Abstract
Venous congestion and endothelial and neurohormonal activation are known to occur in acute decompensated heart failure (ADHF), yet the temporal role of these processes in the pathophysiology of decompensation is not fully understood. Conventional wisdom presumes congestion to be a consequence of worsening cardiovascular function; however, the biomechanically driven effects of venous congestion are biologically plausible contributors to ADHF that remain largely unexplored in vivo. Recent experimental evidence from human models suggests that fluid accumulation and venous congestion are not simply consequences of poor cardiovascular function, but rather are fundamental pro-oxidant, pro-inflammatory, and hemodynamic stimuli that contribute to acute decompensation. The latest advances in the monitoring of volume status using implantable devices allow for the detection of venous congestion before symptoms arise. This may ultimately lead to improved treatment strategies including not only diuretics, but also specific, adjuvant interventions to counteract endothelial and neurohormonal activation during early preclinical decompensation.
Keywords: Endothelial, Heart failure
Introduction
Patients with heart failure (HF) consume considerable health care resources due to frequent hospitalizations for acute de-compensated HF (ADHF). These hospitalizations are primarily driven by signs and symptoms of venous congestion, which has been shown to be an important hemodynamic predictor of worsening renal failure, rehospitalization, and post-discharge mortality in ADHF [1–9]. Two mechanisms have been proposed for the pathogenesis of venous congestion in ADHF: fluid redistribution and fluid accumulation [10–12]. The former is a primarily vascular pathway in which increased systemic resistance leads to increased afterload. Simultaneously, this leads to reduced capacitance in large veins, increased venous return to the heart, and increased preload. This mismatch between increased load and impaired cardiac function cannot be tolerated by the failing heart, ultimately leading to clinical decompensation [10, 12]. In the latter pathway, fluid progressively accumulates in the setting of cardiorenal dysfunction, leading to increased total body volume and venous congestion. In addition, medication and dietary non-compliance may cause increased sodium and water retention. Accumulating evidence suggests that venous congestion begins to develop weeks before the overt clinical decompensation that brings patients to medical attention, suggesting that it may itself be a primary contributor to disease in ADHF rather than merely a consequence of poor cardiac function [13].
On a molecular level, ADHF in humans is characterized by endothelial and neurohormonal activation, which are known to enhance oxidative stress, inflammation, and vasoconstriction. These processes are associated with elevated levels of circulating biomarkers of endothelial activation including adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) [14–18], as well as cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) [19–27]. In addition, neurohormones including endothelin-1 (ET-1) [28, 29], angiotensin II (A II), and norepinephrine (NE) are also elevated [30–32]. These markers acutely increase in patients with signs and symptoms of venous congestion who are hospitalized for ADHF and then decline as patients clinically improve, though it is not clear whether their elevation is causal or a secondary effect [33–35].
While the negative pathophysiological impact and prognostic relevance of endothelial and neurohormonal activation in ADHF is widely accepted, the biological forces driving these pathophysiologic changes remain a source of intense debate. Recent experimental evidence suggests that biomechanical forces generated by fluid retention and venous congestion are important contributors to endothelial and neurohormonal activation. The following discussion will review (i) the biomechanical modulation of the endothelial phenotype, (ii) the translational strategies that have been used to study endothelial activation in patients with ADHF, (iii) the biophysical models that allowed investigation of the effects of venous congestion on endothelial and neurohormonal activation, and (iv) the working concept that fluid accumulation and venous congestion in ADHF are not just epiphenomena, resulting secondary to poor cardiac (and renal) function, but rather fundamental pro-oxidant, pro-inflammatory, and hemo-dynamic stimuli that contribute to acute decompensation.
Biomechanical Stress and Endothelial Activation
The endothelium is a cellular monolayer that lines the entire cardiovascular system and, from this strategic position, regulates many processes including vascular tone, thrombosis, angiogenesis, and inflammation. Endothelial cells have been shown to be phenotypically dynamic and, in response to a variety of local and systemic stimuli, are able to transition between quiescent and activated states. In recent years, emerging research has demonstrated that endothelial dysfunction is a major contributor to cardiovascular disease, including hyper-tension, atherosclerosis, and more recently, congestive heart failure [36–38]. One of the most potent endothelial stimuli is the biomechanical force from hydrostatic pressure, shear, or circumferential wall stress. This force is detected by specialized mechanosensors on the endothelial cell membrane, including integrins, tyrosine kinase receptors, ion channels, and intercellular junction proteins [39]. Activation of these sensors by mechanical stress triggers an intracellular signaling cascade and causes endothelial cells to undergo a phenotypic switch to a pro-oxidant, pro-inflammatory, vasoconstricted state, both in vitro and in vivo.
Nitric oxide (NO) is a key regulator of the vascular environment through its vasodilatory, anti-oxidant and anti-inflammatory properties [40]. Released in proximity to smooth muscle cells, NO leads to increased cyclic GMP and activation of GMP-dependent kinases that decrease intracellular calcium and lead to vasorelaxation [41]. In endothelial cells, NO is primarily produced by the action of the constitutively expressed endothelial NO synthase (eNOS) [42]. In addition, inducible NO synthase (iNOS) is expressed by endothelial cells and circulating inflammatory cells in response to environmental cues [39, 43]. In both cultured endothelial cells and intact vessels ex vivo, application of biomechanical stress leads to the production of reactive oxygen species (ROS) and the concomitant upregulation of the expression and activity of both eNOS and iNOS [44, 45]. Excess NO can cause nitrosylation of proteins, leading to altered redox signaling. ROS and NO can also interact to produce reactive nitrogen species and toxic metabolites such as peroxynitrate that nitrosylates proteins on tyrosine residues and worsens local cellular damage. When endothelial cells are chronically exposed to biomechanical stress, it can generate excessive reactive oxygen species, which ultimately quench NO activity by oxidative degradation making it less bioavailable and leading to vasoconstriction [38, 46].
