Abstract
Diffuse large B cell lymphoma (DLBCL) is a complex disease comprising diverse subtypes and genetic profiles. Possibly due to the prevalence of genetic alterations activating canonical NF-κB activity, a role for oncogenic lesions that activate the alternative NF-κB pathway in DLBCL has remained elusive. Here we show that deletion/mutation of TRAF3, a negative regulator of the alternative NF-κB pathway, occurs in ∼15% of DLBCLs, and that it often coexists with BCL6 translocation, which prevents terminal B cell differentiation. Accordingly, in a mouse model constitutive activation of the alternative NF-κB pathway cooperates with BCL6 deregulation in DLBCL development. This work demonstrates a key oncogenic role for the alternative NF-κB pathway in DLBCL development.
Introduction
DLBCL, the most common form of non-Hodgkin's lymphoma, is a genetically, phenotypically and clinically heterogeneous disease. Various DLBCL subtypes have been revealed by gene expression profile analysis using distinct classification schemes, that is according to their putative cell of origin or the coordinated expression of consensus clusters (Alizadeh et al., 2000; Monti et al., 2005). In the “cell of origin” classification, two main subtypes of DLBCL have been identified whose transcriptional programs resemble normal B cells at particular developmental stages. These are the germinal center B cell (GCB)-like DLBCL, presumably derived from a GC B cell, and the activated B cell (ABC)-like DLBCL, whose cell of origin is less clear but may correspond to a cell undergoing plasmacytic differentiation (Lenz and Staudt, 2010; Wright et al., 2003).
Analysis of the coding genome of DLBCL has identified various genetic lesions and revealed their association with the GCB or ABC subtype. Inactivating mutations and deletions of BLIMP1/PRDM1, a key gene in terminal B cell differentiation, are found exclusively in the ABC subtype (∼30% of cases) (Mandelbaum et al., 2010; Pasqualucci et al., 2006; Tam et al., 2006). Similarly BCL6 expression is deregulated by chromosomal translocation more frequently in the ABC (∼26% of cases) than in the GCB subtype, where BCL6 expression is high a priori (Iqbal et al., 2007; Mandelbaum et al., 2010; Pasqualucci et al., 2011). Interestingly, BCL6 translocations are mutually exclusive with BLIMP1 structural alterations in ABC-DLBCL (Mandelbaum et al., 2010). Given that BCL6 can directly suppress BLIMP1 expression (Tunyaplin et al., 2004), it has been hypothesized that Balthough BCL6 controls multible additional functions in GC B cells CL6 translocations represent an alternative mechanism for BLIMP1 inactivation in ABC-DLBCL, although BCL6 controls multiple additional functions in GC B cells (Mandelbaum et al., 2010). Another group of mutations promote constitutive NF-κB activation, such as those affecting TNFAIP3 (A20), CD79B and MYD88, predominantly in the ABC subtype (Compagno et al., 2009; Davis et al., 2010; Ngo et al., 2011; Pasqualucci et al., 2011), and CARD11 mutations occurring in both subtypes (Lenz et al., 2008; Pasqualucci et al., 2011).
Notably, NF-κB activating mutations in DLBCLs, including the ones described above, predominantly involve the NF-κB canonical pathway (Compagno et al., 2009; Davis et al., 2010; Lenz et al., 2008; Ngo et al., 2011; Pasqualucci et al., 2011; Staudt, 2010). As a consequence, a role for putative genetic lesions involving the NF-κB alternative pathway remained largely overlooked. Supporting a role of the NF-κB alternative pathway in DLBCL pathogenesis, ∼10% of DLBCLs were found to stain positive for NF-κB2 p52 but not NF-κB1 p50 in the nucleus, and another 20% of cases exhibited both NF-κB1 and NF-κB2 nuclear staining (Compagno et al., 2009); furthermore, a recent study revealed that roughly 10% of DLBCLs carry deletions or mutations of TRAF3 or TRAF2 (Pasqualucci et al., 2011). TRAF3 and TRAF2 control the degradation of NF-κB inducing kinase (NIK) and consequently restrain activation of the alternative NF-κB pathway (Gardam et al., 2008; Hacker et al., 2011; Sasaki et al., 2008).
While an oncogenic role for constitutive canonical NF-κB activity has been demonstrated in a mouse model of DLBCL (Calado et al., 2010), a functional link between the activation of alternative NF-κB pathway and the pathogenesis of DLBCL remained to be established. In this study, we performed complementary human and mouse studies to investigate mutations activating the alternative NF-κB pathway and concurrent genetic events, and developed a genetic system in the mouse to test the role of constitutive alternative NF-κB signaling in the pathogenesis of DLBCL.
Results
TRAF3 Gene Lesions Coexist with BCL6 Translocation in Human DLBCL
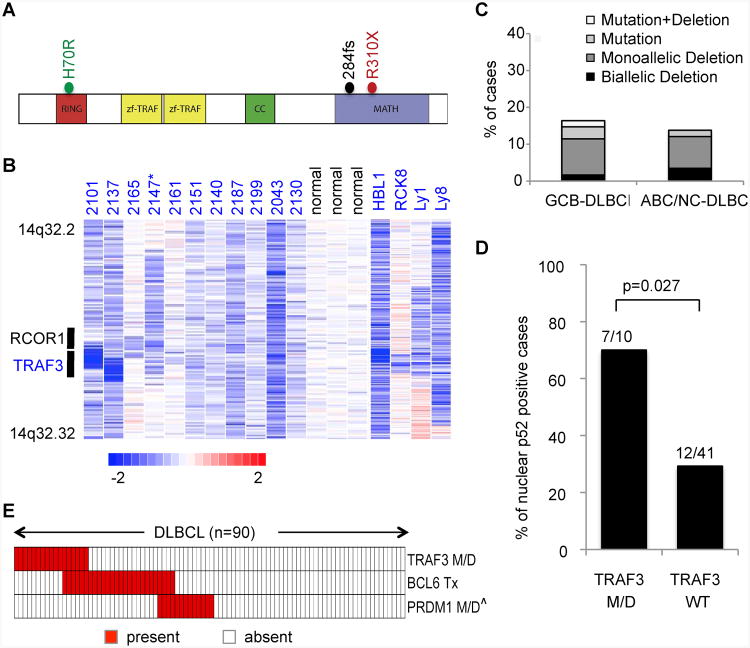
Deletions and mutations of TRAF3 have been found in human DLBCLs (Pasqualucci et al., 2011). To have a deeper look into TRAF3 genetic lesions and their distribution in DLBCL subtypes, we analyzed the TRAF3 sequences for the presence of point mutations and copy number aberrations in 119 DLBCL samples, including 98 biopsies and 21 cell lines whose phenotypic subtype was known. This analysis revealed missense, frameshift, and nonsense mutations (the two mutations tested being both somatic in origin) in functional domains, which are required for TRAF3 to negatively regulate NIK protein stability (Fig. 1 A; Annunziata et al., 2007; Hacker et al., 2011; He et al., 2007; Keats et al., 2007). Specifically, we identified one DLBCL case carrying a frameshift mutation (284fs) and one carrying a nonsense mutation (R310X), both of which are predicted to disrupt the MATH domain, required for the interaction between TRAF3 and NIK (Hacker et al., 2011; He et al., 2007). One additional DLBCL harbored a missense mutation (H70R) that may alter the function of the RING domain, required for the negative regulation of NIK by TRAF3 (He et al., 2007). Notably, a missense mutation affecting the H70 residue has been previously reported in a multiple myeloma patient (Keats et al., 2007). The present analysis also identified biallelic or monoallelic deletions involving the TRAF3 locus, including two focal homozygous losses that encompass TRAF3 and its neighboring gene RCOR1 (Figure 1B). A similar spectrum of deletions has been observed in human multiple myeloma and was shown to stabilize the NIK protein (Annunziata et al., 2007; Keats et al., 2007). TRAF3 deletions/mutations occurred similarly in GCB and ABC DLBCL (Figure 1C), and significantly correlated with alternative NF-κB activation, indicated by nuclear p52 staining (Figure 1D).
