Abstract
Background
The Single Ventricle Reconstruction (SVR) trial demonstrated a transplant-free survival advantage at 12 month follow up for patients with right-ventricle-pulmonary-artery shunt (RVPAS) at Norwood procedure versus modified Blalock-Taussig shunt (MBTS) but similar survival and decreased global right ventricular (RV) function on longer term follow-up. The impact of the required ventriculotomy for the RVPAS remains unknown. We compared echo-derived RV deformation indices after stage 2 procedure in survivors with single RV anomalies enrolled in the SVR trial.
Methods
Global and regional RV systolic longitudinal and circumferential strain and strain rate, ejection fraction, and short axis % fractional area change were all derived by speckle tracking echocardiography from protocol echocardiograms performed at 14.3±1.2 months. Student’s t-test or Wilcoxon rank sum test was used to compare groups.
Results
The cohort included 275 subjects (129 MBTS and 146 RVPAS). Longitudinal deformation could be quantified in 214 (78%) subjects and circumferential measures in 182 (66%) subjects. RV ejection fraction and % fractional area change did not differ between groups. There were no significant differences between groups for global or regional longitudinal deformation. Circumferential indices showed abnormalities in deformation in the RVPAS group with decreased global circumferential strain (p=0.05), strain rate (p=0.09) and anterior regional strain rate (p=0.07) that approached statistical significance.
Conclusion
RV myocardial deformation at 14 months, after stage 2 procedure, is not significantly altered by the type of initial shunt placed. However, abnormal trends were appreciated in circumferential deformation for the RVPAS group in the area of ventriculotomy that may represent early myocardial dysfunction. These data provide a basis for longer-term RV deformation assessment in survivors after Norwood procedure.
Keywords: Single ventricle, Norwood, Strain, Strain rate, Deformation
INTRODUCTION
Patients undergoing staged repair for single right ventricle (RV) anomalies are at high risk for adverse outcomes with reported mortality rates of 17–34% during the first year of life(1–3). The Pediatric Heart Network Single Ventricle Reconstruction (SVR) trial compared outcomes in 549 infants undergoing a Norwood procedure randomized to either a Modified Blalock-Taussig shunt (MBTS) or right-ventricle-pulmonary-artery shunt (RVPAS) at 15 North American centers(2). The primary result of the trial found better one-year transplant-free survival in subjects who received a RVPAS compared to those who had a MBTS. This advantage, however, appears to diminish over time(2). At 3 years of age, transplant-free survival was similar between the groups, due to worse transplant-free survival in the RVPAS group between 1 and 3 years, and RV systolic function as assessed by echocardiography was worse for the survivors in the RVPAS group(4). The long term effect of the ventriculotomy required for RVPAS placement at the time of Norwood procedure has been suggested as a potential cause of this deterioration in RV function and increase in the hazard risk for late adverse outcome, but a direct correlation has not been demonstrated(2, 4, 5).
Qualitative assessment of systemic RV function has shown significant intra-observer variability and poor correlation with cardiac magnetic resonance imaging (CMR)(6). Due to the complex geometry of the RV, quantitative 2D and Doppler measures by echocardiography are difficult to perform, correlate modestly at best with CMR estimates of ejection fraction and are not routinely used in clinical practice(7–9). Prior analysis of this SVR cohort has shown that standard echocardiographic measures of RV systolic and diastolic function are similar between shunt groups at 14 months(10).
Emerging technologies have shown more promise in the evaluation of RV function. Speckle tracking echocardiography has provided reliable assessment of global and regional myocardial deformation without dependence on the angle of interrogation. It has been applied to the systemic RV and has shown good correlation with CMR-derived RV ejection fraction (11–13). The SVR cohort presents a unique opportunity to evaluate a large group of single RV patients randomly assigned to the initial surgical shunt type. We sought to compare global and regional RV deformation indices in SVR survivors at 14 months of age after the stage 2 procedure to assess for shunt-related differences in RV function.