Endothelins are a family of potent vasoconstrictor molecules produced selectively by endothelial cells to oppose the vasodilatory effects of NO. Cultured endothelial cells exposed to circumferential stress quickly upregulate the production of ET-1 [47]. Oxidative stress, as well as other neurohormones including A II, NE, vasopressin, and IL-6, also promotes endothelin expression. Beyond endothelins, the production of other neurohormones is also modulated by vascular stretch. In particular, mechanosensors that are present in the renal vasculature are responsible for the activation of the reninangiotensin-aldosterone axis and generation of A II that in turn promote vasoconstriction both directly and indirectly [48].
Biomechanical stress also induces cytokine production by endothelial cells. In vitro, cultured endothelial cells exposed to circumferential or longitudinal stretch increase production of a variety of vasoactive mediators including TNF-α, IL-6, ICAM-1, and VCAM-1 [49–51]. In mice, the inflammatory effect of non-laminar flow on arterial endothelium has been well studied. In areas of the vessel wall that are exposed to flow reversal, oscillating shear stress, or turbulence, there is significant upregulation of pro-inflammatory genes including VCAM-1 [39, 52]. Reactive oxygen species and cytokines may also trigger an inflammatory response through activation of nuclear factor (NF)-kB [53], a transcription factor that promotes expression of iNOS and other pro-inflammatory genes such as cyclo-oxygenase-2 (COX-2), TNF-α, and ICAM-1 [54, 55].
In summary, substantial evidence from in vitro and animal studies demonstrates that the endothelium may become activated in response to mechanical stretch in order to become a primary source of oxidative stress, vasoactive peptides, and cytokine production. Whether mechanical stretch is sufficient to activate the vascular endothelium in humans, such as in the setting of venous congestion, remains unclear.
Assessment of In Vivo Endothelial Phenotype
In an attempt to bridge the gap between in vitro experiments, animal studies, and clinical science, we have introduced and validated a safe, minimally invasive method to study the venous endothelium in humans. In order to sample the venous endothelium, Bpediatric^ J-shaped endovascular wires are sequentially inserted into a superficial arm vein through a small 20-gauge angiocatheter and advanced for approximately 5 cm to dislodge endothelial cells and trap them in the serrated surface of the wire. Endothelial cells are rinsed from the wires and then processed in the laboratory using molecular techniques.
We first employed this technique to study venous endothelial activation in a cohort of 15 patients hospitalized for ADHF [56, 57]. Quantitative immunofluorescence analysis of protein expression in harvested endothelial cells revealed that nitrotyrosine (a marker of oxidative stress), COX-2, and iNOS (markers of inflammation) were significantly increased in venous endothelial cells of ADHF patients when compared with age-matched healthy subjects. Return to a steady compensated state after diuresis was associated with a substantial reduction in these same endothelial markers of oxidative stress and inflammation [58, 59]. Concurrently, plasma levels of 8-isoprostane (a systemic marker of oxidative stress) that were elevated on admission also declined after resolution of the decompensation episode.
While these findings were suggestive of an association between ADHF and endothelial activation, they did not reveal a causal link between the two. To address this mechanistic question, additional model systems were necessary in which the transition from compensated to decompensated HF could be readily studied in a more controlled experimental environment.
Venous Congestion Causes Endothelial and Neurohormonal Activation: Animal and Human Models
Experimental Systemic Venous Congestion in Dogs
We first utilized a canine model to study systemic venous congestion in a controlled in vivo system. Six normal dogs were studied at baseline and 1 h after fluid load to a target CVP ≥20 mmHg. Blood and endothelial cells were collected from jugular veins and mRNA expression was analyzed by reverse transcription polymerase chain reaction (RT-PCR). Administration of the fluid load caused endothelial activation as evidenced by significantly increased expression of several pro-oxidant and pro-inflammatory genes such as iNOS, COX-2, and TNF-α. Importantly, this experimental model of systemic venous congestion also caused neurohormonal activation as evidenced by significantly increased plasma NE and ET-1. Biomarkers of inflammation such as IL-6 and TNF-α were also increased. Overall, this experimental model provided the first evidence that systemic venous congestion is sufficient to cause endothelial and neurohormonal activation [60].