Figure 1. Recurrent TRAF3 and BCL6 Lesions in DLBCL.
(A) Diagram of the TRAF3 protein, with its relevant functional domains. The mutations found in DLBCL are indicated.
(B) Inferred log2 copy number (CN) data from representative DLBCL cases carrying deletions encompassing TRAF3, compared to normal controls. The position of TRAF3 and its neighboring gene RCOR1 is indicated. In the red-blue scale, white corresponds to a normal (diploid) CN log-ratio, blue is deletion and red is gain. *Case 2147 harbored a point mutation in the residual allele, leading to biallelic inactivation.
(C) Overall frequency of TRAF3 genetic lesions (point mutations and deletions) in DLBCL phenotypic subtypes. 47 GCB and 51 ABC/NC-DLBCL biopsies, 14 GCB and 7 ABC-DLBCL cell lines were included in this analysis (the primary tumors and cell lines of each DLBCL subtype displayed similar frequencies of TRAF3 lesions). Note that among the 119 samples, 29 lack copy number data, thus the frequencies shown may represent an underestimate.
(D) Percentage of DLBCL primary cases showing nuclear p52 staining, as a readout of non-canonical NF-κB activation, in TRAF3 M/D versus TRAF3 WT cases. The cutoff used to score cases as nuclear positive was ≥ 20% (Compagno et al., 2009).
(E) Relative distribution of genetic lesions affecting TRAF3, BCL6 and PRDM1 in individual DLBCLs. Each column represents one patient, with color codes indicating the presence or absence of the corresponding feature (Tx, translocation; M/D, mutations and/or deletions). Only the 90 samples with full information on TRAF3 M/D, BCL6 Tx, and PRDM1 M/D are included. ˆPRDM1 genetic lesions only include biallelic deletions or point mutations, since the functional significance of large 6q monoallelic deletions is unclear. Details of PRDM1 and BCL6 lesions have been described previously (Mandelbaum et al., 2010; Pasqualucci et al., 2006).
See also Figure S1.
We previously observed that constitutive canonical NF-κB activation promotes DLBCL development upon disruption of terminal B cell differentiation via inactivating BLIMP1 (Calado et al., 2010). With these observations in mind, we searched whether TRAF3 mutations in DLBCL associate with BCL6 or BLIMP1 genetic lesions, either of which would presumably disrupt terminal B cell differentiation (Mandelbaum et al., 2010). While none of the 17 DLBCLs carrying TRAF3 deletions/mutations exhibited biallelic BLIMP1 deletion/mutation, 6 of them (35%) had concurrent BCL6 translocation (the small number of cases analyzed did not provide statistical power to assess whether the co-occurrence is significant) (Figure 1E; Figure S1).
Taken together, these data show that roughly 15% of DLBCLs carry TRAF3 genetic alterations and that these lesions often coexist with BCL6 translocations.
Impact of Alternative NF-κB Activation and/or BCL6 Deregulation on the GC Reaction
To study the impact of enforced alternative NF-κB activation and BCL6 deregulation on the pathogenesis of DLBCL, we used a system of conditional gain- and/or loss-of-function mutagenesis in mice. Given that DLBCL arises from a GC or post-GC B cell (Shaffer et al., 2012), we decided to perform targeted mutagenesis in GC B cells, using the Cγ1-cre transgene, from which Cre is expressed in B cells at an early stage of the GC reaction (Casola et al., 2006). To induce activation of the alternative NF-κB pathway, we combined this transgene with a ROSA26 allele harboring a cDNA encoding NIK, preceded by a loxP flanked STOP cassette (hereafter called NikstopFL) (Sasaki et al., 2008). NIK expression from the mutant ROSA26 allele is indicated by a GFP reporter controlled by an internal ribosome entry site (Sasaki et al., 2008). For BCL6 deregulated expression, we used an HA-tagged BCL6 transgene inserted into the immunoglobulin (Ig) heavy chain locus downstream of Iμ promoter (hereafter called IμBcl6), mimicking the observed BCL6/IgH translocation in DLBCL (Cattoretti et al., 2005). To monitor Cre-mediated recombination in cells of compound mutant mice not carrying the NikstopFL allele, we used a conditional YFP reporter allele in the ROSA26 locus designated YFPstopFL (Srinivas et al., 2001). Mice carrying the Cγ1-cre and YFPstopFL alleles served as controls.
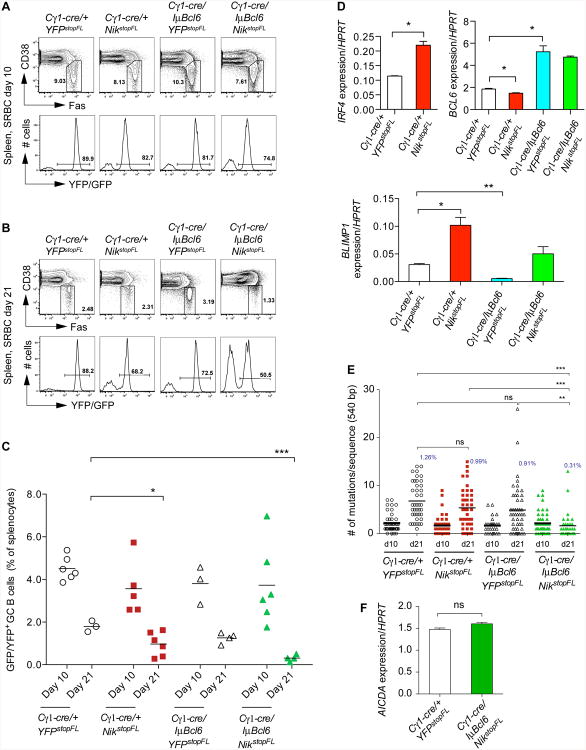
To test the impact of alternative NF-κB pathway activation, alone or together with enforced BCL6 expression, on GC B cell formation, we immunized experimental and control mice with sheep red blood cells (SRBCs). Analysis 10 days after immunization revealed expression of the reporter, GFP or YFP, in the majority of GC B cells, indicating efficient Cre-mediated recombination in mice of all genotypes analyzed (Figure 2A). Control mice and mice with enforced expression of NIK and/or BCL6 also showed similar fractions of GC B cells at day 10 after primary immunization (Figures 2A and 2C). However, at day 21 post-immunization, mice with enforced NIK expression and thus constitutive alternative NF-κB activation alone had a significantly reduced fraction of GC B cells compared to control mice (Figures 2B and 2C), similar to what is seen in mice with constitutive canonical NF-κB activation in GC B cells (Calado et al., 2010). Enforced NIK expression in GC B cells led to increased expression of IRF4, which might, in turn, account for upregulation of BLIMP1 and downregulation of BCL6 in these cells (Figure 2D; Saito et al., 2007; Sciammas et al., 2006). We considered the possibility that the premature termination of the GC reaction in mice with enforced NIK expression might be due to the altered expression of BLIMP1 or BCL6 (Calado et al., 2010; Martins and Calame, 2008; Ye et al., 1997), however, concomitant BLIMP1 deletion (data not shown) or BCL6 enforced expression did not prevent GC early termination in these mice (Figures 2B and 2C). We next looked if activation of the alternative NF-κB pathway alone or together with enforced BCL6 expression affects physiological processes of GC B cells, such as somatic hypermutation. At day 21 post-immunization, GC B cells from mice with enforced NIK or BCL6 expression alone carried slightly reduced numbers of somatic mutations in their Ig heavy chain (IgH) V regions, compared to controls, while those from mice with enforced expression of both NIK and BCL6 had significantly fewer mutations (Figure 2E). The reduced somatic mutation load in the latter group is likely due to premature termination of the GC reaction, as similar levels of somatic mutation and AICDA expression were detected in GC B cells from these mice and controls when analyzed at day 10 after immunization, the peak time of the GC reaction (Figures 2C, 2E and 2F). Collectively, these results demonstrate that constitutive activation of NF-κB signaling through enforced NIK expression negatively impacts the GC reaction and that this effect is independent of BCL6 regulation. Hence, constitutive NF-κB activation is incompatible with the maintenance of a GC B cell phenotype, and may in a similar way impact the phenotype of lymphoma cells arising in the context of the GC reaction (see Discussion).