METHODS
Study population
The SVR trial enrolled patients with a single morphologic RV lesion undergoing the Norwood procedure from 15 centers in the United States and Canada between May 2005 and July 2008. Inclusion and exclusion criteria have been previously described(14). Protocol echocardiographic data were collected at specific intervals during the first year as well as at 14 months of age(14). All protocol echocardiograms were maintained at the echocardiography core lab at the Medical College of Wisconsin, and the 14 month echocardiograms were selected for this analysis after removal of the initial shunt as part of the stage 2 procedure. Those with inadequate imaging for speckle tracking were excluded.
Echocardiographic analysis
Digital Imaging and Communications in Medicine (DICOM) image clips from the parasternal short axis (SAX) below the level of the atrioventricular valve(s) at the midventricular level and from the apical 4 chamber (A4C) view at the cardiac crux were selected for analysis when the endocardium of the RV was displayed throughout the cardiac cycle. These DICOM images were stored at a frame rate of 30 frames per second. Analysis was initially performed with manual tracing of ventricular subendocardium by a single blinded reviewer using commercially available off-line echocardiographic speckle tracking software (TomTec Imaging Systems GmbH, Unterschleissheim, Germany). The subendocardial border was automatically tracked through the cardiac cycle by the software and the overlayed map of the subendocardial tracking was then reviewed by 2 senior pediatric cardiologists with experience in speckle tracking to confirm accuracy of the tracking and adjust as necessary for a consensus best tracing. Once confirmed, the software automatically calculates global circumferential (from SAX) and longitudinal (from A4C) strain and strain rate as well as regional strain and strain rate from 6 pre-determined segments of the RV (Figure 1). In addition, the software automatically calculates the fractional area change from the SAX and single plane Simpson’s ejection fraction from the A4C tracing. For the purposes of this study we elected not to use the apical longitudinal segments due to poor reliability.
Figure 1. Regional segments.
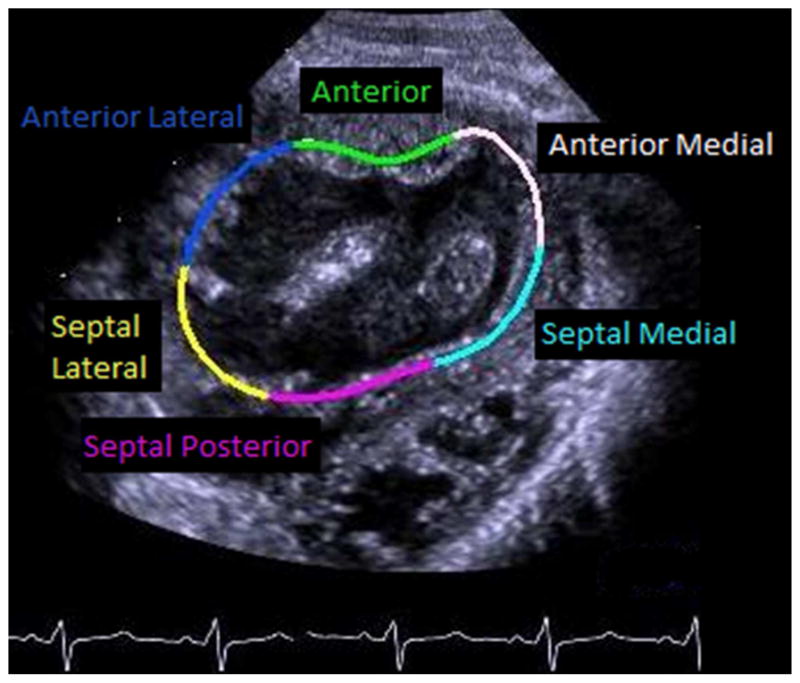
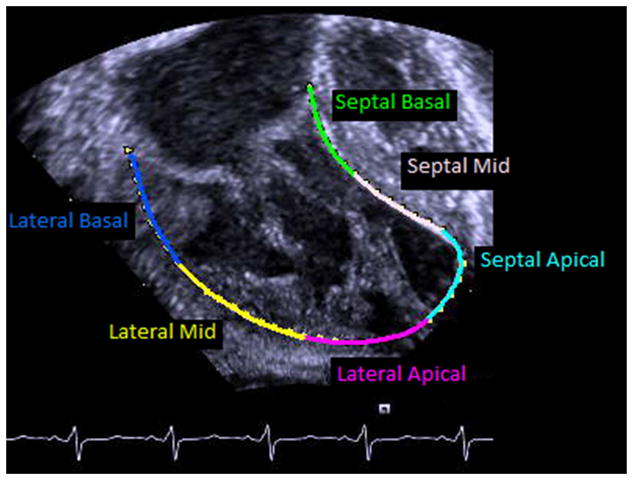
Regional segments from the A) parasternal short axis and B) apical 4 chamber imaging planes.
