Supplemental Digital Content is Available in the Text.
Key Words: cornea, preservation time, Descemet stripping automated endothelial keratoplasty
Abstract
Purpose:
The aim of this study was to describe the aims, methods, donor and recipient cohort characteristics, and potential impact of the Cornea Preservation Time Study (CPTS).
Methods:
The CPTS is a randomized clinical trial conducted at 40 clinical sites (70 surgeons) designed to assess the effect of donor cornea preservation time (PT) on graft survival 3 years after Descemet stripping automated endothelial keratoplasty (DSAEK). Eyes undergoing surgery for Fuchs endothelial corneal dystrophy or pseudophakic/aphakic corneal edema were randomized to receive donor corneas stored ≤7 days or 8 to 14 days. Donor and patient characteristics, tissue preparation and surgical parameters, recipient and donor corneal stroma clarity, central corneal thickness, intraocular pressure, complications, and a reading center-determined central endothelial cell density were collected. Surveys were conducted to evaluate pre-CPTS PT practices.
Results:
The 1330 CPTS donors were: 49% >60 years old, 27% diabetic, had a median eye bank–determined screening endothelial cell density of 2688 cells/mm2, and 74% eye bank prepared for DSAEK. A total of 1090 recipients (1330 eyes including 240 bilateral cases) had: median age of 70 years, were 60% female, 90% white, 18% diabetic, 52% phakic, and 94% had Fuchs endothelial corneal dystrophy. Before the CPTS, 19 eye banks provided PT data on 20,852 corneas domestically placed for DSAEK in 2010 to 2011; 96% were preserved ≤7 days. Of 305 American Academy of Ophthalmology members responding to a pre-CPTS survey, 233 (76%) set their maximum PT preference at 8 days or less.
Conclusions:
The CPTS will increase understanding of factors related to DSAEK success and, if noninferiority of longer PT is shown, will have great potential to extend the available pool of endothelial keratoplasty donors.
Clinical Trial Registration—URL: http://www.clinicaltrials.gov. Unique identifier: NCT01537393.
Despite the success of the Cornea Donor Study1–4 (CDS) in expanding the donor pool by establishing the effective range of donor ages for successful penetrating keratoplasty (PKP) in endothelial failure conditions, the donor pool remains threatened. The successor to the CDS was thus conceived; the Cornea Preservation Time Study (CPTS), a prospective multicenter randomized trial designed to potentially increase the available donor supply by proving to the surgeon and eye bank community that comparable graft success and endothelial cell loss can be achieved with the use of longer-preserved donor tissue beyond 7 days up to the maximum Food and Drug Administration (FDA)-approved preservation time (PT) of 14 days. The short-term and long-term threats to the donor pool include: (1) increasing number of donors with common viral infections such as hepatitis B5 (2) emerging infections such as West Nile virus and Chagas disease5; (3) prion agents; (4) an increasing number of potential donors with a compromised medical–social history6 or possible or proven sepsis7; (5) expanding demand on the donor pool with an aging recipient population and greater number of Fuchs endothelial corneal dystrophy (FECD) cases; (6) greater number of FECD cases also due to surgery performed at an earlier stage of the disease before there is structural damage to the stroma5; and (7) a greater demand for donors as a result of the tremendous growth of Descemet stripping automated endothelial keratoplasty (DSAEK) over the past 8 years coupled with a higher donor failure rate in the perioperative period (primary failures, those related to surgical and/or early postoperative complications) than after PKP.5
The design of the CPTS was modeled on the CDS, which was a multicenter study randomizing 1090 participants to receive donor tissue from donors 12 to <66.0 and 66.0 to <76.0 years old.1,4 However, the CDS preceded the advent of DSAEK in the United States and did not address the question of extended PT (beyond 7–14 days) and its impact on graft success and cell loss postoperatively; median death to surgery time in the CDS was only 4 days (25th, 75th percentiles: 3, 5 days).8
Before the CPTS initiation in 2012, the general consensus among corneal surgeons and eye banks in the United States was not to use donor corneal tissue preserved for more than 7 to 8 days for domestic PKP and/or endothelial keratoplasty (EK), but no formal survey had been conducted to specifically determine a practice pattern. This practice was prevalent despite US FDA approval of 4°C storage solutions for up to 14 days since Optisol GS (Bausch & Lomb, Rochester, NY) was approved in the early 1990s9,10 and successful export programs by many eye banks with donor tissue commonly being used successfully beyond 8 days internationally [Tissue Banks International (TBI, Baltimore, MD; Jerry Cole, written communication, June 2009) and SightLife (Seattle, WA; Monty Montoya, written communication, May 2009)]. FDA approval only required ex vivo endothelial survival in culture data11,12 and no clinical trial to show safety and efficacy. With limited reports on the relationship of PT to graft success after PKP8,13–15 and EK,16–19 there was an opportunity for a well-designed trial to address the question of PT and graft success. Thus, the CPTS was designed to determine whether the 3-year graft failure rate after EK performed with donor corneas with a PT of 8 to 14 days is noninferior to the failure rate when donor corneas with a PT of 7 or fewer days are used.