Figure 2. Impact of Constitutive NIK and BCL6 Expression on the GC Reaction.
(A and B) Representative FACS analysis of splenic GC B cells at day 10 (A) and day 21 (B) after primary immunization with SRBCs, respectively. Upper panels show the GC B cell population (within the gate; CD19+FashiCD38lo). Lower panels show reporter expression in GC B cells.
(C) Summary of FACS analysis of GC B cells as in (A) and (B). Black bar represents mean for each genotype of mice at the indicated time points.
(D) Real-time PCR analysis of the expression levels of the indicated genes in reporter-positive GC B cells at day 10 after primary immunization with SRBCs. Values represent normalized levels to HPRT. Data are represented as mean ± standard error of the mean (SEM).
(E) IgH somatic mutation in reporter-positive GC B cells at day 10 (13-16 sequences per mouse from 2-3 mice per genotype) and day 21 (12-16 sequences per mouse from 2-3 mice per genotype) after primary immunization with SRBCs. Black bar represents mean. Average mutation frequency at day 21 is shown in graph.
(F) Real-time PCR analysis of AICDA transcript levels in reporter-positive GC B cells at day 10 after primary immunization with SRBCs. Values represent normalized levels to HPRT. Data are represented as mean ± SEM.
Enforced Activity of the Alternative NF-κB Pathway Enhances B Cell Proliferation and Survival
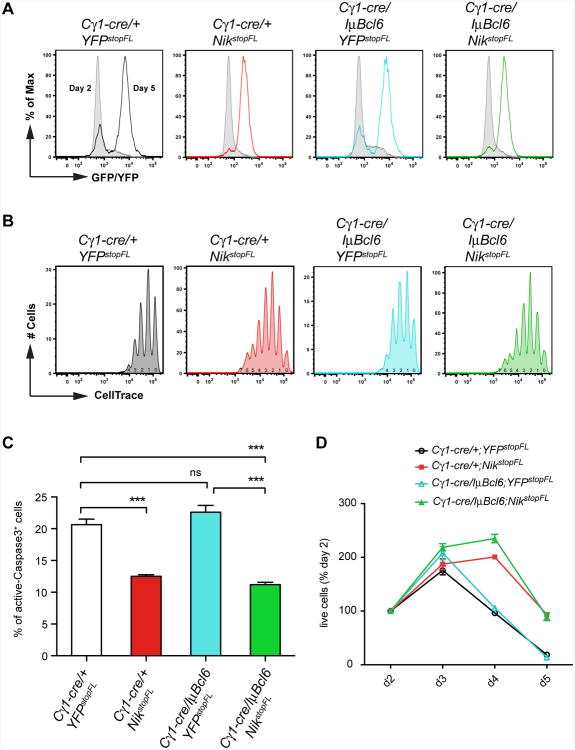
To evaluate the impact of the constitutive expression of NIK and/or BCL6 on B cell proliferation and survival, we used an in vitro cell culture system where Cre-mediated recombination is induced in B cells upon treatment with anti-CD40 and interleukin 4 (IL4), mimicking T cell-dependent B cell activation (Calado et al., 2010). NF-κB activation through enforced expression of NIK not only improved survival of activated B cells but also increased their proliferation (Figures 3A-3C). Interestingly, in this experimental system concurrent BCL6 expression did not further enhance these effects (Figures 3A-3C). Overall, constitutive NIK expression led to the accumulation of increased numbers of cells in culture, with concomitant BCL6 expression having no additional effect (Figure 3D).
Figure 3. Enhanced Cellular Proliferation and Survival as A Result of Constitutive NIK Expression.
(A) Cre-mediated recombination efficiency in purified B cells from mice of the indicated genotypes, cultured in vitro in the presence of anti-CD40 plus IL4, measured by expression of reporter genes at day 2 and day 5 of the culture.
(B) Proliferation of in vitro cultured B cells treated as in (A), measured by CellTrace dilution of reporter-positive cells at day 5 of the culture. The numbers under CellTrace peaks indicate the number of cell divisions.
(C) Frequency of apoptotic cells, at day 5, within in vitro cultured B cells treated as in (A), measured by active Caspase3 staining.
(D) Relative number of live cells at the indicated time points in in vitro culture of splenic B cells treated as in (A), normalized to day 2.
Data in (A-D) are representative of two independent experiments performed in triplicate; data in (C) are shown as mean ± SEM of triplicates; data in (D) are shown as mean ± standard deviation (SD) of triplicates.
BCL6 enforced expression through a BCL6/IgH translocation Blocks Plasma Cell Differentiation Induced by Constitutive Alternative NF-κB Signaling
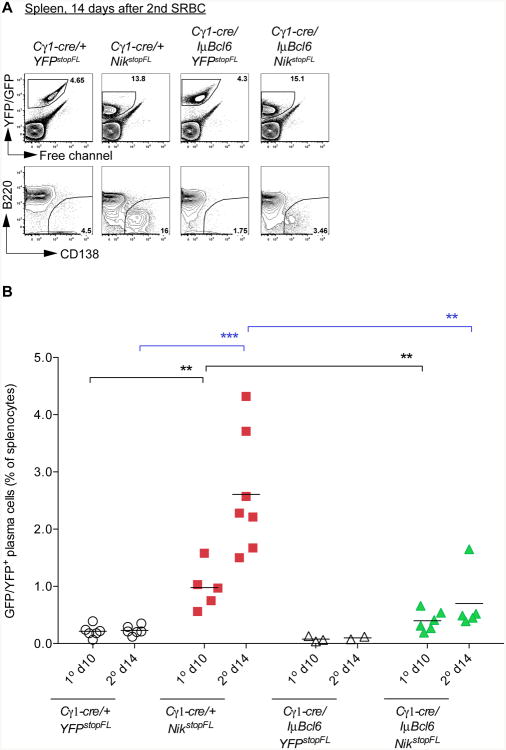
Enforced expression of NIK in GC B cells promoted transcription of IRF4 (Figure 2D), a key transcription factor for plasma cell differentiation (Klein et al., 2006). Accordingly, SRBC-immunized Cγ1-cre/+;NikstopFL mice displayed a significantly enlarged plasma cell compartment after primary and secondary immunization, compared to controls (Figures 4A and 4B; Figure S2A). In accord with the notion that BCL6 represses BLIMP1 transcription (Figure 2D; Tunyaplin et al., 2004) and the latter is essential for plasma cell differentiation (Martins and Calame, 2008), the plasma cell compartment was significantly reduced in Cγ1-cre/IμBcl6;NikstopFL mice, compared to Cγ1-cre/+;NikstopFL mice (Figures 4A and 4B; Figure S2A). In line with the in vitro data that enforced alternative NF-κB activity enhances B cell proliferation and survival, while concurrent BCL6 expression has no additive effect (Figure 3), Cγ1-cre/+;NikstopFL mice displayed increased numbers of total reporter positive cells (containing both plasma cells and B cells) in spleen compared to controls (Cγ1-cre/+;YFPstopFL), and these numbers were not further increased in mice with concurrently enforced expression of BCL6 (Cγ1-cre/IμBcl6;NikstopFL mice) (Figure 4A; Figure S2B). Collectively, these data suggest that a major effect of the deregulated BCL6 expression in GC B cells is a block of plasma cell differentiation.
Figure 4. Plasma Cell Differentiation Induced by NIK Expression Is Largely Abolished upon Coexpression of BCL6.
(A) Representative FACS analysis of plasma cells in spleen at day 14 after secondary immunization. Reporter-positive splenic cells from mice of the indicated genotypes were gated (upper panel) for the analysis of plasma cells (B220loCD138+; lower panel).
(B) Summary of FACS analysis of plasma cells in spleen at day 10 after primary immunization and day 14 after secondary immunization. Black bar represents mean.