Statistical analysis
Descriptive data are presented as mean ± standard deviation, median with range or number with percent of total. Comparisons between groups were made using Student’s t test or Wilcoxon rank sum test based on distribution using SAS OnDemand 4.3 (SAS Institute, Cary, NC). Because the presence of a ventriculotomy and its effect on RV function was the primary objective, the groups were compared based on shunt in place at the completion of the Norwood procedure (non-intention to treat). For all differences a P value <0.05 was considered significant.
RESULTS
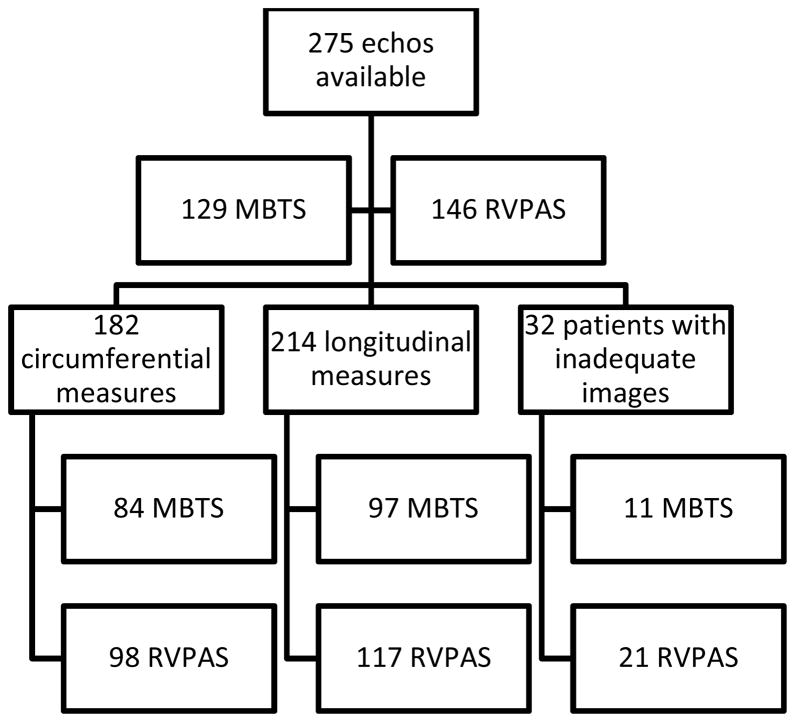
The cohort included 275 survivors, with 174 (63%) males, with echocardiograms at a mean age of 14.3 ± 1.2 months available for retrospective review. There were 129 MBTS and 146 RVPAS subjects. These groups were similar in gender distribution and age and had similar ejection fraction and % fractional area change (Table 1). Thirty-two subjects (12%) had inadequate endocardial display from both the SAX and A4C views for speckle tracking and were excluded from analysis. The excluded group included 11 (34%) in the MBTS group and 21 (66%) in the RVPAS group; 19 of the 32 excluded subjects (59%) were male. SAX circumferential measures could be obtained in 182 (66%) and longitudinal measures could be obtained in 214 (78%) of the original 275 subjects (Figure 2). There was no statistically significant difference between the shunt groups in either global or regional circumferential strain or strain rate (Table 2). Some circumferential indices, including global RV circumferential strain (p=0.05), strain rate (p=0.09) and anterior regional strain rate (p=0.07), approached statistical significance for worse deformation in the RVPAS group. There were no differences in the global or regional longitudinal strain or strain rate between shunt types (Table 3).