This report describes the methods of the CPTS, the demographics of the donor and patient cohorts enrolled in the CPTS, PT data from 19 eye banks in the United States before the CPTS initiation, and a survey of surgeon attitudes toward PT, which provides a baseline view of practices that could serve as better measures of study impact than occurred with the CDS.20
MATERIALS AND METHODS
Site Qualifications, Recipient, Donor, Eye Bank, Operative, and Postoperative Parameters Captured
Site Qualifications
All surgeons were required to have performed at least 50 DSAEK cases before study entry with a primary donor failure rate in the year before certification into the CPTS of no greater than 3% and a dislocation rate requiring repositioning and/or rebubbling of less than 15%. All eye banks were accredited by the Eye Bank Association of America (EBAA).
Recipient Inclusion and Exclusion Criteria
The recipient inclusion and exclusion criteria are listed in Table 1 and were developed to use DSAEK as a model to test the question regarding the impact of donor cornea PT on graft success. The CPTS recipient criteria included patients requiring DSAEK for conditions with a moderate risk for graft rejection [FECD, pseudophakic/aphakic corneal edema (PCE/ACE)], and therefore failed PKP or EK grafts were excluded, as were subjects with glaucoma tube shunts, uncontrolled glaucoma, anterior chamber intraocular lenses, and synechiae. Finally, subjects with preoperative central subepithelial or stromal scarring that could impact postoperative recipient stroma clarity assessment were excluded. If eligible, both eyes of a single patient could be entered into the study with randomization assuring that each eye would be in a different study group. The study was approved by the University Hospitals Case Medical Center Institutional Review Board (IRB) and other university-affiliated IRBs, and is listed on www.clinicaltrials.gov (NCT01537393). Informed consent was obtained from all recipients.
TABLE 1.
CPTS Recipient Inclusion and Exclusion Criteria

Randomization to PT Group and Tissue Assignment Procedures
Randomization to PT group and tissue assignment was directed by the CPTS Data Management and Analysis Center at the Jaeb Center for Health Research (Tampa, FL); the participants, clinical sites, Coordinating Center, and Study Chair remained masked to these assignments. A study participant could have both eyes enrolled in the study; the eye scheduled for surgery first was randomly assigned to a PT group (donor cornea preserved 7 days or less vs. 8–14 days), and the second eye scheduled for surgery was assigned to the alternate group. Randomization through an automated computer program was stratified by the surgeon using a permuted blocks design, and occurred at the time of the tissue request and entry of scheduled surgery date into the study Web site. Surgeries were required to be scheduled at least 10 days in advance to allow randomization to either PT group. Eye banks received automated e-mail notifications of pending assignments along with a randomly designated tissue assignment date to promote better distribution of PTs within each PT group. At the time of tissue assignment, the eye banks listed all available donor corneas for which the preservation date fell in the correct PT window for the scheduled surgery date on the study Web site; a computer program used a minimization algorithm to select the tissue assignment from the list to attempt a balance of preplanned subgroups (0–4 days, 5–7 days, 8–11 days, and 12–14 days from preservation to surgery).
Donor Criteria
All donor corneas were required to meet the current EBAA standards for DSAEK surgery.21 Other criteria included: (1) age of donor at the time of death 10 to 75 years; (2) death to PT up to 20 hours if the donor body was refrigerated or ice placed over the eyes, and death to PT ≤10 hours, if not refrigerated; (3) eye bank-determined minimum endothelial cell density (ECD) of 2300 cells/mm2; (4) none to no more than mild (slight) polymorphism/polymegethism; (5) no true guttae present; and (6) no evidence of central endothelial cell damage/trauma or dystrophy, such as FECD.