Experimental Local Venous Congestion in Humans
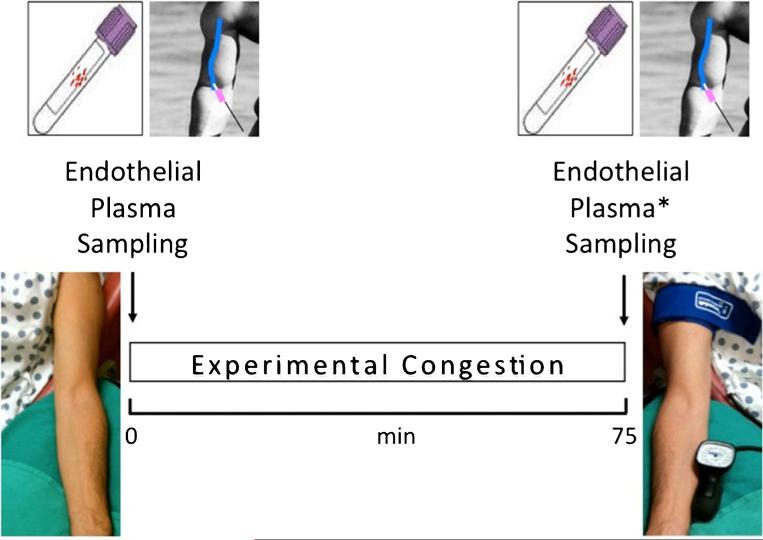
To experimentally model peripheral venous congestion in humans, we then developed a new model, termed the venous stress test in 24 healthy subjects [61••]. Venous arm pressure was increased to ~30 mmHg above baseline by inflating a tourniquet cuff around the dominant arm (test arm). At baseline and after 75 min of venous congestion, blood and endothelial cells were sampled from the test and control arm (lacking an inflated cuff) (Fig. 1). The induction of venous congestion was associated with a 60 % increase in plasma levels of IL-6 and ET-1 in the test arm compared to the control arm (Table 1). VCAM-1, ICAM-1, and A II were also significantly increased in the test arm. In contrast to our earlier finding in the canine model, plasma TNF-α levels did not change. Differences in experimental models (i.e., systemic vs. local venous congestion) and between animal species (i.e., dogs vs. humans) may account for this discrepancy. A major logistical step forward in the analysis of the endothelial phenotype was achieved with the move from single candidate gene analyses such as RT-PCR to high-throughput molecular biological techniques such as microarray. Experimental local venous congestion was sufficient to cause endothelial and neurohormonal activation in healthy humans. More than 3000 mRNA probe sets were differentially expressed in venous endothelial cells between samples from test versus control arms, including ET-1 and VCAM-1. Preliminary results from 42 compensated patients with heart failure with reduced ejection fraction (HFrEF) on optimal medical treatment suggest that this new experimental model of local congestion can also promote endothelial and neurohormonal activation, as evidenced by an increase in plasma ET-1, IL-6, and VCAM-1 in this patient population [62]. In this more recent study, we also provided the first direct evidence that peripheral experimental venous congestion causes local release of 8-isoprostane (a marker of oxidative stress) into the blood stream. Additional preliminary data on plasma ET-1 suggest the magnitude of activation to be dependent on the severity and duration of the congestion protocol [63]. Future studies using venous stress test are warranted to investigate (i) whether a similar pattern of endothelial and neurohormonal activation occurs in patients with heart failure with preserved ejection fraction (HFpEF) and (ii) whether changes in endothelial cell phenotype differ between healthy individuals and HF patients.
Fig. 1.
Venous stress test. Blood and endothelial cells were sampled from the antecubital or basilic vein of the non-dominant arm (control arm) at baseline and of the dominant arm (test arm) after 75 min of local venous congestion using angiocatheters and endovascular wires. Peripheral venous pressure was increased ~30 mmHg above baseline levels by inflating a tourniquet cuff around the test arm, proximally, just below the shoulder. Blood was also obtained at 75 min from the control arm that was not exposed to venous congestion, thus serving as a control (asterisk) [61••]
Table 1.
Plasma measurements (mean±s.e.m.) before and after 75 min of venous congestion
| Variable | Baseline (B) (0 min) | Control arm (C) (75 min) | Test arm (T) (75 min) | P(Tvs. B) | P(Tvs. C) |
|---|---|---|---|---|---|
| IL-6 (pg/mL) | 1.37±0.44 | 1.79±0.53 | 2.26±0.58 | <0.01 | <0.01 |
| TNF-α (pg/mL) | 1.35±0.08 | 1.27±0.08 | 1.35±0.11 | 0.75 | 0.22 |
| ET-1 (pg/mL) | 1.46±0.19 | 1.26±0.13 | 2.43±0.27 | <0.0001 | <0.0001 |
| AII (pg/mL) | 27±3 | 25±3 | 32±4 | 0.01 | <0.01 |
| VCAM-1 (ng/mL) | 557±26 | 544±24 | 589±25 | <0.01 | <0.01 |
| ICAM-1 (ng/mL) | 158±9 | 158±7 | 167±9 | 0.09 | 0.06 |
| vWF:Ag (%) | 105±9 | 100±6 | 113±9 | 0.37 | 0.15 |
From Colombo PC, Onat D, Harxhi A, Demmer RT, Hayashi Y, Jelic S, et al. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. European heart journal. 2014;35(7):448-54, with permission from Oxford University Press
IL-6 interleukin-6, TNF-α tissue necrosis factor-α, ET-1 endothelin-1, AII angiotensin II, VCAM-1 vascular adhesion molecule–1, ICAM-1 intercellular adhesion molecule–1, vWF:Ag vonWillebrand factor antigen
An alternative model of local venous congestion involves the infusion of normal saline in the veins of an occluded vascular bed in the human forearm. Venous distention in this model evoked systemic sympathetic activation in 19 healthy subjects, as evidenced by a large increase in muscle sympathetic nerve activity (MSNA) and arterial blood pressure [64••]. Stimulation of muscle afferent nerves, which are located in proximity to small limb veins, has been proposed as the responsible mechanism [65]. In additional studies, the same group reported that the magnitude of the MSNA and blood pressure responses depended on the volume and the rate of the saline infused [66]. Of note, a systemic sympathetic response was specific to the peripheral venous compartment, as distention of central great veins did not increase MSNA [67••].
Overall, results from the above models indicate that experimental congestion is sufficient to cause endothelial and neurohormonal activation as well as inflammation in large animals and humans. Importantly, the pro-oxidant, pro-inflammatory, and vasoconstrictive phenotype characterized in these models mimics, at least in part, notable aspects of the neurohormonal and hemodynamic phenotype that is typical of patients with ADHF.