Table 1. Comparison of shunt groups.
Comparison of demographic data and standard assessments of right ventricular function by shunt type.
| MBTS | RVPAS | p value | |
|---|---|---|---|
| Male, N | 74 (67%) | 81 (61%) | 0.42 |
| Mean age, months (SD) | 14.3 (±1.1) | 14.3 (±1.3) | 0.98 |
| Ejection fraction, % (SD) | 46.7 (±8.9) | 45.2 (±11.1) | 0.28 |
| Short axis area change, % (SD) | 33.6 (±8.9) | 31.2 (±9.3) | 0.09 |
MBTS Modified Blalock-Taussig Shunt; RVPAS Right ventricular to pulmonary artery shunt; SD Standard deviation.
Figure 2. Study Flow Chart.
Distribution of available echocardiograms by shunt type and number of studies adequate for speckle tracking. MBTS Modified Blalock-Taussig Shunt; RVPAS Right ventricular to pulmonary artery shunt.
Table 2. Circumferential indices by shunt type.
Comparison of global and regional circumferential strain and strain rate by shunt type as assessed from the short axis window.
| MBTS | RVPAS | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p value | |
| Circumferential strain | |||||
| Anterior medial | −13.1 | 6.7 | −11.9 | 6.0 | 0.19 |
| Septal medial | −13.6 | 6.9 | −11.8 | 6.7 | 0.09 |
| Septal posterior | −13.2 | 7.8 | −11.1 | 5.9 | 0.14 |
| Septal lateral | −16.0 | 7.0 | −15.4 | 7.4 | 0.79 |
| Anterior lateral | −14.9 | 5.9 | −13.7 | 6.0 | 0.19 |
| Anterior | −9.8 | 5.1 | −9.3 | 5.4 | 0.31 |
| Global | −13.4 | 4.3 | −12.2 | 4.2 | 0.05 |
| Circumferential strain rate | |||||
| Anterior medial | −1.01 | 0.51 | −0.92 | 0.46 | 0.20 |
| Septal medial | −1.03 | 0.53 | −0.92 | 0.49 | 0.14 |
| Septal posterior | −1.06 | 0.64 | −0.97 | 0.48 | 0.74 |
| Septal lateral | −1.26 | 0.55 | −1.20 | 0.59 | 0.63 |
| Anterior lateral | −1.15 | 0.53 | −1.03 | 0.46 | 0.26 |
| Anterior | −0.76 | 0.38 | −0.68 | 0.39 | 0.07 |
| Global | −1.04 | 0.37 | −0.95 | 0.34 | 0.09 |
MBTS Modified Blalock-Taussig Shunt; RVPAS Right ventricular to pulmonary artery shunt; SD Standard deviation.
Table 3.
Longitudinal indices by shunt type
Comparison of global and regional longitudinal strain and strain rate by shunt type as assessed from the apical 4 chamber window.
| MBTS | RVPAS | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p value | |
| Longitudinal strain | |||||
| Septal basal | −8.9 | 5.5 | −9.4 | 5.7 | 0.44 |
| Septal mid | −12.3 | 6.2 | −12.6 | 6.5 | 0.72 |
| Lateral mid | −12.3 | 3.7 | −12.5 | 4.8 | 0.71 |
| Lateral basal | −17.1 | 5.5 | −16.1 | 6.4 | 0.10 |
| Global | −12.4 | 3.3 | −12.4 | 3.9 | 0.97 |
| Longitudinal strain rate | |||||
| Septal basal | −0.77 | 0.47 | −0.81 | 0.48 | 0.66 |
| Septal mid | −0.94 | 0.43 | −0.98 | 0.51 | 0.88 |
| Lateral mid | −0.85 | 0.32 | −0.85 | 0.33 | 0.99 |
| Lateral basal | −1.20 | 0.46 | −1.16 | 0.52 | 0.36 |
| Global | −0.96 | 0.27 | −0.96 | 0.33 | 0.99 |
MBTS Modified Blalock-Taussig Shunt; RVPAS Right ventricular to pulmonary artery shunt; SD Standard deviation.