Eye Bank Procedures
The eye bank collected donor age, cause of death, time of death, time of cooling, time of preservation, storage solution, slit-lamp biomicroscopic findings, 3 images of the central endothelium at screening (used for donor eligibility), as well as 3 images after lamellar dissection of donor tissue by the eye bank, or 3 images before shipping for tissues to be prepared by the surgeon. Those eye banks performing lamellar dissection preparation of the donor were allowed to follow their individual protocol for this preparation, while capturing preparation parameters. Observations were captured during eye bank tissue preparation (eg, tearing of Descemet membrane, endothelial touch during mounting or removal from the artificial anterior chamber, significant increase in stress lines, cap decentration, variation in graft thickness), along with pre- and post-lamellar dissection thickness. Surgeons were masked to all donor and donor cornea data with the exception of the storage solution, residual bed thickness, and observations captured during eye bank tissue preparation.
Preoperative and Operative Procedures
Preoperative care, surgical technique, and postoperative care (including prescription of medications) are provided according to each clinical investigator's customary routine. For the preoperative examination, assurance of study eligibility according to the inclusion and exclusion criteria was determined and documented. In addition, a history of ocular surgery (in particular glaucoma surgery), diabetes, smoking, and FECD or other corneal dystrophies in the patient and family members, and current medications (including antiglaucoma medication and antiinflammatories) was obtained. If FECD was present, the severity of the dystrophy was graded as previously described22; for example, grade 4 (>2–5 mm of confluent central/paracentral guttae) advancing to grade 6 (>5 mm of confluent central/paracentral guttae with stromal and/or epithelial edema).
During surgery, the surgeon recorded incision size and location, donor cornea diameter, donor cornea insertion method, stab incision for venting or not, peripheral scraping of the recipient bed, air fill duration, other procedures (eg, cataract surgery), trainee participation (eg, tissue preparation, donor cornea insertion, and positioning), and operative complications (eg, difficult tissue preparation, difficult placement). If the donor cornea tissue was surgeon prepared, lamellar dissection technique and pre-cut and post-cut thicknesses were recorded. A donor cornea rim culture was not mandatory, but if obtained, the results were collected including any antimicrobial therapy that was prescribed.
Postoperative Procedures and Complications
Slit-lamp examinations have been and will be performed 1 day, 1 week, and 1, 6, 12, 24, and 36 months postoperatively. On these examinations, recipient and donor corneal stromal clarity are assessed. Each study visit also includes measurement of intraocular pressure and documentation of topical medications including topical corticosteroid, other antirejection medication (eg, cyclosporine) and antiglaucoma medication. Ultrasonic pachymetry of the central cornea, using the same calibrated instrument for all sites (Pachette 3, DGH 555 Ultrasonic Pachymeter; DGH Technology, Inc, Exton, PA) is obtained beginning at the 1 week postoperative visit and for all study visits thereafter and performed by an operator certified on the use of the instrument, either the investigator or technician. In the first week and out to the first month, donor cornea positioning and location and extent of interface fluid were assessed, as well as the need for air injection alone and/or external and/or internal repositioning.
Significant complications tracked include the occurrence of microbial keratitis, endophthalmitis, stromal and/or epithelial edema, partial and free-floating graft detachments, interface haze, graft rejection (possible, definite-mild/severe), and graft failure.
Recipient Stroma Clarity
The resolution of recipient stromal edema and establishment of recipient stroma clarity is a clinically accepted measure of donor function after DSAEK23; this assessment was viewed as important in determining the possible short-term and long-term effect of PT on the donor cornea function. Therefore, a grading scale of recipient stroma clarity was developed (clear, equivocal, and cloudy; Supplemental Digital Content 3, http://links.lww.com/ICO/A266) and validated before use by investigators in the study.
Repeatability of grading recipient stroma clarity between 2 cornea fellowship-trained ophthalmologists was conducted on images of 32 non-CPTS postoperative DSAEK patients. Each ophthalmologist reader graded each of the 32 image sets (including at least 1 slit-lamp photograph crossing the central cornea over the graft and at least 1 diffuse photograph per set), and one of the ophthalmologist readers read the entire image set a second time on a different occasion. Intrarater agreement was determined between 2 readings carried out by one reader, followed by interrater agreement between the 2 readers. The kappa statistic was calculated, which measures agreement beyond that occurring by chance alone.