Cross-talk Between Fluid Accumulation Pathway and Fluid Redistribution (Vascular) Pathway in the Pathophysiology of ADHF
Pulmonary venous congestion is found in the majority of patients hospitalized for ADHF. Two mechanisms have previously been suggested for the pathophysiology of cardiogenic pulmonary edema (and adopted by the European Society of Cardiology guidelines on the diagnosis and treatment of ADHF): a vascular (fluid redistribution) pathway and a cardiorenal (fluid accumulation) pathway. The first pathway results from vasoconstriction causing an increase in preload and afterload with redistribution of intravascular volume from the periphery to the pulmonary circulation. The second mechanism results from impaired cardiac (and renal) function, de novo accumulation of fluid, and increase in preload leading to progressive pulmonary congestion and worsening symptoms [10, 11, 68, 69].
While both pathways have been individually postulated to play an important role in the development of ADHF, herein we have highlighted the animal and human models of venous congestion that provide evidence for a new pathophysiological link between these two pathways (Fig. 2, red arrow). Endothelial stretch and tissue edema can cause vasoconstriction through a local increase in oxidative stress and the systemic release of ET-1 and A II. In addition, peripheral venous dis-tension promotes an acute inflammatory response that (i) may worsen arterial stiffness [70] and (ii) directly evokes systemic sympathetic activation through a powerful autonomic reflex, further linking venous congestion to vasoconstriction [64••, 65, 66, 67••, 71]. Importantly, because veins represent a low-pressure reservoir that contains >70 % of the systemic blood volume, even relatively small volume changes can cause substantial alterations in central blood volume, and thus in cardiac filling pressures [72].
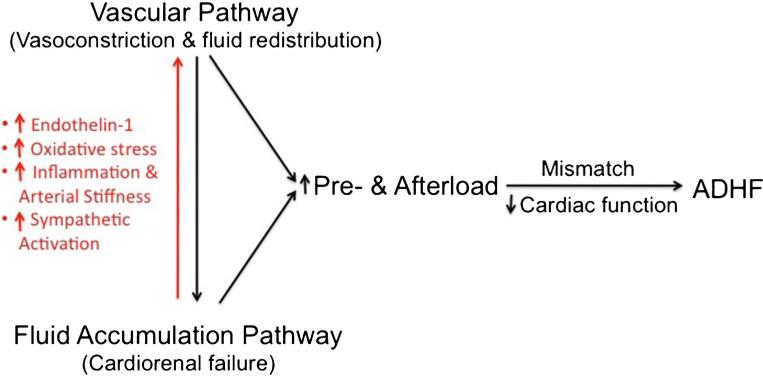
Fig. 2.
Hypothesized causal link between fluid accumulation and fluid redistribution pathways in the pathophysiology of ADHF. The red and black arrows represent a positive feedback loop between fluid accumulation and redistribution pathways. While the black arrow represents a known mechanism, the red arrow represents a novel component of ADHF pathophysiology suggesting fluid accumulation as a potential cause of vasoconstriction
Figure 2 details this working concept. The mismatch between increased load (both preload and afterload) and impaired cardiac function, which is typical of ADHF, may primarily stem from (i) vasoconstriction and fluid redistribution (vascular pathway) and/or (ii) enhanced de novo fluid accumulation (cardiorenal pathway). However initiated, the interaction of these two pathways may provoke a vicious cycle as fluid accumulation causes vasoconstriction while vasoconstriction increases cardiovascular filling pressures. The resulting progressive deterioration in clinical symptoms may eventually lead to hospitalization for ADHF.
Conclusions
Collectively, the findings of the past decade linking fluid retention and venous congestion to endothelial and neurohormonal activation have provided a new conceptual framework to better understand the pathophysiology of ADHF. While unquestionably endothelial and neurohormonal activation from venous congestion represent the effect of poor cardiac function, they concurrently cause the progressive hemodynamic deterioration that is typical of ADHF. Chronically implanted devices such as the recently FDA-approved cardioMEMS [73•, 74•] will provide a valuable tool for the early detection and treatment of pulmonary congestion in the setting of impending decompensation. An early treatment strategy with diuretics and, hypothetically, adjuvant means (e.g., short-term anti-oxidant and/or anti-inflammatory therapies) may not only improve symptoms but also restore the state of endothelial and neurohormonal quiescence that keeps patients compensated and out of the hospital.
Acknowledgments
This study was supported by the A. L. Mailman Family Foundation, NIH Grant Number HL092144 and NIH Grant Number DE018739.
Footnotes
This article is part of the Topical Collection on Decompensated Heart Failure
Compliance with Ethics Guidelines
Conflict of Interest Paolo C. Colombo, Amanda C. Doran, Duygu Onat, Ka Yuk Wong, Myra Ahmad, Hani N. Sabbah, and Ryan T. Demmer declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Paolo C. Colombo, Division of Cardiology, College of Physicians & Surgeons, Department of Medicine, Columbia University, 622 West 168th Street, PH 12-134, New York, NY 10032, USA
Amanda C. Doran, Division of Cardiology, College of Physicians & Surgeons, Department of Medicine, Columbia University, 622 West 168th Street, PH 12-134, New York, NY 10032, USA
Duygu Onat, Division of Cardiology, College of Physicians & Surgeons, Department of Medicine, Columbia University, 630 West 168th Street, P&S 17-401, New York, NY 10032, USA.
Ka Yuk Wong, Division of Cardiology, College of Physicians & Surgeons, Department of Medicine, Columbia University, 630 West 168th Street, P&S 8-510, New York, NY 10032, USA.
Myra Ahmad, Division of Cardiology, College of Physicians & Surgeons, Department of Medicine, Columbia University, 630 West 168th Street, P&S 8-510, New York, NY 10032, USA.