See also Figure S2.
Mice with Enforced NIK and BCL6 Expression in GC B cells Display a Shortened Life Span
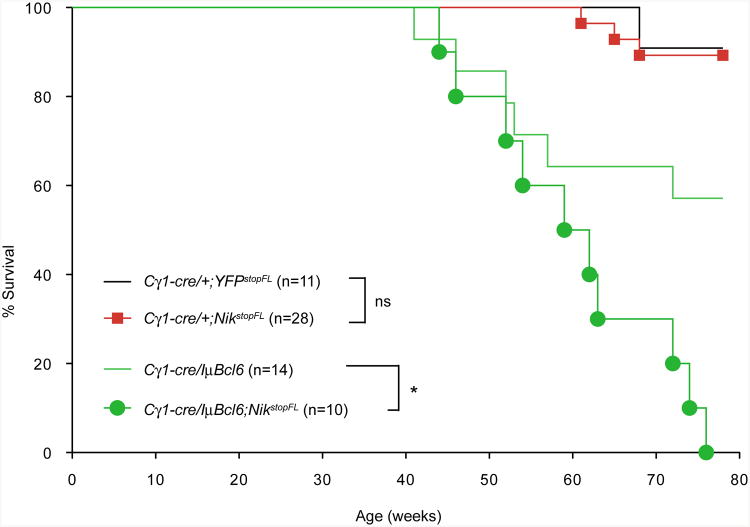
We next assessed the role of alternative NF-κB activation with or without concomitant BCL6 deregulation in B cell malignant transformation, by monitoring the mice for tumor development over a period of 1.5 years (78 weeks). Mice with enforced expression of NIK displayed a similar life span as controls, while ∼40% of Cγ1-cre/IμBcl6 mice died prematurely (Figure 5), consistent with a previous report (Cattoretti et al., 2005). In contrast, all mice with concurrent NIK and BCL6 enforced expression died within the observation period, suggesting a cooperative role (Figure 5).
Figure 5. Mice with Enforced NIK and BCL6 Expression Display a Shortened Life Span.
Kaplan-Meier survival curves of mice of the indicated genotypes.
Plasma Cell Hyperplasia in Mice with Constitutive Alternative NF-κB Signaling
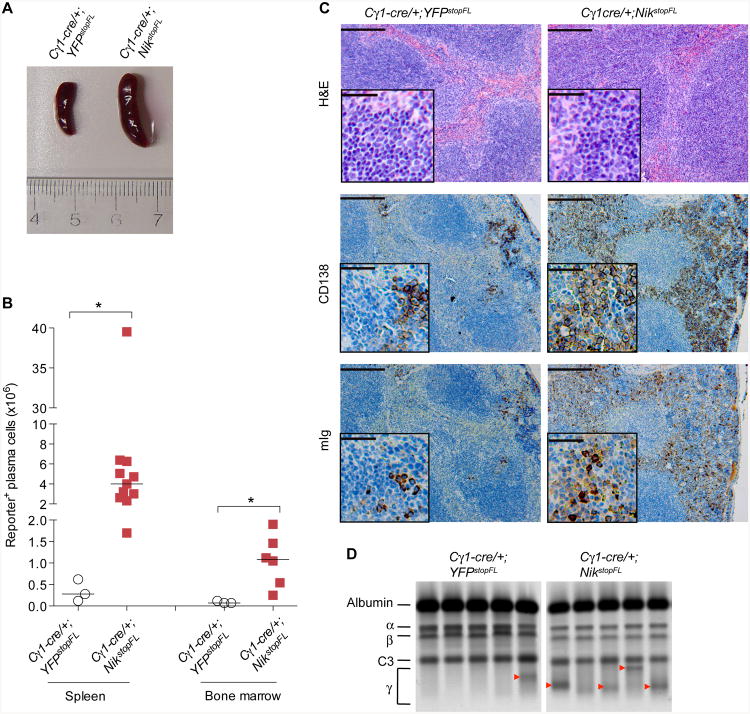
We decided to analyze the Cγ1-cre/+;NikstopFL mice in more detail and sacrificed them at the end of the observation period (1.5 years). Mice with enforced NIK expression displayed enlarged spleens (Figure 6A), and had a significant hyperplasia of both B cells and plasmablasts/plasma cells in spleen and bone marrow, compared to age-matched control animals (Figure 6B; Figure S3). Histological analysis revealed dramatically increased numbers of spleen cells expressing the plasma cell marker CD138 and intracellular Ig (Figure 6C). Serum protein electrophoresis further revealed that 8/9 of Cγ1-cre/+;NikstopFL mice displayed a distinct band in the γ-globulin region of the gel (M-spike), in contrast to 3/9 of controls (Figure 6D; data not shown), indicative of clonal plasma cell expansion. We conclude that enforced activation of alternative NF-κB signaling promotes B cell hyperplasia, in accord with previous work (Sasaki et al., 2008), as well as an expansion of the plasma cell compartments in spleen and bone marrow.
Figure 6. Plasma Cell Hyperplasia in Mice with Constitutive Alternative NF-κB Signaling.
(A) Representative picture of spleens from aged mice (≥ 60 weeks) of the indicated genotypes. Three or more mice per genotype were analyzed.
(B) Number of reporter-positive plasma cells (B220loCD138+) in spleen and bone marrow of aged mice of the indicated genotypes. Black bar represents median. The statistics was analyzed using unpaired, nonparametric Mann-Whitney test (comparing ranks).
(C) Representative histological (H&E) and immunohistochemical (CD138 and Ig) staining of spleens from aged mice of the indicated genotypes. Three or more mice per genotype were analyzed. Scale bar, 1000 μm; inset, 200 μm.
(D) Serum protein electrophoresis of representative samples from aged mice of the indicated genotypes. The position of albumin and of various globulin components of the serum is indicated. Red arrowhead indicates M-spike. In total, 3/9 (33%) of Cγ1-cre/+YFPstopFL mice and 8/9 (89%) of Cγ1-cre//+YFPstopFL mice display M-spike, respectively.
See also Figure S3.
Alternative NF-κB Signaling Cooperates with Deregulated BCL6 in DLBCL Pathogenesis
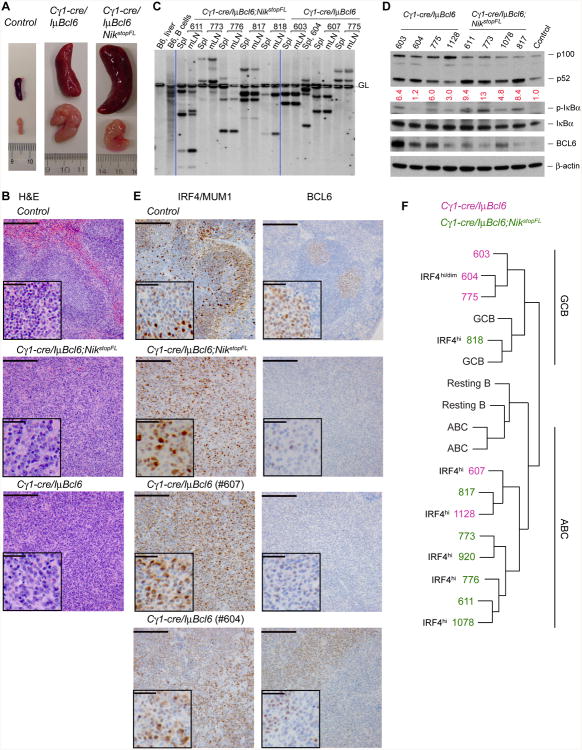
Macroscopic examination of terminally ill Cγ1-cre/IμBcl6 (6 cases) and Cγ1-cre/IμBcl6;NikstopFL (10 cases) mice revealed splenomegaly and lymphadenopathy in all cases (Figure 7A). Histological examination of the enlarged lymphoid organs showed that 5 of the 6 Cy1-cre/IμBcl6 mice analyzed had a DLBCL-like disease, characterized by a diffuse growth pattern of large cells, while the remaining mouse had a tumor with a plasmacytic morphology. Diseased Cγ1-cre/IγμBcl6;NikstopFL mice on the other hand all showed histological features of DLBCL (Figure 7B; table S1; data not shown). By flow cytometry these tumors were all negative for the plasma cell marker CD138, and displayed a mature B cell phenotype (CD19+AA4-1−IgM+ or occasionally IgG+; table S2). Analysis of IgH gene rearrangements by Southern blot revealed that the DLBCLs were of clonal B cell origin, and that the same tumor clone was present in both spleen and mesenteric lymph nodes of each mouse examined (Figure 7C), indicative of an aggressive phenotype. We next amplified the rearranged IgH V regions from clonal B cell tumors of Cγ1-cre/IμBcl6 and Cγ1-cre/IμBcl6;NikstopFL mice (4 cases each). Sequence analysis revealed somatically mutated Ig genes in 3 of the 4 Cγ1-cre/IμBcl6 tumors, and 2 of the 4 Cγ1-cre/IμBcl6;NikstopFL tumors (Table S3), suggesting that a fraction of the tumors derived from GC or post-GC B cells.