As part of the certification process, all investigators were trained on this assessment with the same photographic guide (Supplemental Digital Content 3, http://links.lww.com/ICO/A266) and then tested with examples of each grade. During the slit-lamp biomicroscopic examination, the central (4-mm-diameter zone overlying the graft) recipient stroma clarity is assessed with the 3-point scale by judging clarity of the slit beam passing through the recipient stroma and how this clarity relates to recipient stromal thickness and difficulty viewing iris details.
Graft Failure
As the primary endpoint measure, graft failure is defined as the occurrence of one of the following: (1) cornea that requires regrafting for any reason; (2) cornea that remains cloudy [per the grading scale of recipient stroma clarity (Supplemental Digital Content 3, http://links.lww.com/ICO/A266)] without clearing either if (a) cloudy on the first postoperative day that does not clear within 8 weeks, or (b) initially clear postoperatively but becomes and remains cloudy for 3 months. The principal causes of graft failure in the CPTS are presented in Table 2. The intention of the categories in the early postoperative period is to distinguish early graft failure associated with perioperative complications from “true” primary failure and to be consistent with the EBAA classification for primary donor failure.21 Detailed categories for late failures are also provided. These distinctions will support the determination of the effect of PT on graft failure both in the early and late postoperative periods.
TABLE 2.
CPTS Graft Failure Classification

Central Endothelial Imaging
All eye banks obtained a screening image of the central donor cornea endothelium and determined ECD using their usual analysis method for eligibility. They then obtained 3 images either after lamellar dissection or if the donor cornea was to be prepared by the surgeon, before shipment. Postoperative specular microscopic images of the central corneal endothelium of the graft are obtained at 6 month, and 1, 2, and 3 years. The preoperative and postoperative recipient images are evaluated for quality and ECD by a central reading center, the Cornea Image Analysis Reading Center (CIARC) at Case Western Reserve University and University Hospitals Eye Institute (Cleveland, OH), using a previously described variable frame analysis method.24,25
Sample Size Determination and Study Aims
To evaluate the impact of longer donor cornea PT on graft success, 2 randomization groups were created with PT of 7 days or less and of 8 to 14 days. Graft survival and function up to 3 years postoperatively was considered to be sufficient time to observe a potential effect of PT. Recipient diagnoses were conditions with endothelial dysfunction, FECD, and PCE/ACE, as in the CDS.1,4 DSAEK was chosen as the sole procedure to simplify the sample size calculation and data analysis as well as assure recruitment goals based on its emergence as the most common form of keratoplasty being performed in the United States.26
The sample size was calculated based on a noninferiority (a single 1-sided test) design, to determine whether the graft failure rate up to 3 years of the recipient corneas of tissue transplanted 8 to 14 days after donor death was not worse than the graft failure rate of recipient corneas of donor corneas transplanted ≤7 days after death. Based on a 3-year CDS failure rate of 8%1 and the primary and late graft failure rates after DSAEK for FECD and PCE/ACE,27 a 3-year failure rate of 6% was assumed. Based on equal allocation of recipients to each group and a type I error of 5%, a sample size of 1208 eyes was estimated, providing 90% power for a noninferiority limit of 4%. The 4% noninferiority limit was selected as the largest clinically acceptable difference in graft failure rates up to 3 years to consider the 8- to 14-day PT group to be no worse than the 0- to 7-day PT group. Based on the CDS results1,4 with a comparable recipient cohort (age, recipient diagnoses), approximately 10% of subjects were estimated to not reach 3 years of follow-up because of death, withdrawal, or loss to follow-up. Increasing the calculated sample size by this amount gave a total of 1330 eyes (665 per group). This sample size calculation was not adjusted for potentially correlated outcomes of both corneas from the same donor or study enrollment of both eyes of the same participant, which was permitted. Any correlation that does potentially exist would increase statistical power.