DISCUSSION
In this study, we describe the first multi-center application of speckle tracking echocardiography technology for analysis of RV performance in children with single RV anomalies. The 275 survivors randomized to either a MBTS or RVPAS, who proceeded through initial staged repair, had no statistically significant differences in global or regional indices of RV deformation identified between shunt types at 14 months of age. This dataset also provides important information on the range of values for systolic circumferential and longitudinal strain and strain rate as this vulnerable population transitions from infancy to childhood.
Identification of RV dysfunction is critically important in the child with a single RV anomaly. RV function has a vital impact on both short- and long-term outcomes since it is the only effective pump, providing flow to the systemic, pulmonary, and coronary circulations. Yet no consistently reproducible and quantifiable echocardiographic measure of RV function has been devised. Using standard 2D and Doppler echocardiographic measures, Frommelt et al described shunt-based comparisons of systolic, diastolic and global RV function in this same SVR cohort at baseline, early after Norwood, prior to the stage 2 procedure, and at 14 months. They also found no shunt-related differences in any traditional 2D echocardiographic index at 14 months(10). In longer term follow-up, other reports have identified diminished qualitative RV systolic function prior to and after the final stage Fontan procedure in patients who had the RVPAS(15, 16). This is consistent with the findings in the SVR cohort that there is diminished RV function and higher mortality in the RVPAS group compared to the MBTS group in those surviving beyond 1 year of age(4). It has been hypothesized that the ventriculotomy associated with the RVPAS could potentially impact ventricular performance because of myocardial injury, scar, or aneurysm formation and therefore be at least partially responsible for these observations(17).
Speckle tracking echocardiography could provide additional insights into the mechanics of RV dysfunction in patients with a single RV anomaly. Using speckle tracking echocardiography in patients after stage 2 procedure with prior Norwood and RVPAS, Menon et al demonstrated lower velocity, strain and strain rate in the anterior region, where a ventriculotomy scar would be located, compared with the septal, left lateral and right lateral regions(18). Our study also showed decreased regional anterior wall circumferential strain rate (p=0.07) as well as decreased global circumferential strain (p=0.05), strain rate (p=0.09), and % fractional area change (p=0.09) in the RVPAS than the MBTS group at 14 months that did not reach statistical significance. These differences were not seen for any longitudinal indices, which do not assess the RV anterior wall, suggesting a possible subtle early impact of the RVPAS ventriculotomy on RV circumferential deformation, particularly of the anterior wall. The lack of statistical significance for circumferential strain and strain rate may reflect technical issues, such as higher variation between patients in RV strain measurement(13) or variability in image acquisition.
The subtle changes identified in single RV circumferential mechanics could reflect early changes in RV function that contribute to the loss of survival benefit for the RVPAS cohort after the first year of life(4). Although normal RV systolic shortening in a biventricular circulation is predominantly longitudinal, the systemic RV adopts a more circumferential contraction pattern similar to the normal left ventricle. A lack of this transition has been associated with pathologically increased ventricular dilatation and increased ventricular mass (12, 19). Based on this, any change that decreases circumferential deformation might impact global function in the long term. Continued assessment of RV deformation in the SVR cohort may determine whether these changes become more pronounced over time. An ongoing Pediatric Heart Network-sponsored SVR extension study will provide additional clinical and echocardiographic surveillance through age 6 years and may be able to answer the question of whether changes in circumferential deformation are progressive and of clinic significance.