Figure 7. Constitutive Alternative NF-κB Activation Synergizes with BCL6 in DLBCL Formation and Progression.
(A) Representative pictures of spleens and mesenteric lymph nodes from mice of the indicated genotypes.
(B) Representative H&E staining of spleens from compound mutant and control mice. Scale bar, 1000 μm; inset, 200 μm. See a summary of histological findings in table S1.
(C) Southern blot analysis of tumor clonality using a JH4 probe. Dashed line, germline IgH configuration. Spl, spleen; mLN, mesenteric lymph node. Clonal tumors usually exhibit two non-germline bands corresponding to VDJ and DJ rearrangements in IgH alleles.
(D) Immunoblot analysis of various proteins in tumor tissues from the indicated compound mutant mice or in normal spleen from a Cγ1-cre/+ mouse. Tumor tissues, spleens of tumor-bearing mice (only those containing ∼50% of lymphoma B cells, as determined by FACS analysis, were selected for this assay). β-actin serves as loading control.
(E) Representative immunohistochemical (IHC) staining for IRF4/MUM1 and BCL6 on spleen sections of lymphoma-bearing compound mutants and an SRBC-immunized control mouse. Scale bar, 1000 μm; inset, 200 μm. Note that BCL6 expression from the transgene is lower than that from the endogenous loci of normal GC B cells (Cattoretti et al., 2005); two Cγ1-cre/IμBcl6 tumors are shown, and tumor #604 shows at least two populations, one of which shows relatively low IRF4 staining and high BCL6 (IRF4dimBCL6hi) while the other exhibits higher IRF4 and lower BCL6 staining (IRF4hiBCL6dim). See the summary of IHC results in table S1.
(F) Comparison of gene expression profiles of the indicated mouse tumors to that of GC B cells (GCB), resting B cells (Resting B), or activated B cells (ABC). The protein levels of IRF4 detected by IHC staining are noted next to the corresponding tumors [see also (E) and table S1], which complement gene expression profiling in classification of DLBCL subtypes.
See also Figure S4 and Tables S1-S3.
The activation of the alternative NF-κB pathway was confirmed in all tumors from Cγ1-cre/IμBcl6;NikstopFL mice by the enhanced processing of p100 to p52 on the immunoblot, compared to tumors from Cγ1-cre/IμBcl6 mice (Figure 7D).
In an attempt to subclassify the lymphomas occurring in our mouse cohorts according to the COO classification scheme, we performed immunohistochemistry (IHC) and gene expression profiling (GEP) by RNA sequencing (RNAseq). By IHC, expression of IRF4/MUM1 usually segregates with ABC-DLBCL, while high levels of BCL6 expression often associate with GCB-DLBCL (Choi et al., 2009; Hans et al., 2004), although a fraction of ABC-DLBCLs also expresses BCL6 in part due to BCL6 translocation to the IgH locus (Iqbal et al., 2007; Lenz and Staudt, 2010). All tumors analyzed were BCL6 positive, in line with the presence of the IμBcl6 allele mimicking the BCL6/IgH chromosomal translocation (Figure 7E; Table S1). The expression was however varied and particularly low in the cases where NIK expression was enforced (Figures 7D and 7E; Table S1). These observations suggest that the alternative NF-κB pathway was able to inhibit BCL6 expression from its endogenous loci, but not from the transgene, similar to what had been observed for the canonical pathway (Figure 2D; Cattoretti et al., 2005; Saito et al., 2007). IHC for IRF4 on the other hand revealed that all lymphomas (5/5) arising in Cγ1-cre/IμBcl6;NikstopFL mice were IRF4hi, while a more varied pattern was observed in tumors arising in Cγ1-cre/IμBcl6 mice, with two IRF4hi tumors; one tumor displaying both IRF4dim and IRF4hi cells; and one IRF4lo/neg tumor (Figure 7E; Table S1). To subclassify these DLBCLs, we compared their GEP to that of GC B cells and in vitro activated B cells (ABCs), in analogy to the strategy used for classification of human DLBCLs (Alizadeh et al., 2000). This analysis revealed that three (#603, #604, #775) out of five DLBCLs arising in Cγ1-cre/IμBcl6;NikstopFL mice displayed a GEP similar to GC B cells, indicative of the GCB subtype; while the GEP of the remaining two (#607, #1128) Cγ1-cre/IμBcl6 lymphomas resembled that of ABCs. Of note, tumors #607 and #1128 were also IRF4hi by IHC, further supporting an ABC subtype classification. In contrast, most DLBCLs (6/7; #817, #773, #920, #776, #611, #1078) occurring upon activation of the alternative NF-κB pathway together with enforced BCL6 expression (Cγ1-cre/IμBcl6;NikstopFL mice) displayed a GEP similar to that of ABCs (Figure 7F). The remaining Cγ1-cre/IμBcl6;NikstopFL DLBCL (#818) had a GEP resembling that of GC B cells, but because it stained highly positive for IRF4 we considered it “non-classified”. Thus, in our experimental system, the alternative NF-κB pathway and BCL6 synergize in the development of lymphomas that, in most cases, resemble ABC-DLBCL.
Mutations in Genes of the Canonical NF-κB Pathway in a Fraction of Mouse DLBCLs
Our genetic analysis revealed that a fraction (6/17) of human DLBCLs carrying a mutated TRAF3 gene have additional mutation(s) in genes of the canonical NF-κB pathway (Figure S1). In the tumors of Cγ1-cre/IμBcl6;NikstopFL mice we detected variable levels of phospho-IκBα at Ser32/26 (Figure 7D). Phosphorylation at these residues indicates canonical NF-κB activation (Brown et al., 1995; Traenckner et al., 1995). For that reason we decided to analyze the RNAseq data of the DLBCLs arising in the compound mutant mice for acquired mutations affecting genes within the canonical NF-κB pathway (For the list of genes, see SUPPLEMENTAL EXPERIMENTAL PROCEDURES). Interestingly we found such mutations in 2 out of 7 DLBCLs arising in Cγ1-cre/IμBcl6;NikstopFL mice (Figure S4). More specifically, DLBCLs #773 and #776 harbored the same mutation (R218H) in the MYD88 gene, and increased levels of phospho-IκBα were seen in DLBCL #773 compared to other tumors of the same genotype and normal splenic tissue from a Cγ1-cre/+ mouse (Figure 7D), suggesting a functional role for this mutation (Ngo et al., 2011). We also analyzed RNAseq data of the DLBCLs arising in mice with enforced BCL6 expression alone for the presence of mutations in genes of both canonical and alternative pathways of NF-κB (For the list of genes, see SUPPLEMENTAL EXPERIMENTAL PROCEDURES). We found that 2 out of 5 such DLBCLs had acquired mutations in either the CK1α kinase or CARD11 gene (Figure S4; Bidere et al., 2009; Lenz et al., 2008). DLBCL #1128 had a mutation in the CK1α kinase domain, and displayed elevated phospho-IκBα compared to the normal spleen control (Figure 7D). These observations are consistent with the observed IRF4 expression in this tumor and classification as ABC-DLBCL (Figure 7F). Another Cγ1-cre/IμBcl6;NikstopFL derived DLBCL (#603) displayed a mutation in the CARD11 (D401N) coiled-coil domain, to which the CARD11 mutations in human DLBCL are confined (Lenz et al., 2008), and this lymphoma also showed elevated levels of phospho-IκBα compared to the normal spleen control (Figure 7D). Despite canonical NF-κB pathway activation, this tumor was classified as a GCB-DLBCL by GEP profiling. Interestingly, the same exact mutation has been found in a human GCB-DLBCL (Morin et al., 2011). This is consistent with previous observations in human DLBCL, where canonical NF-κB activation could be detected in ∼20% of GCB-DLBCLs (Compagno et al., 2009). Collectively, our data show that a fraction of the tumors arising in both Cγ1-cre/IμBcl6 and Cγ1-cre/IμBcl6;NikstopFL mice have acquired mutations in genes of the canonical NF-κB pathway that are also affected in human DLBCL (Compagno et al., 2009; Pasqualucci et al., 2011).
