Pentoxifylline in the treatment of radiation-induced fibrosis. J Clin Oncol. 2004;22(11):2207–2213. doi: 10.1200/JCO.2004.09.101. [DOI] [PubMed] [Google Scholar]
- 31.Simone NL, Soule BP, Gerber L, et al. Oral pirfenidone in patients with chronic fibrosis resulting from radiotherapy: a pilot study. Radiation oncology. 2007;2:19. doi: 10.1186/1748-717X-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Figueroa JD, Flanders KC, Garcia-Closas M, et al. Expression of TGF-beta signaling factors in invasive breast cancers: relationships with age at diagnosis and tumor characteristics. Breast cancer research and treatment. 2010;121(3):727–735. doi: 10.1007/s10549-009-0590-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen JL, Tata PV, Fore MS, et al. Increased BCR responsiveness in B cells from patients with chronic GVHD. Blood. 2014;123(13):2108–2115. doi: 10.1182/blood-2013-10-533562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ando Y, Tsunoda T, Beck Y, Takayama T, Tahara H. Effect of imatinib (STI571) on metastatic gastrointestinal stromal tumors: report of a case. Surgery today. 2005;35(2):157–160. doi: 10.1007/s00595-004-2890-6. [DOI] [PubMed] [Google Scholar]
- 35.Olivieri A, Locatelli F, Zecca M, et al. Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood. 2009;114(3):709–718. doi: 10.1182/blood-2009-02-204156. [DOI] [PubMed] [Google Scholar]
- 36.Olivieri A, Cimminiello M, Corradini P, et al. Long-term outcome and prospective validation of NIH response criteria in 39 patients receiving imatinib for steroid-refractory chronic GVHD. Blood. 2013;122(25):4111–4118. doi: 10.1182/blood-2013-05-494278. [DOI] [PubMed] [Google Scholar]
- 37.Chen GL, Arai S, Flowers ME, et al. A phase 1 study of imatinib for corticosteroid-dependent/refractory chronic graft-versus-host disease: response does not correlate with anti-PDGFRA antibodies. Blood. 2011;118(15):4070–4078. doi: 10.1182/blood-2011-03-341693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magro L, Mohty M, Catteau B, et al. Imatinib mesylate as salvage therapy for refractory sclerotic chronic graft-versus-host disease. Blood. 2009;114(3):719–722. doi: 10.1182/blood-2009-02-204750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Masson A, Bouaziz JD, Peffault de Latour R, et al. Limited efficacy and tolerance of imatinib mesylate in steroid-refractory sclerodermatous chronic GVHD. Blood. 2012;120(25):5089–5090. doi: 10.1182/blood-2012-09-453928. [DOI] [PubMed] [Google Scholar]
- 40.Stadler M, Ahlborn R, Kamal H, et al. Limited efficacy of imatinib in severe pulmonary chronic graft-versus-host disease. Blood. 2009;114(17):3718–3719. doi: 10.1182/blood-2009-07-231159. author reply 3719–3720. [DOI] [PubMed] [Google Scholar]
- 41.Clark J, Yao L, Pavletic SZ, et al. Magnetic resonance imaging in sclerotic-type chronic graft-vs-host disease. Archives of dermatology. 2009;145(8):918–922. doi: 10.1001/archdermatol.2009.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francois A, Chatelus E, Wachsmann D, et al. B lymphocytes and B-cell activating factor promote collagen and profibrotic markers expression by dermal fibroblasts in systemic sclerosis. Arthritis research & therapy. 2013;15(5):R168. doi: 10.1186/ar4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inamoto Y, Pidala J, Chai X, et al. Assessment of joint and fascia manifestations in chronic graft-versus-host disease. Arthritis & rheumatology. 2014;66(4):1044–1052. doi: 10.1002/art.38293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inamoto Y, Flowers ME, Sandmaier BM, et al. Failure-free survival after initial systemic treatment of chronic graft-versus-host disease. Blood. 2014;124(8):1363–1371. doi: 10.1182/blood-2014-03-563544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Plasma TGF-β1 levels normalized to PF4 during treatment with imatinib.
Patients 2,5,7,8 (cohort 1) were initially treated with 400mg daily and all required dose reduction due to adverse events. Patients 8–20 began treatment at 100mg daily and increased to 200mg daily at one month.
Supplementary Figure 2. Lymphocyte immunophenotyping.
(A) Absolute and relative percentage (B) of Treg, Th1, Th17 and Th2 cells before imatinib therapy and after 6 months of treatment based on non-responders, partial responders, and progressive disease. (C) Cytokine assays failed to show a consistent pattern of expression or change during treatment with imatinib (patients 14 and 19, partial responders).
Supplementary Figure 3. B cell activation before and after imatinib.
A significant increase in B cell activation and B Cell Receptor (BCR) signaling via spleen tyrosine kinase (Syk) has been associated with active cGVHD. As imatinib is known to bind to Syk35 and attenuate BTK signaling in CML, we used a phosphoflow assay to study four patients with sufficient numbers of viably frozen PBMCs to determine if BCR signaling was altered in treated patients. Intracellular staining of Syk, BLNK, PLCγ and BTK, molecules is known to be stimulated sequentially after BCR engagement. Samples were taken before, three, and/or nine months after start of imatinib therapy. Total PBMC were thawed and rested before stimulation with anti-μ for 5 minutes. The parent gate was CD19+ B cells and the positive staining gate for each phosphoantibody was set based on the unstimulated controls (not shown). The frequency of Syk, BLNK, PLCγ, or BTK phosphorylation is marked by the rectangular gates.
The proportion of B cells with phosphorylation of Syk and 3 downstream molecules (BLNK, PLCg and BTK), tended to decrease in 2/3 patients tested who achieved a PR. Also consistent with findings in CML patients, BTK activation was decreased by 23–41% in patients 19 and 13 after imatinib administration. In 2/3 patients with a PR, BCR signaling decreased, despite decreasing immunosuppression in these patients. Together, these findings suggest that imatinib may affect Syk-mediated BCR signaling in ScGVHD B cells.
Supplemental Table 1. MRI Imaging of ScGVHD.
Supplemental Table 2. Phospho-Smad2 staining of ScGVHD skin