There are limitations to this study. The cohort included only survivors free from transplantation at 14 months of age. Given the differential transplant-free survival at 12 months of age between the groups, the deformation indices could be skewed. Not all patients had adequate images for analysis, emphasizing the challenge of multi-institutional echocardiographic trials that require extensive data acquisition, particularly when obtaining the imaging from infants and young children with complex heart disease who may have challenges with acoustic windows and cooperation. Importantly, the assessment of regional and global deformation was not a part of the original echocardiographic SVR protocol. Given that the lack of adequate imaging was likely random and not related to any patient-specific co-morbidity, extrapolation of this data as representative of the entire cohort is appropriate. Regional RV myocardial assessment was also limited by the lack of focused high-frame rate captures in these studies. Lower frame rates may have negatively impacted accuracy of speckle tracking, and did not allow quantitation of regional RV dyssynchrony. The deformational analysis of the RV did not include the outflow tract but this should not be impacted by shunt type. Intra- and inter-observer variability was not performed in this study but has been previously assessed for speckle tracking in a single RV cohort(12). Instead, we intentionally were highly selective in our acceptance of adequate images in order to ensure reliable data by reviewing each subject’s dataset by 2 experienced pediatric echocardiographers with experience in speckle tracking. It has been observed that the values obtained by vendor-independent software do not consistently correlate with the values obtained using vendor-dependent packages acquired from different ultrasound systems(20). For this study, a single off-line software analysis tool was used to obtain the deformation indices to ensure consistency.
CONCLUSIONS
Speckle tracking echocardiography assessment of circumferential and longitudinal deformation indices are available for the majority of survivors at 14 months of age in the SVR cohort from 2D echocardiograms, even when imaging is not targeted to optimize the assessment of deformation. No significant shunt-related differences were identified, though there were trends of decreased global and regional circumferential anterior wall deformation in the RVPAS compared to MBTS group that correlate with the location of the ventriculotomy. Long-term serial evaluations are needed to assess whether changes in RV deformation become clear over time and correlate with poorer outcomes prior to and after Fontan procedure.
Highlights.
Shunt types at Norwood procedure were compared using speckle tracking echocardiography
Global and regional deformation were compared at 14 months of age
There are no significant differences between shunt groups
Some differences approached significance for decreased indices in the RVPAS group
These differences may represent early changes resulting from the ventriculotomy
Acknowledgments
Supported by grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288, HL085057).
Footnotes
This work is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fortuna RS, Ruzmetov M, Geiss DM. Outcomes of the Modified Norwood Procedure: Hypoplastic Left Heart Syndrome Versus Other Single-Ventricle Malformations. Pediatr Cardiol. 2013 doi: 10.1007/s00246-013-0747-8. [DOI] [PubMed] [Google Scholar]
- 2.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaynor JW, Mahle WT, Cohen MI, Ittenbach RF, DeCampli WM, Steven JM, et al. Risk factors for mortality after the Norwood procedure. Eur J Cardiothorac Surg. 2002;22:82–89. doi: 10.1016/s1010-7940(02)00198-7. [DOI] [PubMed] [Google Scholar]
- 4.Newburger JW, Sleeper LA, Frommelt PC, Pearson GD, Mahle WT, Chen S, et al. Transplantation-free survival and interventions at 3 years in the single ventricle reconstruction trial. Circulation. 2014;129:2013–2020. doi: 10.1161/CIRCULATIONAHA.113.006191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barron DJ. The Norwood procedure: in favor of the RV-PA conduit. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2013;16:52–58. doi: 10.1053/j.pcsu.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Bellsham-Revell HR, Simpson JM, Miller OI, Bell AJ. Subjective evaluation of right ventricular systolic function in hypoplastic left heart syndrome: how accurate is it? J Am Soc Echocardiogr. 