Discussion
A Causal Role of Alternative NF-κB Activation in DLBCL
Recent studies of human DLBCLs have identified various genetic lesions that activate NF-κB through the canonical pathway, and revealed their association predominantly with the ABC over the GCB subtype (Compagno et al., 2009; Davis et al., 2010; Kato et al., 2009; Lenz et al., 2008; Ngo et al., 2011; Pasqualucci et al., 2011), in accord with the observation that ABC- but not GCB-DLBCL cell lines rely on constitutive canonical NF-κB signaling for survival (Davis et al., 2001; Staudt, 2010). Using a mouse model, we had previously established an oncogenic role for constitutive canonical NF-κB activity in ABC-DLBCL pathogenesis (Calado et al., 2010). In contrast, the question whether enforced activation of the alternative NF-κB pathway can be functionally involved in DLBCL pathogenesis has not been addressed, despite several observations suggesting a role for this pathway in the disease. Thus, mutations affecting genes (TRAF3 and TRAF2) of the alternative NF-κB pathway have been observed in a subset of human DLBCLs (Pasqualucci et al., 2011); the NF-κB2 gene, encoding the core molecule for this signaling pathway, was originally identified by virtue of its translocation to the IgH locus in a case of DLBCL (Neri et al., 1991); and IHC data revealed nuclear NF-κB2 p52, reflecting activation of the alternative pathway, in a subset of both GCB and ABC-DLBCL (Compagno et al., 2009). While these data demonstrated alternative NF-κB activity in a subset of DLBCLs, a paper by Pham et al. (2011) claimed that in DLBCLs of all subtypes both the canonical and alternative NF-κB pathways are activated, through constitutive BAFF-R (BR3) signaling. However, in most DLBCL cell lines analyzed there was no evidence for robust degradation of p100 to p52.
To clarify these matters, and in particular to obtain functional evidence for a contribution of alternative NF-κB signaling to DLBCL pathogenesis, we first extended the analysis of TRAF3 mutations by studying a larger number of DLBCL primary tumors and examining the association of TRAF3 mutations with the ABC or GCB subtype. These analyses demonstrated that biallelic or monoallelic deletions/mutations of TRAF3 occur recurrently in similar fractions (∼15%) of ABC- and GCB-DLBCL, and correlate with alternative NF-κB activity in these cases. We then developed a mouse model system that allows conditional activation of the alternative NF-κB pathway in a GC B cell restricted manner, and found that activation of this pathway, in concert with BCL6 deregulation, leads to the development of DLBCL. This indicates a causal role of deregulated alternative NF-κB signaling in DLBCL pathogenesis. Interestingly, in this scenario the deregulation of alternative NF-κB activity appears to be required in the context of GC B cell differentiation. Deletion of TRAF3 in mouse B cells from early developmental stages via CD19-cre leads to the formation of B1 and marginal zone B cell lymphomas, not DLBCL (Moore et al., 2012). These tumors resemble human splenic marginal zone lymphoma (SMZL), where inactivating mutations of TRAF3 have also been found (Rossi et al., 2011). Together, these observations highlight the importance of ontogenetic timing in the acquisition of oncogenic somatic mutations driving different classes of lymphomas. TRAF3 mutations/deletions are absent in follicular lymphoma, Burkitt lymphoma and B cell chronic lymphocytic leukemia (data not shown).
Of note, we observed that most Cγ1-cre/IμBcl6;NikstopFL mice developed DLBCLs of the ABC subtype. This observation is likely related to the fact that activation of the alternative NF-κB pathway interferes with the GC reaction even when BCL6 expression is deregulated and suggests that, in NF-κB positive human GCB-DLBCLs, additional mutation(s) allowing maintenance of the GCB phenotype must exist. Future studies comparing genetic lesions in the GCB vs. ABC types of DLBCLs carrying TRAF3 lesions may lead to the identification of those latter events.
Interference with Terminal B cell Differentiation Is Required for the Pathogenesis of ABC-DLBCL: A Role for BCL6
Constitutive NF-κB signaling in B cells promotes their differentiation towards plasma cells, through induction of IRF4 (Grumont and Gerondakis, 2000; Klein et al., 2006; Saito et al., 2007; Sciammas et al., 2006), in line with the presence of genetic lesions leading to constitutive NF-κB signaling in multiple myeloma (Annunziata et al., 2007; Keats et al., 2007). The observations that ABC-DLBCLs express some key genes characteristic of a plasmablast (Lenz and Staudt, 2010; Wright et al., 2003), but often carry genetic lesions interfering with plasma cell differentiation, suggest that the transformation of an ABC into DLBCL requires interference with terminal B cell differentiation (Lenz and Staudt, 2010; Staudt, 2010). Indeed, mice with specific activation of the canonical NF-κB signaling pathway in GC B cells developed plasma cell hyperplasia and had an overall normal life span, but succumbed to ABC-like DLBCL when B cell terminal differentiation was abolished by deletion of Blimp1 (Calado et al., 2010). Similarly, we show here that mice with activation of the alternative NF-κB pathway alone in GC B cells do not succumb to tumors in the time-frame of this study but display overt plasma cell hyperplasia, and that the oncogenic role of the alternative NF-κB pathway is revealed upon interference with plasma cell differentiation through enforced BCL6 expression, in accord with the coexistence of TRAF3 and BCL6 mutations in human DLBCL.
Our work indicates that the role of BCL6 in the development of DLBCLs exhibiting constitutive NF-κB signaling is at least in part due to its ability to inhibit BLIMP1 expression, which in turn limits the terminal differentiation of B cells. This is supported by data showing that in the mouse, loss of BLIMP1 cooperates with alternative NF-κB signaling in DLBCL formation (data not shown). However, the fact that human DLBCLs with alternative NF-κB activation are often concurrent with BCL6 translocation but not BLIMP1 inactivation, suggests that other functions of BCL6, such as repression of the DNA damage response (Basso and Dalla-Favera, 2010) may also be critically required to complement alternative NF-κB signaling in DLBCL development.