Surveys of PT Experience Pre-CPTS
To assess the impact of the CPTS on PT experience in the United States, CPTS eye banks and eye banks not participating in the CPTS (Supplemental Digital Content 2, http://links.lww.com/ICO/A266) were solicited through an EBAA mass mailing to contribute demographic and preparation data (PT, tissue preparation by eye bank or surgeon, donor age, and cause of death) on all donor corneas placed for DSAEK in 2010 and 2011. PT distribution was reviewed overall and stratified by the factors noted as well as by the year and eye bank. Univariate least squares regression models were used to select factors with a P < 0.01 for subsequent inclusion in a multivariable model that included backward selection, with the final model consisting of factors with P < 0.01. Variability of PT among eye banks was further evaluated in a random-effects model. Analyses used SAS version 9.4 (SAS Institute, Cary, NC). A similar data collection is planned after study results are published to determine whether the PT distribution changes pre- to post-CPTS.
Additionally, a survey instrument was developed to determine surgeon attitudes surrounding PT and was sent to every member of the American Academy of Ophthalmology (AAO) in 2011 who listed subspecialty interest in corneal and external diseases. This survey was developed to complement the eye bank survey on PT practices.
RESULTS
Description of CPTS Recipient and Donor Cohort
A total of 1330 recipient corneas of 1090 recipient patients (240 bilateral cases) were randomized and completed surgery between May 2012 and April 2014. A description of the recipient patient and eyes cohort is summarized in Table 3. The median recipient patient age was 70 years; 60% were female and 90% were white. The most common indication for DSAEK was FECD (94%). The FECD severity grade22 distribution of recipient eyes before surgery is shown in Table 3 with a similar distribution of severity grade for those recipient patients with only eye enrolled in the CPTS with those recipient patients with both eyes enrolled. Of the 1254 total number of recipient eyes with FECD, 228 (18%) had grade 4 or less (>2–5 mm of confluent central/paracentral guttae or less). Of the 1330 recipient eyes, 690 were phakic (52%). Eighteen percent of the recipient patients were diabetic by history.
TABLE 3.
CPTS Recipient Characteristics
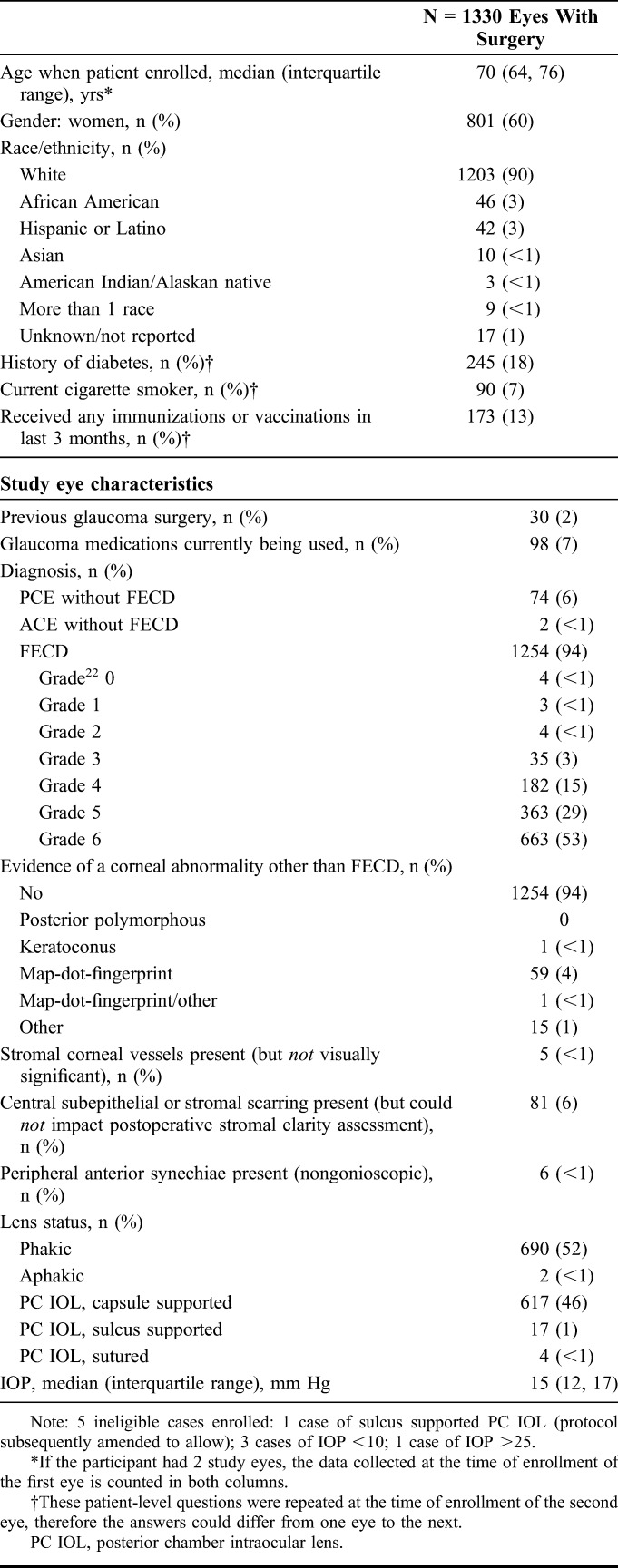
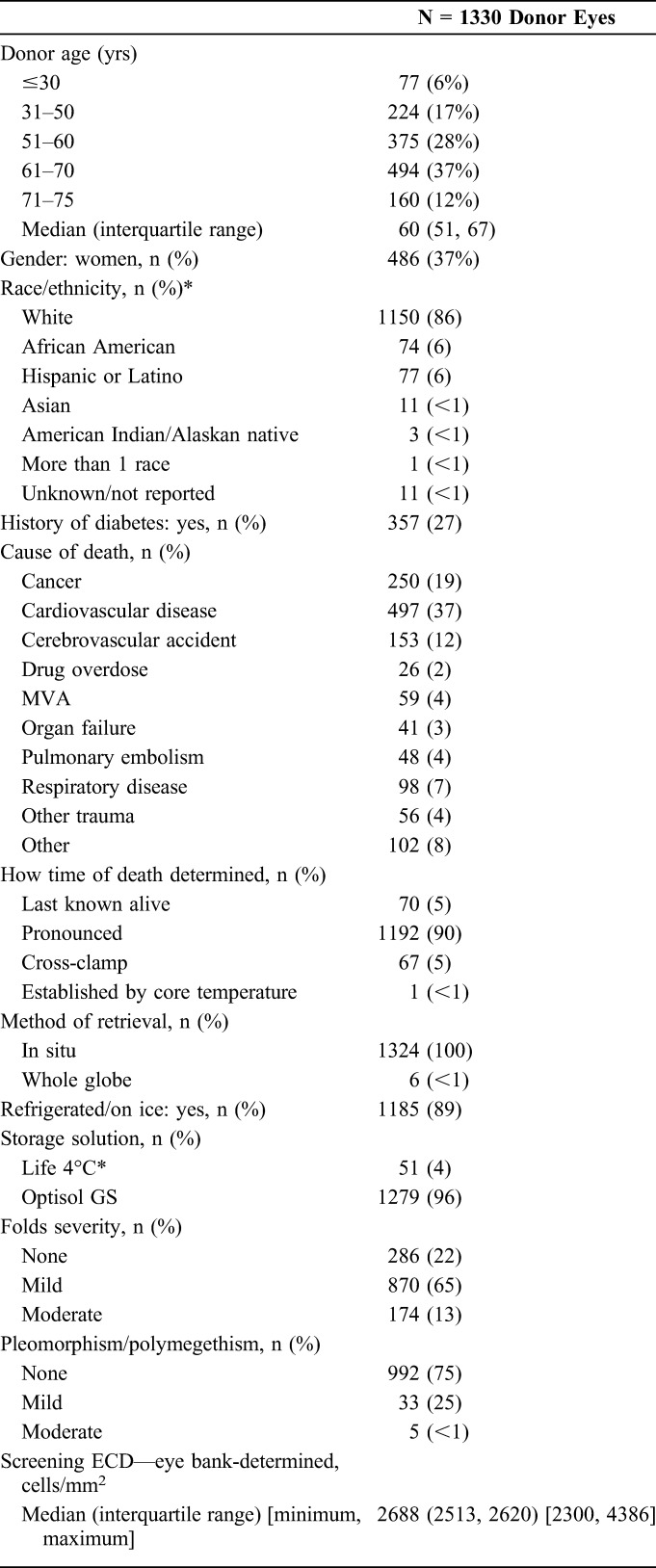
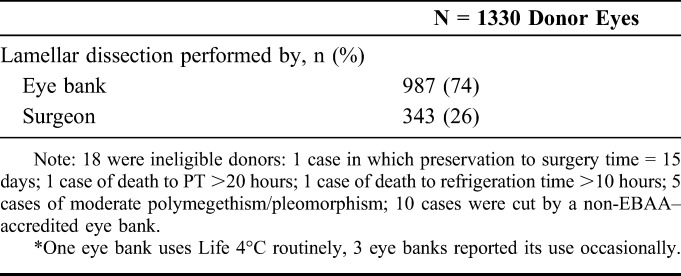
A description of the donor and donor cornea characteristics is provided in Table 4. Of the 1330 donor corneas provided for CPTS surgeries, median donor age was 60 years (range, 11–75 years), on average 10 years younger than the recipients. Twenty-seven percent of the donors were diabetic. Tissue was prepared by the eye bank in 74% of cases and by the surgeon in 26%. The median eye bank–determined ECD at the time of screening was 2688 cells/mm2 (range, 2300–4386 cells/mm2). Because the Coordinating Center, CIARC, investigators, and coordinators remain masked as to other donor and donor cornea characteristics, most importantly PT, these data will be reported at the conclusion of the study early in 2017.