2013;26:52–56. doi: 10.1016/j.echo.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iriart X, Horovitz A, van Geldorp IE, Barnetche T, Lederlin M, De Guillebon M, et al. The role of echocardiography in the assessment of right ventricular systolic function in patients with transposition of the great arteries and atrial redirection. Arch Cardiovasc Dis. 2012;105:432–441. doi: 10.1016/j.acvd.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Khattab K, Schmidheiny P, Wustmann K, Wahl A, Seiler C, Schwerzmann M. Echocardiogram versus cardiac magnetic resonance imaging for assessing systolic function of subaortic right ventricle in adults with complete transposition of great arteries and previous atrial switch operation. Am J Cardiol. 2013;111:908–913. doi: 10.1016/j.amjcard.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 9.Margossian R, Schwartz ML, Prakash A, Wruck L, Colan SD, Atz AM, et al. Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study) Am J Cardiol. 2009;104:419–428. doi: 10.1016/j.amjcard.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frommelt PC, Guey LT, Minich LL, Bhat M, Bradley TJ, Colan SD, et al. Does initial shunt type for the Norwood procedure affect echocardiographic measures of cardiac size and function during infancy?: the Single Vventricle Reconstruction trial. Circulation. 2012;125:2630–2638. doi: 10.1161/CIRCULATIONAHA.111.072694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow PC, Liang XC, Cheung EW, Lam WW, Cheung YF. New two-dimensional global longitudinal strain and strain rate imaging for assessment of systemic right ventricular function. Heart. 2008;94:855–859. doi: 10.1136/hrt.2007.131862. [DOI] [PubMed] [Google Scholar]
- 12.Khoo NS, Smallhorn JF, Kaneko S, Myers K, Kutty S, Tham EB. Novel insights into RV adaptation and function in hypoplastic left heart syndrome between the first 2 stages of surgical palliation. JACC Cardiovasc Imaging. 2011;4:128–137. doi: 10.1016/j.jcmg.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Petko C, Uebing A, Furck A, Rickers C, Scheewe J, Kramer HH. Changes of right ventricular function and longitudinal deformation in children with hypoplastic left heart syndrome before and after the Norwood operation. J Am Soc Echocardiogr. 2011;24:1226–1232. doi: 10.1016/j.echo.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Ohye RG, Gaynor JW, Ghanayem NS, Goldberg CS, Laussen PC, Frommelt PC, et al. Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2008;136:968–975. doi: 10.1016/j.jtcvs.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padalino MA, Castellani C, Toffoli S, Della Barbera M, Milanesi O, Thiene G, et al. Pathological changes and myocardial remodelling related to the mode of shunting following surgical palliation for hypoplastic left heart syndrome. Cardiol Young. 2008;18:415–422. doi: 10.1017/S1047951108002461. [DOI] [PubMed] [Google Scholar]
- 16.Ballweg JA, Dominguez TE, Ravishankar C, Gaynor JW, Nicolson SC, Spray TL, et al. A contemporary comparison of the effect of shunt type in hypoplastic left heart syndrome on the hemodynamics and outcome at Fontan completion. J Thorac Cardiovasc Surg. 2010;140:537–544. doi: 10.1016/j.jtcvs.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 17.Graham EM, Zyblewski SC, Phillips JW, Shirali GS, Bradley SM, Forbus GA, et al. Comparison of Norwood shunt types: do the outcomes differ 6 years later? Ann Thorac Surg. 2010;90:31–35. doi: 10.1016/j.athoracsur.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 18.Menon SC, Minich LL, Casper TC, Puchalski MD, Hawkins JA, Tani LY. Regional myocardial dysfunction following Norwood with right ventricle to pulmonary artery conduit in patients with hypoplastic left heart syndrome. J Am Soc Echocardiogr. 2011;24:826–833. doi: 10.1016/j.echo.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Tham EB, Smallhorn JF, Kaneko S, Valiani S, Myers KA, Colen TM, et al. Insights into the Evolution of Myocardial Dysfunction in the Functionally Single Right Ventricle between Staged Palliations Using Speckle-Tracking Echocardiography. J Am Soc Echocardiogr. 2014;27:314–322. doi: 10.1016/j.echo.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Koopman LP, Slorach C, Manlhiot C, McCrindle BW, Jaeggi ET, Mertens L, et al. Assessment of myocardial deformation in children using Digital Imaging and Communications in Medicine (DICOM) data and vendor independent speckle tracking software. J Am Soc Echocardiogr. 2011;24:37–44. doi: 10.1016/j.echo.2010.09.018. [DOI] [PubMed] [Google Scholar]