Alternative and Canonical NF-κB Pathway Activation in DLBCLs
While mechanisms of aberrant NF-κB activation in DLBCL can in the major fraction of the cases be attributed to the presence of oncogenic mutations in genes related to the canonical NF-κB pathway (Compagno et al., 2009; Davis et al., 2010; Lenz et al., 2008; Ngo et al., 2011; Pasqualucci et al., 2011), previous data and the present work show that genetic lesions activating the alternative NF-κB pathway occur in up to 15% of DLBCLs. It is worth noting in this context that besides the ∼10% DLBCL cases demonstrating nuclear NF-κB activity exclusively for the alternative pathway (indicated by nuclear staining of p52 but not p50), ∼20% of DLBCLs display nuclear staining for both p50 and p52 (Compagno et al., 2009), suggesting the activation of both canonical and alternative NF-κB pathways. Indeed, our genetic analysis showed that a fraction (6/17) of TRAF3 mutated DLBCLs carry concurrent mutation(s) in genes of the canonical NF-κB pathway. Likewise, while the present mouse model suggests that the alternative pathway can by itself drive DLBCL development if combined with a lesion preventing plasma cell differentiation, it became apparent that in a small fraction (2/7) of the resulting tumors additional mutations accumulated which resulted in the activation of canonical NF-κB signaling. We have previously observed redundancy between the canonical and alternative NF-κB pathways to replace BAFF-mediated survival signals in B cells (Sasaki et al., 2008; Sasaki et al., 2006), and there is evidence in the human that NIK activation can also trigger canonical NF-κB activity (Annunziata et al., 2007; O'Mahony et al., 2000). Given that the NIK allele used in the present study yields only a moderate cell survival advantage, in sharp contrast to a NIK allele lacking the TRAF3 binding site (Sasaki et al., 2008), we speculate that in a NIK-expressing B cell acquisition of an activating canonical NF-κB mutation may confer a further survival advantage, enabling the cell to outcompete its siblings during the clonal evolution of lymphoma. A similar mechanism may operate in human DLBCL pathogenesis. Overall, the current work provides a rationale for the design of therapies targeting the alternative NF-κB pathway in a fraction of DLBCL patients, and suggests that for those human DLBCLs that display both canonical and alternative NF-κB mutations (Compagno et al., 2009; Pasqualucci et al., 2011), targeting both arms of NF-κB signaling may be required for therapeutic intervention, as recently demonstrated for multiple myeloma (Fabre et al., 2012).
Experimental Procedures
Sequencing analysis and High-density SNP Array Analysis of Human DLBCLs
119 DLBCL samples, including 98 biopsies (47 GCB and 51 ABC/NC-DLBCLs) and 21 cell lines (14 GCB and 7 ABC-DLBCLs), were analyzed as described previously (Pasqualucci et al., 2011). Oligonucleotides and conditions used for TRAF3 amplification are available upon request.
Mice, Immunization, and Tumor Cohorts
Cγ1-cre, NikstopFL, Blimp1FF, IμBcl6 and YFPstopFL alleles have been described (Casola et al., 2006; Cattoretti et al., 2005; Ohinata et al., 2005; Sasaki et al., 2008; Srinivas et al., 2001). 8-10 weeks old mice were immunized i.v. with 1 × 109 SRBCs (Cedarlane) in PBS. Mouse cohorts for tumor development were given monthly antigenic stimulation by SRBC immunization for 7 additional times, and then monitored twice a week for tumor development and euthanized if signs of tumor development occurred. All animal care and procedures followed National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC 03341) of Harvard University and the Immune Disease Institute.
Statistical Analysis
Unless otherwise indicated, data were analyzed using unpaired two-tailed Student's t test; a p value ≤0.05 was considered significant. A single asterisk (*) in the graphs of figures represents a p value ≤0.05, double asterisks (**) a p value ≤0.01, and triple asterisks (***) a p value ≤0.001, and “ns” stands for not statistically significant, i.e., a p value >0.05. Survival curves were compared using the log rank test. Data in text and figures are represented as mean ± standard error of the mean (SEM), unless otherwise indicated.
Supplementary Material
Significance.
In DLBCL the mechanisms of aberrant NF-κB activation have been predominantly attributed to oncogenic mutations in genes related to the canonical NF-κB pathway. Hence genetic lesions that lead to activation of the alternative NF-κB signaling have been less studied. We find through the analysis of human DLBCLs and genetic modeling in mice that the alternative NF-κB pathway also plays a key role in DLBCL development. Similar to canonical NF-κB activity, the oncogenic role of alternative NF-κB signaling is revealed upon block of terminal B cell differentiation. This suggests that therapeutic targeting of DLBCLs should take both arms of the NF-κB pathway in consideration. The mouse model presented here should prove useful in developing such therapies.
Acknowledgments
We thank J. Xia, X. Chen, D. Ghitza, A. Pellerin, J. Grundy and C. Langnick for technical assistance; M. Bamberg, M. Ottaviano, H.-L. Cheng, J. Needham and L. Lynch for administrative assistance; K.R. laboratory members and M. Janz for critical comments and suggestions. This work was supported by the National Cancer Institute grants P01 CA092625 and R01 CA098285, a Leukemia & Lymphoma Society SCOR grant and by the European Research Council, ERC Advanced Grant ERC-AG-LS6 to K.R.; the National Cancer Institute grant CA172492 to L.P.; the Leukemia & Lymphoma Society fellowships to B.Z. and D.P.C; the Dana-Farber Cancer Institute Faculty Startup Funds to B.Z.; core funding from Cancer Research UK, and a MRC career development award MR/J008060/1 to D.C.
Footnotes
Accession Numbers: The Gene Expression Omnibus accession number for the RNA sequencing data is GSE65422.
Author Contributions: B.Z., D.P.C. and K.R. conceived and supervised the study. B.Z., D.P.C. and Z.W. designed, performed and analyzed the main experiments. L.P. and R.D-F. were responsible for the human DLBCL analysis. F.W.A supervised some aspects of the study. S.F., K.K., Y.Q., S.B.K., C.U., S.R. and W.C performed further experiments. M.S-S. and Y.S. generated the NIK transgenic mice and helped conceive the study. B.Z., D.P.C. and K.R. interpreted the results and wrote the paper. All authors read and contributed to the finalization of the paper.
The authors declare no conflict of interest.
Supplemental Information: Supplemental Information includes Supplemental Experimental Procedures, four figures, and three tables.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, Lenz G, Hanamura I, Wright G, Xiao W, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso K, Dalla-Favera R. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Advances in immunology. 2010;105:193–210. doi: 10.1016/S0065-2776(10)05007-8. [DOI] [PubMed] [Google Scholar]
- Bidere N, Ngo VN, Lee J, Collins C, Zheng L, Wan F, Davis RE, Lenz G, Anderson DE, Arnoult D, et al. Casein kinase 1alpha governs antigen-receptor-induced NF-kappaB activation and human lymphoma cell survival. Nature. 2009;458:92–96. doi: 10.1038/nature07613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- Calado DP, Zhang B, Srinivasan L, Sasaki Y, Seagal J, Unitt C, Rodig S, Kutok J, Tarakhovsky A, Schmidt-Supprian M, et al. Constitutive canonical NF-kappaB activation cooperates with disruption of BLIMP1 in the pathogenesis of activated B cell-like diffuse large cell lymphoma. Cancer cell. 2010;18:580–589. doi: 10.1016/j.ccr.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casola S, Cattoretti G, Uyttersprot N, Koralov SB, Seagal J, Hao Z, Waisman A, Egert A, Ghitza D, Rajewsky K. Tracking germinal center B cells expressing germ-line immunoglobulin gamma1 transcripts by conditional gene targeting. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7396–7401. doi: 10.1073/pnas.0602353103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattoretti G, Pasqualucci L, Ballon G, Tam W, Nandula SV, Shen Q, Mo T, Murty VV, Dalla-Favera R. Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer cell. 2005;7:445–455. doi: 10.1016/j.ccr.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Choi WW, Weisenburger DD, Greiner TC, Piris MA, Banham AH, Delabie J, Braziel RM, Geng H, Iqbal J, Lenz G, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5494–5502. doi: 10.1158/1078-0432.CCR-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagno M, Lim WK, Grunn A, Nandula SV, Brahmachary M, Shen Q, Bertoni F, Ponzoni M, Scandurra M, Califano A, et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459:717–721. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. The Journal of experimental medicine. 2001;194:1861–1874. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre C, Mimura N, Bobb K, Kong SY, Gorgun G, Cirstea D, Hu Y, Minami J, Ohguchi H, Zhang J, et al. Dual inhibition of canonical and noncanonical NF-kappaB pathways demonstrates significant antitumor activities in multiple myeloma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4669–4681. doi: 10.1158/1078-0432.CCR-12-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Grumont RJ, Gerondakis S. Rel induces interferon regulatory factor 4 (IRF-4) expression in lymphocytes: modulation of interferon-regulated gene expression by rel/nuclear factor kappaB. The Journal of experimental medicine. 2000;191:1281–1292. doi: 10.1084/jem.191.8.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker H, Tseng PH, Karin M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nature reviews Immunology. 2011;11:457–468. doi: 10.1038/nri2998. [DOI] [PubMed] [Google Scholar]
- Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Muller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- He JQ, Saha SK, Kang JR, Zarnegar B, Cheng G. Specificity of TRAF3 in its negative regulation of the noncanonical NF-kappa B pathway. The Journal of biological chemistry. 2007;282:3688–3694. doi: 10.1074/jbc.M610271200. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Greiner TC, Patel K, Dave BJ, Smith L, Ji J, Wright G, Sanger WG, Pickering DL, Jain S, et al. Distinctive patterns of BCL6 molecular alterations and their functional consequences in different subgroups of diffuse large B-cell lymphoma. Leukemia. 2007;21:2332–2343. doi: 10.1038/sj.leu.2404856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Sanada M, Kato I, Sato Y, Takita J, Takeuchi K, Niwa A, Chen Y, Nakazaki K, Nomoto J, et al. Frequent inactivation of A20 in B-cell lymphomas. Nature. 2009;459:712–716. doi: 10.1038/nature07969. [DOI] [PubMed] [Google Scholar]
- Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, Van Wier S, Tiedemann R, Shi CX, Sebag M, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U, Casola S, Cattoretti G, Shen Q, Lia M, Mo T, Ludwig T, Rajewsky K, Dalla-Favera R. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nature immunology. 2006;7:773–782. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- Lenz G, Davis RE, Ngo VN, Lam L, George TC, Wright GW, Dave SS, Zhao H, Xu W, Rosenwald A, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319:1676–1679. doi: 10.1126/science.1153629. [DOI] [PubMed] [Google Scholar]
- Lenz G, Staudt LM. Aggressive lymphomas. The New England journal of medicine. 2010;362:1417–1429. doi: 10.1056/NEJMra0807082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbaum J, Bhagat G, Tang H, Mo T, Brahmachary M, Shen Q, Chadburn A, Rajewsky K, Tarakhovsky A, Pasqualucci L, et al. BLIMP1 is a tumor suppressor gene frequently disrupted in activated B cell-like diffuse large B cell lymphoma. Cancer cell. 2010;18:568–579. doi: 10.1016/j.ccr.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins G, Calame K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annual review of immunology. 2008;26:133–169. doi: 10.1146/annurev.immunol.26.021607.090241. [DOI] [PubMed] [Google Scholar]
- Monti S, Savage KJ, Kutok JL, Feuerhake F, Kurtin P, Mihm M, Wu B, Pasqualucci L, Neuberg D, Aguiar RC, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005;105:1851–1861. doi: 10.1182/blood-2004-07-2947. [DOI] [PubMed] [Google Scholar]
- Moore CR, Liu Y, Shao C, Covey LR, Morse HC, 3rd, Xie P. Specific deletion of TRAF3 in B lymphocytes leads to B-lymphoma development in mice. Leukemia. 2012;26:1122–1127. doi: 10.1038/leu.2011.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin RD, Mendez-Lago M, Mungall AJ, Goya R, Mungall KL, Corbett RD, Johnson NA, Severson TM, Chiu R, Field M, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri A, Chang CC, Lombardi L, Salina M, Corradini P, Maiolo AT, Chaganti RS, Dalla-Favera R. B cell lymphoma-associated chromosomal translocation involves candidate oncogene lyt-10, homologous to NF-kappa B p50. Cell. 1991;67:1075–1087. doi: 10.1016/0092-8674(91)90285-7. [DOI] [PubMed] [Google Scholar]
- Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, Kohlhammer H, Xu W, Yang Y, Zhao H, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony A, Lin X, Geleziunas R, Greene WC. Activation of the heterodimeric IkappaB kinase alpha (IKKalpha)-IKKbeta complex is directional: IKKalpha regulates IKKbeta under both basal and stimulated conditions. Molecular and cellular biology. 2000;20:1170–1178. doi: 10.1128/mcb.20.4.1170-1178.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohinata Y, Payer B, O'Carroll D, Ancelin K, Ono Y, Sano M, Barton SC, Obukhanych T, Nussenzweig M, Tarakhovsky A, et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436:207–213. doi: 10.1038/nature03813. [DOI] [PubMed] [Google Scholar]
- Pasqualucci L, Compagno M, Houldsworth J, Monti S, Grunn A, Nandula SV, Aster JC, Murty VV, Shipp MA, Dalla-Favera R. Inactivation of the PRDM1/BLIMP1 gene in diffuse large B cell lymphoma. The Journal of experimental medicine. 2006;203:311–317. doi: 10.1084/jem.20052204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualucci L, Trifonov V, Fabbri G, Ma J, Rossi D, Chiarenza A, Wells VA, Grunn A, Messina M, Elliot O, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nature genetics. 2011;43:830–837. doi: 10.1038/ng.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham LV, Fu L, Tamayo AT, Bueso-Ramos C, Drakos E, Vega F, Medeiros LJ, Ford RJ. Constitutive BR3 receptor signaling in diffuse, large B-cell lymphomas stabilizes nuclear factor-kappaB-inducing kinase while activating both canonical and alternative nuclear factor-kappaB pathways. Blood. 2011;117:200–210. doi: 10.1182/blood-2010-06-290437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D, Deaglio S, Dominguez-Sola D, Rasi S, Vaisitti T, Agostinelli C, Spina V, Bruscaggin A, Monti S, Cerri M, et al. Alteration of BIRC3 and multiple other NF-kappaB pathway genes in splenic marginal zone lymphoma. Blood. 2011;118:4930–4934. doi: 10.1182/blood-2011-06-359166. [DOI] [PubMed] [Google Scholar]
- Saito M, Gao J, Basso K, Kitagawa Y, Smith PM, Bhagat G, Pernis A, Pasqualucci L, Dalla-Favera R. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer cell. 2007;12:280–292. doi: 10.1016/j.ccr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Calado DP, Derudder E, Zhang B, Shimizu Y, Mackay F, Nishikawa S, Rajewsky K, Schmidt-Supprian M. NIK overexpression amplifies, whereas ablation of its TRAF3-binding domain replaces BAFF:BAFF-R-mediated survival signals in B cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10883–10888. doi: 10.1073/pnas.0805186105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Derudder E, Hobeika E, Pelanda R, Reth M, Rajewsky K, Schmidt-Supprian M. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM, Singh H. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25:225–236. doi: 10.1016/j.immuni.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, 3rd, Young RM, Staudt LM. Pathogenesis of human B cell lymphomas. Annual review of immunology. 2012;30:565–610. doi: 10.1146/annurev-immunol-020711-075027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC developmental biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt LM. Oncogenic activation of NF-kappaB. Cold Spring Harbor perspectives in biology. 2010;2:a000109. doi: 10.1101/cshperspect.a000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam W, Gomez M, Chadburn A, Lee JW, Chan WC, Knowles DM. Mutational analysis of PRDM1 indicates a tumor-suppressor role in diffuse large B-cell lymphomas. Blood. 2006;107:4090–4100. doi: 10.1182/blood-2005-09-3778. [DOI] [PubMed] [Google Scholar]
- Traenckner EB, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. The EMBO journal. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunyaplin C, Shaffer AL, Angelin-Duclos CD, Yu X, Staudt LM, Calame KL. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. Journal of immunology. 2004;173:1158–1165. doi: 10.4049/jimmunol.173.2.1158. [DOI] [PubMed] [Google Scholar]
- Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A, Staudt LM. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9991–9996. doi: 10.1073/pnas.1732008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, de Waard R, Leung C, Nouri-Shirazi M, Orazi A, Chaganti RS, et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nature genetics. 1997;16:161–170. doi: 10.1038/ng0697-161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.