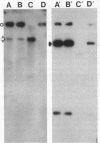
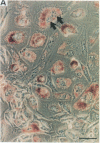
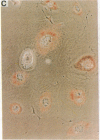
Abstract
To learn about adipose differentiation of precursors from postnatal adipose tissue of lean and massively obese subjects, human omental adipocyte precursor-murine renal adenocarcinoma cell (RAG) hybrids were formed by fusion with polyethylene glycol, and cultured selectively with 50 microM ouabain in hypoxanthine aminopterin thymidine (HAT) medium. Under conditions in which the parent cells did not differentiate, a number of hybrids, which were cloned, revealed morphologic and biochemical evidence of differentiation. In addition to activation of human genes within the common nucleus of the hybrids, murine cytoplasmic activators are probably also involved because heterocaryons (fused cells with two interspecific nuclei) revealed the same phenomenon. Hybrids composed of precursors from massively obese subjects disclosed more frequent and prominent differentiation. Since these hybrids, in contrast to those from the lean, recapitulate this phenomenon in subcultures, they provide the potential system for mapping the human gene(s) responsible for adipose differentiation and its exaggeration in massive obesity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blau H. M., Chiu C. P., Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983 Apr;32(4):1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Donjacour A. A., Sugimura Y. Stromal-epithelial interactions and heterogeneity of proliferative activity within the prostate. Biochem Cell Biol. 1986 Jun;64(6):608–614. doi: 10.1139/o86-084. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., Benda P. Regulation of specific functions of glial cells in somatic hybrids. II. Control of inducibility of glycerol-3-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1870–1877. doi: 10.1073/pnas.67.4.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. L., O'Malley K. A., Wheeler T. B. Polyethylene glycol-induced mammalian cell hybridization: effect of polyethylene glycol molecular weight and concentration. Somatic Cell Genet. 1976 May;2(3):271–280. doi: 10.1007/BF01538965. [DOI] [PubMed] [Google Scholar]
- Edwards Y., McMillan S. L., Kielty C., Shaw M. A. The expression of human glycerol-3-phosphate dehydrogenase in human/rodent somatic-cell hybrids. Biochem Genet. 1985 Jun;23(5-6):405–422. doi: 10.1007/BF00499083. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Geiser A. G., Der C. J., Marshall C. J., Stanbridge E. J. Suppression of tumorigenicity with continued expression of the c-Ha-ras oncogene in EJ bladder carcinoma-human fibroblast hybrid cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5209–5213. doi: 10.1073/pnas.83.14.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan T. V., Anderson W. F. Epigenetic activation of phenylalanine hydroxylase in mouse erythroleukemia cells by the cytoplast of rat hepatoma cells. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3932–3936. doi: 10.1073/pnas.76.8.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan T. V., Thompson E. B., Anderson W. F. Extinction of hemoglobin inducibility in Friend erythroleukemia cells by fusion with cytoplasm of enucleated mouse neuroblastoma or fibroblast cells. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1642–1646. doi: 10.1073/pnas.74.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975 May;5(1):19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Hoar D. I., Haslam D. B., Starozik D. M. Improved direct molecular diagnosis and rapid fetal sexing. Prenat Diagn. 1984 Jul-Aug;4(4):241–247. doi: 10.1002/pd.1970040402. [DOI] [PubMed] [Google Scholar]
- Iwakura Y., Nozaki M., Asano M., Yoshida M. C., Tsukada Y., Hibi N., Ochiai A., Tahara E., Tosu M., Sekiguchi T. Pleiotropic phenotypic expression in cybrids derived from mouse teratocarcinoma cells fused with rat myoblast cytoplasts. Cell. 1985 Dec;43(3 Pt 2):777–791. doi: 10.1016/0092-8674(85)90251-x. [DOI] [PubMed] [Google Scholar]
- Lau D. C., Roncari D. A., Hollenberg C. H. Release of mitogenic factors by cultured preadipocytes from massively obese human subjects. J Clin Invest. 1987 Feb;79(2):632–636. doi: 10.1172/JCI112859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mével-Ninio M., Weiss M. C. Immunofluorescence analysis of the time-course of extinction, reexpression, and activation of albumin production in rat hepatoma-mouse fibroblast heterokaryons and hybrids. J Cell Biol. 1981 Aug;90(2):339–350. doi: 10.1083/jcb.90.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlath G. K., Blau H. M. Expression of muscle genes in heterokaryons depends on gene dosage. J Cell Biol. 1986 Jan;102(1):124–130. doi: 10.1083/jcb.102.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncari D. A., Kindler S., Hollenberg C. H. Excessive proliferation in culture of reverted adipocytes from massively obese persons. Metabolism. 1986 Jan;35(1):1–4. doi: 10.1016/0026-0495(86)90087-9. [DOI] [PubMed] [Google Scholar]
- Russell T. R. Growth and cytodifferentiation of 3T3-L1 preadipocytes. Methods Enzymol. 1981;72:720–723. doi: 10.1016/s0076-6879(81)72062-7. [DOI] [PubMed] [Google Scholar]
- Rutter W. J., Pictet R. L., Morris P. W. Toward molecular mechanisms of developmental processes. Annu Rev Biochem. 1973;42:601–646. doi: 10.1146/annurev.bi.42.070173.003125. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Baker R. M. Isolation of mutants of cultured mammalian cells. Methods Cell Biol. 1973;6:209–281. doi: 10.1016/s0091-679x(08)60052-7. [DOI] [PubMed] [Google Scholar]
- Van R. L., Bayliss C. E., Roncari D. A. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J Clin Invest. 1976 Sep;58(3):699–704. doi: 10.1172/JCI108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van R. L., Roncari D. A. Complete differentiation of adipocyte precursors. A culture system for studying the cellular nature of adipose tissue. Cell Tissue Res. 1978 Dec 28;195(2):317–329. doi: 10.1007/BF00236728. [DOI] [PubMed] [Google Scholar]
- Weiss M. C., Chaplain M. Expression of differentiated functions in hepatoma cell hybrids: reappearance of tyrosine aminotransferase inducibility after the loss of chromosomes. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3026–3030. doi: 10.1073/pnas.68.12.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise L. S., Green H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J Biol Chem. 1979 Jan 25;254(2):273–275. [PubMed] [Google Scholar]