Hani N. Sabbah, Division of Cardiovascular Medicine, Department of Medicine, Henry Ford Heart and Vascular Institute, 2799 West Grand Boulevard, Detroit, MI 48202, USA
Ryan T. Demmer, Department of Epidemiology, Mailman School of Public Health, Columbia University, 722 West 168th Street, 7th floor, New York, NY 10032, USA
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Allen LA, Metra M, Milo-Cotter O, Filippatos G, Reisin LH, Bensimhon DR, et al. Improvements in signs and symptoms during hospitalization for acute heart failure follow different patterns and depend on the measurement scales used: an international, prospective registry to evaluate the evolution of Measures of Disease Severity in Acute Heart Failure (MEASURE-AHF). J Card Fail. 2008;14(9):777–84. doi: 10.1016/j.cardfail.2008.07.188. doi:10.1016/j.cardfail.2008.07.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53(7):582–8. doi: 10.1016/j.jacc.2008.08.080. doi:10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 3.Felker GM, Adams KF, Jr, Konstam MA, O'Connor CM, Gheorghiade M. The problem of decompensated heart failure: nomenclature, classification, and risk stratification. Am Heart J. 2003;145(2 Suppl):S18–25. doi: 10.1067/mhj.2003.150. doi:10.1067/mhj.2003.150. [DOI] [PubMed] [Google Scholar]
- 4.Fonarow GC, Abraham WT, Albert NM, Gattis Stough W, Gheorghiade M, Greenberg BH, et al. Influence of a performance-improvement initiative on quality of care for patients hospitalized with heart failure: results of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Arch Intern Med. 2007;167(14):1493–502. doi: 10.1001/archinte.167.14.1493. doi:10.1001/archinte.167.14.1493. [DOI] [PubMed] [Google Scholar]
- 5.Fonarow GC, Heywood JT, Heidenreich PA, Lopatin M, Yancy CW. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2007;153(6):1021–8. doi: 10.1016/j.ahj.2007.03.012. doi:10.1016/j.ahj.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med. 2006;119(12 Suppl 1):S3–10. doi: 10.1016/j.amjmed.2006.09.011. doi:10.1016/j.amjmed.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Gattis WA, O'Connor CM, Adams KF, Jr, Elkayam U, Barbagelata A, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA. 2004;291(16):1963–71. doi: 10.1001/jama.291.16.1963. doi: 10.1001/jama.291.16.1963. [DOI] [PubMed] [Google Scholar]
- 8.Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, et al. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005;112(25):3958–68. doi: 10.1161/CIRCULATIONAHA.105.590091. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 9.Lucas C, Johnson W, Hamilton MA, Fonarow GC, Woo MA, Flavell CM, et al. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am Heart J. 2000;140(6):840–7. doi: 10.1067/mhj.2000.110933. doi:10.1067/mhj.2000.110933. [DOI] [PubMed] [Google Scholar]
- 10.Cotter G, Metra M, Milo-Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure—re-distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008;10(2):165–9. doi: 10.1016/j.ejheart.2008.01.007. doi:10.1016/j.ejheart.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Dorhout Mees EJ. Diastolic heart failure: a confusing concept. Heart Fail Rev. 2013;18(4):503–9. doi: 10.1007/s10741-012-9344-9. doi:10.1007/s10741-012-9344-9. [DOI] [PubMed] [Google Scholar]
- 12.Felker GM, Cotter G. Unraveling the pathophysiology of acute heart failure: an inflammatory proposal. Am Heart J. 2006;151(4):765–7. doi: 10.1016/j.ahj.2005.07.004. doi:10.1016/j.ahj.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Yu CM, Wang L, Chau E, Chan RH, Kong SL, Tang MO, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112(6):841–8. doi: 10.1161/CIRCULATIONAHA.104.492207. doi:10.1161/CIRCULATIONAHA.104.492207. [DOI] [PubMed] [Google Scholar]
- 14.Tsutamoto T, Hisanaga T, Fukai D, Wada A, Maeda Y, Maeda K, et al. Prognostic value of plasma soluble intercellular adhesion molecule-1 and endothelin-1 concentration in patients with chronic congestive heart failure. Am J Cardiol. 1995;76(11):803–8. doi: 10.1016/s0002-9149(99)80231-8. [DOI] [PubMed] [Google Scholar]
- 15.Andreassen AK, Nordoy I, Simonsen S, Ueland T, Muller F, Froland SS, et al. Levels of circulating adhesion molecules in congestive heart failure and after heart transplantation. Am J Cardiol. 1998;81(5):604–8. doi: 10.1016/s0002-9149(97)00972-7. [DOI] [PubMed] [Google Scholar]
- 16.Klein RM, Breuer R, Mundhenke M, Schwartzkopff B, Strauer BE. Circulating adhesion molecules (cICAM-1, lcVCAM-1) in patients with suspected inflammatory heart muscle disease. Z Kardiol. 1998;87(2):84–93. doi: 10.1007/s003920050158. [DOI] [PubMed] [Google Scholar]
- 17.Yin WH, Chen JW, Jen HL, Chiang MC, Huang WP, Feng AN, et al. The prognostic value of circulating soluble cell adhesion molecules in patients with chronic congestive heart failure. Eur J Heart Fail. 2003;5(4):507–16. doi: 10.1016/s1388-9842(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 18.Bonomini M, Reale M, Santarelli P, Stuard S, Settefrati N, Albertazzi A. Serum levels of soluble adhesion molecules in chronic renal failure and dialysis patients. Nephron. 1998;79(4):399–407. doi: 10.1159/000045084. [DOI] [PubMed] [Google Scholar]