TABLE 4.
CPTS Donor and Donor Cornea Characteristics
The grading of recipient stroma clarity is an integral part of defining the types of graft failures in the CPTS (Supplemental Digital Content 3, http://links.lww.com/ICO/A266) as mentioned above. The intraobserver reliability of the recipient stroma clarity grading scale as tested before implementation in the CPTS was high (kappa 0.80), as was interobserver reliability for distinguishing a cloudy cornea from equivocal or clear corneas (kappa 0.72). Therefore, the grading scale is a key element in the CPTS to determine the primary endpoint of graft failure, indirectly monitoring graft function and the effect of PT over the 3-year observation period.
PT Experience Pre-CPTS
Eye Banks
Nineteen eye banks provided data on 20,852 donor corneas placed for DSAEK in the United States in 2010 and 2011 (Supplemental Digital Content 2, http://links.lww.com/ICO/A266). The mean (SD) PT was 4.7 (1.6) days, with 96% preserved no more than 7 days. The distribution of PTs is shown in Figure 1. After adjusting for all other factors in a multivariate model, PT was significantly (P < 0.01) associated with eye bank processing and distributing donor tissue, cause of death, survey year, and tissue preparation source (individual mean PTs ranged from 3.5 to 5.5 days, 4.6 to 4.8 days, 4.6 in 2010 vs. 4.7 in 2011, and 4.7 by eye bank and 4.4 by surgeon, for each associated parameter, respectively). Observed differences, however, were not meaningful (<1 day) with the exception of the range across individual eye banks. The SD among eye banks from a random-effects model was 0.6 days, with 2 eye banks averaging 1 day above and 1 eye bank averaging 1 day below the overall average.
FIGURE 1.
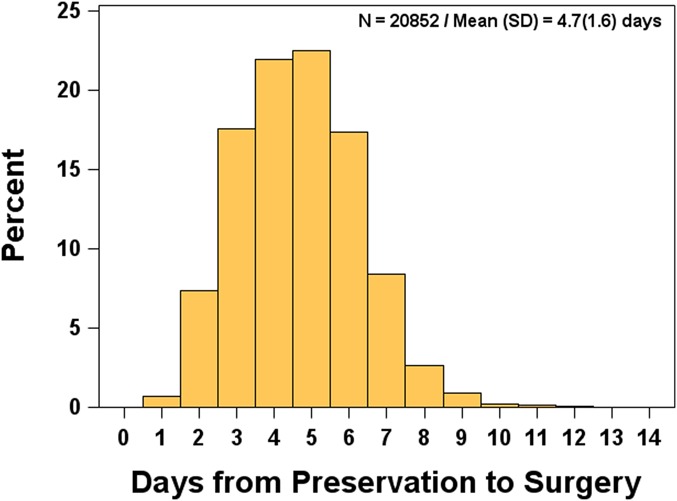
Preservation time for domestic placements used for DSAEK in 2010–2011 via 19 eye banks.