- 19.Munger MA, Johnson B, Amber IJ, Callahan KS, Gilbert EM. Circulating concentrations of proinflammatory cytokines in mild or moderate heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1996;77(9):723–7. doi: 10.1016/s0002-9149(97)89206-5. [DOI] [PubMed] [Google Scholar]
- 20.Testa M, Yeh M, Lee P, Fanelli R, Loperfido F, Berman JW, et al. Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J Am Coll Cardiol. 1996;28(4):964–71. doi: 10.1016/s0735-1097(96)00268-9. [DOI] [PubMed] [Google Scholar]
- 21.Packer M. Is tumor necrosis factor an important neurohormonal mechanism in chronic heart failure? Circulation. 1995;92(6):1379–82. doi: 10.1161/01.cir.92.6.1379. [DOI] [PubMed] [Google Scholar]
- 22.Carlstedt F, Lind L, Lindahl B. Proinflammatory cytokines, measured in a mixed population on arrival in the emergency department, are related to mortality and severity of disease. J Intern Med. 1997;242(5):361–5. doi: 10.1046/j.1365-2796.1997.00209.x. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto M, Tsujino T, Lee-Kawabata M, Naito Y, Sakoda T, Ohyanagi M, et al. Serum interleukin-6 and C-reactive protein are markedly elevated in acute decompensated heart failure patients with left ventricular systolic dysfunction. Cytokine. 2010;49(3):264–8. doi: 10.1016/j.cyto.2009.11.006. doi:10.1016/j.cyto.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Barreto DV, Barreto FC, Liabeuf S, Temmar M, Lemke HD, Tribouilloy C, et al. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010;77(6):550–6. doi: 10.1038/ki.2009.503. doi:10.1038/ki.2009.503. [DOI] [PubMed] [Google Scholar]
- 25.Descamps-Latscha B, Herbelin A, Nguyen AT, Roux-Lombard P, Zingraff J, Moynot A, et al. Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J Immunol. 1995;154(2):882–92. [PubMed] [Google Scholar]
- 26.Francis SE, Holden H, Holt CM, Duff GW. Interleukin-1 in myocardium and coronary arteries of patients with dilated cardiomyopathy. J Mol Cell Cardiol. 1998;30(2):215–23. doi: 10.1006/jmcc.1997.0592. doi:10.1006/jmcc.1997.0592. [DOI] [PubMed] [Google Scholar]
- 27.Sharma A, Hermann DD, Mehta RL. Clinical benefit and approach of ultrafiltration in acute heart failure. Cardiology. 2001;96(3–4):144–54. doi: 10.1159/000047398. [DOI] [PubMed] [Google Scholar]
- 28.Rodeheffer RJ, Lerman A, Heublein DM, Burnett JC., Jr Increased plasma concentrations of endothelin in congestive heart failure in humans. Mayo Clin Proc. 1992;67(8):719–24. doi: 10.1016/s0025-6196(12)60795-2. [DOI] [PubMed] [Google Scholar]
- 29.Cottone S, Mule G, Guarneri M, Palermo A, Lorito MC, Riccobene R, et al. Endothelin-1 and F2-isoprostane relate to and predict renal dysfunction in hypertensive patients. Nephrol Dial Transplant. 2009;24(2):497–503. doi: 10.1093/ndt/gfn489. doi:10.1093/ndt/gfn489. [DOI] [PubMed] [Google Scholar]
- 30.Dzau VJ, Colucci WS, Hollenberg NK, Williams GH. Relation of the renin-angiotensin-aldosterone system to clinical state in congestive heart failure. Circulation. 1981;63(3):645–51. doi: 10.1161/01.cir.63.3.645. [DOI] [PubMed] [Google Scholar]
- 31.Lauten A, Ferrari M, Goebel B, Rademacher W, Schumm J, Uth O, et al. Microvascular tissue perfusion is impaired in acutely decompensated heart failure and improves following standard treatment. Eur J Heart Fail. 2011;13(7):711–7. doi: 10.1093/eurjhf/hfr043. doi:10.1093/eurjhf/hfr043. [DOI] [PubMed] [Google Scholar]
- 32.Paton AM, Lever AF, Oliver NW, Medina A, Briggs JD, Morton JJ, et al. Plasma angiotensin II, renin, renin-substrate and aldosterone concentrations in acute renal failure in man. Clin Nephrol. 1975;3(1):18–23. [PubMed] [Google Scholar]
- 33.Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD). Circulation. 1990;82(5):1724–9. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 34.Milo O, Cotter G, Kaluski E, Brill A, Blatt A, Krakover R, et al. Comparison of inflammatory and neurohormonal activation in cardiogenic pulmonary edema secondary to ischemic versus nonischemic causes. Am J Cardiol. 2003;92(2):222–6. doi: 10.1016/s0002-9149(03)00545-9. [DOI] [PubMed] [Google Scholar]
- 35.White M, Ducharme A, Ibrahim R, Whittom L, Lavoie J, Guertin MC, et al. Increased systemic inflammation and oxidative stress in patients with worsening congestive heart failure: improvement after short-term inotropic support. Clin Sci (Lond) 2006;110(4):483–9. doi: 10.1042/CS20050317. doi:10.1042/CS20050317. [DOI] [PubMed] [Google Scholar]
- 36.Gutierrez E, Flammer AJ, Lerman LO, Elizaga J, Lerman A, Fernandez-Aviles F. Endothelial dysfunction over the course of coronary artery disease. Eur Heart J. 2013;34(41):3175–81. doi: 10.1093/eurheartj/eht351. doi: 10.1093/eurheartj/eht351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60(16):1455–69. doi: 10.1016/j.jacc.2011.11.082. doi:10.1016/j.jacc.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 38.Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, et al. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9(10):1057–69. doi: 10.7150/ijbs.7502. doi:10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol. 2014;34(10):2191–8. doi: 10.1161/ATVBAHA.114.303422. doi:10.1161/ATVBAHA.114.303422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell JA, Ali F, Bailey L, Moreno L, Harrington LS. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp Physiol. 2008;93(1):141–7. doi: 10.1113/expphysiol.2007.038588. doi:10.1113/expphysiol.2007.038588. [DOI] [PubMed] [Google Scholar]
- 41.Cannon RO., 3rd Role of nitric oxide in cardiovascular disease: focus on the endothelium. Clin Chem. 1998;44(8 Pt 2):1809–19. [PubMed] [Google Scholar]
- 42.Drexler H. Nitric oxide synthases in the failing human heart: a doubled-edged sword? Circulation. 1999;99(23):2972–5. doi: 10.1161/01.cir.99.23.2972. [DOI] [PubMed] [Google Scholar]
- 43.Starling RC. Inducible nitric oxide synthase in severe human heart failure: impact of mechanical unloading. J Am Coll Cardiol. 2005;45(9):1425–7. doi: 10.1016/j.jacc.2005.02.021. doi:10.1016/j.jacc.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 44.Boo YC. Shear stress stimulates phosphorylation of protein kinase A substrate proteins including endothelial nitric oxide synthase in endothelial cells. Exp Mol Med. 2006;38(4):453. doi: 10.1038/emm.2006.53. doi:10.1038/emm.2006.53. [DOI] [PubMed] [Google Scholar]
- 45.Jin ZG, Ueba H, Tanimoto T, Lungu AO, Frame MD, Berk BC. Ligand-independent activation of vascular endothelial growth factor receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthase. Circ Res. 2003;93(4):354–63. doi: 10.1161/01.RES.0000089257.94002.96. doi:10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh HJ, Liu CA, Huang B, Tseng AH, Wang DL. Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysio-logical implications. J Biomed Sci. 2014;21:3. doi: 10.1186/1423-0127-21-3. doi:10.1186/1423-0127-21-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasdai D, Holmes DR, Jr, Garratt KN, Edwards WD, Lerman A. Mechanical pressure and stretch release endothelin-1 from human atherosclerotic coronary arteries in vivo. Circulation. 1997;95(2):357–62. doi: 10.1161/01.cir.95.2.357. [DOI] [PubMed] [Google Scholar]
- 48.Delli Gatti C, Osto E, Kouroedov A, Eto M, Shaw S, Volpe M, et al. Pulsatile stretch induces release of angiotensin II and oxidative stress in human endothelial cells: effects of ACE inhibition and AT1 receptor antagonism. Clin Exp Hypertens. 2008;30(7):616–27. doi: 10.1080/10641960802443183. doi:10.1080/10641960802443183. [DOI] [PubMed] [Google Scholar]
- 49.Cheng JJ, Wung BS, Chao YJ, Wang DL. Cyclic strain enhances adhesion of monocytes to endothelial cells by increasing intercellular adhesion molecule-1 expression. Hypertension. 1996;28(3):386–91. doi: 10.1161/01.hyp.28.3.386. [DOI] [PubMed] [Google Scholar]
- 50.Kawai M, Naruse K, Komatsu S, Kobayashi S, Nagino M, Nimura Y, et al. Mechanical stress-dependent secretion of interleukin 6 by endothelial cells after portal vein embolization: clinical and experimental studies. J Hepatol. 2002;37(2):240–6. doi: 10.1016/s0168-8278(02)00171-x. [DOI] [PubMed] [Google Scholar]
- 51.Wang BW, Chang H, Lin S, Kuan P, Shyu KG. Induction of matrix metalloproteinases-14 and -2 by cyclical mechanical stretch is mediated by tumor necrosis factor-alpha in cultured human umbilical vein endothelial cells. Cardiovasc Res. 2003;59(2):460–9. doi: 10.1016/s0008-6363(03)00428-0. [DOI] [PubMed] [Google Scholar]
- 52.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6(1):16–26. doi: 10.1038/ncpcardio1397. doi:10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Canty TG, Jr, Boyle EM, Jr, Farr A, Morgan EN, Verrier ED, Pohlman TH. Oxidative stress induces NF-kappaB nuclear translocation without degradation of IkappaBalpha. Circulation. 1999;100(19 Suppl):II361–4. doi: 10.1161/01.cir.100.suppl_2.ii-361. [DOI] [PubMed] [Google Scholar]
- 54.Boyle EM, Jr, Canty TG, Jr, Morgan EN, Yun W, Pohlman TH, Verrier ED. Treating myocardial ischemia-reperfusion injury by targeting endothelial cell transcription. Ann Thorac Surg. 1999;68(5):1949–53. doi: 10.1016/s0003-4975(99)01033-4. [DOI] [PubMed] [Google Scholar]
- 55.Hung TH, Charnock-Jones DS, Skepper JN, Burton GJ. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am J Pathol. 2004;164(3):1049–61. doi: 10.1016/s0002-9440(10)63192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95(8):773–9. doi: 10.1161/01.RES.0000145728.22878.45. doi:10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 57.Sumpio BE, Riley JT, Dardik A. Cells in focus: endothelial cell. Int J Biochem Cell Biol. 2002;34(12):1508–12. doi: 10.1016/s1357-2725(02)00075-4. [DOI] [PubMed] [Google Scholar]
- 58.Onat D, Jelic S, Schmidt AM, Pile-Spellman J, Homma S, Padeletti M, et al. Vascular endothelial sampling and analysis of gene transcripts: a new quantitative approach to monitor vascular inflammation. J Appl Physiol. 2007;103(5):1873–8. doi: 10.1152/japplphysiol.00367.2007. doi:10.1152/japplphysiol.00367.2007. [DOI] [PubMed] [Google Scholar]
- 59.Colombo PC, Banchs JE, Celaj S, Talreja A, Lachmann J, Malla S, et al. Endothelial cell activation in patients with decompensated heart failure. Circulation. 2005;111(1):58–62. doi: 10.1161/01.CIR.0000151611.89232.3B. doi:10.1161/01.CIR.0000151611.89232.3B. [DOI] [PubMed] [Google Scholar]
- 60.Colombo PC, Rastogi S, Onat D, Zaca V, Gupta RC, Jorde UP, et al. Activation of endothelial cells in conduit veins of dogs with heart failure and veins of normal dogs after vascular stretch by acute volume loading. J Card Fail. 2009;15(5):457–63. doi: 10.1016/j.cardfail.2008.12.006. doi:10.1016/j.cardfail.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61••.Colombo PC, Onat D, Harxhi A, Demmer RT, Hayashi Y, Jelic S, et al. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur Heart J. 2014;35(7):448–54. doi: 10.1093/eurheartj/eht456. doi:10.1093/eurheartj/eht456. [This recent article provides mechanistic in vivo evidence for a link between peripheral venous congestion and activation of the inflammatory/oxidative program within endothelial cells of humans.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colombo PC, Onat D, Harxhi A, Hayashi Y, Wong KY, Uriel N, et al. Acute venous congestion enhances vasoconstriction, inflammation, endothelial activation and oxidative stress in compensated ambulatory patients with systolic heart failure on an optimized medical regimen. Circulation. 2014 (AHA abstract) [Google Scholar]
- 63.Hawk C, Hayashu Y, Kin J, Chudasama N, Ramnauth DS, Wong KY, et al. Peripheral venous congestion causes time- and dose-dependent release of endothelin-1 in humans. Circulation. 2014 doi: 10.14814/phy2.13118. (AHA abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64••.Cui J, McQuillan P, Moradkhan R, Pagana C, Sinoway LI. Sympathetic responses during saline infusion into the veins of an occluded limb. J Physiol. 2009;587(Pt 14):3619–28. doi: 10.1113/jphysiol.2009.173237. doi:10.1113/jphysiol.2009.173237. [This important article demonstrates that peripheral venous congestion is sufficient to evoke systemic sympathetic activation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cui J, McQuillan PM, Blaha C, Kunselman AR, Sinoway LI. Limb venous distension evokes sympathetic activation via stimulation of the limb afferents in humans. Am J Physiol Heart Circ Physiol. 2012;303(4):H457–63. doi: 10.1152/ajpheart.00236.2012. doi:10.1152/ajpheart.00236.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cui J, Leuenberger UA, Gao Z, Sinoway LI. Sympathetic and cardiovascular responses to venous distension in an occluded limb. Am J Physiol Regul Integr Comp Physiol. 2011;301(6):R1831–7. doi: 10.1152/ajpregu.00170.2011. doi:10.1152/ajpregu.00170.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67••.Cui J, Gao Z, Blaha C, Herr MD, Mast J, Sinoway LI. Distension of central great vein decreases sympathetic outflow in humans. Am J Physiol Heart Circ Physiol. 2013;305(3):H378–85. doi: 10.1152/ajpheart.00019.2013. doi:10.1152/ajpheart.00019.2013. [This important study demonstrated that central saline infusion leads to activation of the sympathetic response, increase in blood pressure and decrease in MSNA levels.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mann DL. Mechanisms and models in heart failure: a combinatorial approach. Circulation. 1999;100(9):999–1008. doi: 10.1161/01.cir.100.9.999. [DOI] [PubMed] [Google Scholar]
- 69.Masoumi A, Ortiz F, Radhakrishnan J, Schrier RW, Colombo PC. Mineralocorticoid receptor antagonists as diuretics: can congestive heart failure learn from liver failure? Heart Fail Rev. 2014 doi: 10.1007/s10741-014-9467-2. doi:10.1007/s10741-014-9467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, et al. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112(14):2193–200. doi: 10.1161/CIRCULATIONAHA.105.535435. doi:10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]
- 71.Chen X, Rahman MA, Floras JS. Effects of forearm venous occlusion on peroneal muscle sympathetic nerve activity in healthy subjects. Am J Cardiol. 1995;76(3):212–4. [PubMed] [Google Scholar]
- 72.Colombo PC, Onat D, Sabbah HN. Acute heart failure as “acute endothelitis”—interaction of fluid overload and endothelial dysfunction. Eur J Heart Fail. 2008;10(2):170–5. doi: 10.1016/j.ejheart.2007.12.007. doi:10.1016/j.ejheart.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 73•.Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377(9766):658–66. doi: 10.1016/S0140-6736(11)60101-3. doi:10.1016/S0140-6736(11)60101-3. [This landmark study introduces the advantages of long term invasive hemodynamic monitoring in heart failure.] [DOI] [PubMed] [Google Scholar]
- 74•.Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7(6):935–44. doi: 10.1161/CIRCHEARTFAILURE.113.001229. doi: 10.1161/CIRCHEARTFAILURE.113.001229. [This recent article demonstrates the successful use of invasive hemodynamic monitoring for the long term management of heart failure in order to prevent heart failure hospitalizations.] [DOI] [PubMed] [Google Scholar]