Surgeons
Twenty-three percent (364 of 1609) of the AAO members approached responded to the surgeon survey. The respondent surgeons were on average 51 years of age (range, 31–80 years) in practice for 19 years after completing their training (range, 1–34 years), and performing EK, primarily DSAEK, on average 5 years (range, 1–14 years). Sixty-three percent of respondents performed up to 25 DSAEKs per year, and only approximately 11% were moderate-to-high volume surgeons (>75 DSAEKs per year). More than half of the surgeons were very selective of donor cornea tissue; that is, 58% would cancel a scheduled DSAEK case if the cornea donor did not meet their specified range of parameters even if it met their local eye bank's standards. Of the 305 responding to the question on their current limit for accepting donor tissue from the time the cornea is preserved to DSAEK surgery, 233 (76%) set ≤8 days as their limit. When the PT extended beyond 7 days, the surgeons expected a higher ECD; the expectation for a preoperative ECD of 2800 cells/mm2 went up from 5% (if the donor cornea was preserved within their usual time limit) to 53% (if preserved longer than their usual PT limit).
DISCUSSION
The CPTS addresses for the first time the question of the impact of PT with cold storage at 4°C on EK success in a randomized clinical trial. Numerous studies with this method of storage have shown nearly 100% viability of endothelial cells in vitro and maintenance of corneal thickness for 2 weeks with Optisol9,10,28 and its successor, Optisol GS.12 Endothelial cell viability to 14 days was attributed to the combination of chondroitin sulfate, additional antioxidants, energy sources, and nutritive substrates in the storage solution compared with previous storage solutions such as MK, K-Sol, CSM, and DexSol.11 However, nearly 20 years after the introduction of Optisol GS in the United States, less than 900 donor corneas (4%) preserved beyond 7 days were used for PKP or EK domestically from the 19 eye banks contributing data in a survey for 2010 and 2011, compared with 27% of the 2144 internationally used tissues. This prejudice against the use of longer-preserved tissue pushed eye banks to develop international distribution relationships in which transportation and logistics supported successful keratoplasty often beyond 7 days of PT (Kevin Ross, personal communication, 2014). In 2013, of the 62,274 USA-generated donor tissues for either PKP or EK, 18,355 (29%) were exported.5 The introduction of Life 4°C (Numedis, Isanti, MN) in the United States in 2010, another chondroitin-based intermediate storage medium approved by the FDA for 14-day PT with reportedly improved endothelial viability,29 has also not had an impact on this domestic practice pattern, because it represents only a small percentage of the 4°C storage solutions used by eye banks.
Because no single database exists for corneal surgeons active in the United States before initiation of the CPTS, we conducted a survey of AAO members with reported interest in corneal surgery to assess surgeon attitudes regarding PT. Although this survey is an incomplete view of the attitudes regarding PT in the United States surgeon community, it is a plausible sample of active DSAEK surgeons in the United States acknowledged by the fact that almost 85% of the respondents actively performed DSAEK. Two-thirds of the respondents set 7 days as their maximum PT. Additionally, their criteria for ECD were more stringent if the PT increased beyond their usual limit. These results parallel our eye bank data and provide a reference base to assess change in practice patterns if the CPTS shows no difference in graft success and ECD for the 2 PT groups.
Just as in the CDS and the SMAS in which donor,1–4,30–33 recipient,3,31–34 operative,1–4,34 and postoperative factors1–4,34 were examined to determine their effects on graft failure and endothelial cell loss, the CPTS will conduct similar analyses to examine these same factors on the outcome of DSAEK surgery with a large cohort (Table 3). Single-site studies have played a tremendous role in advancing the field and dissecting the most important factors for success, but are limited by observations that may not necessarily apply to the larger surgeon community.27,35–42 The CPTS will explore, for example, donor age, glaucoma surgery and medication history, graft diameter, insertion technique, rebubbling and repositioning, and graft rejection and their impact on graft success and ECD. The relationship of donor, recipient, and operative factors (eg, PT, post-lamellar dissection thickness, recipient diagnosis, glaucoma history, insertion technique, size of air bubble, duration of air fill) to graft adherence and necessity for repositioning will also be examined. The CPTS will also generate data that will be compared descriptively with the CDS data. In particular, because donor age between the 2 cohorts is virtually the same,1 the relationship of donor age and DSAEK graft success and cell loss will be of interest to compare. There also will be an opportunity to examine the effect of both diabetes in the donor and recipient on graft success and cell loss, an effect that was not noted in the CDS.33 Finally, the study's grading system for recipient stroma clarity (Supplemental Digital Content, http://links.lww.com/ICO/A266) could also set a clinical standard for the reporting of graft success with all forms of EK, including DSAEK, and the evolving EK procedure, Descemet membrane endothelial keratoplasty (DMEK).
A trend to perform surgery for FECD at an earlier stage is also most apparent in the CPTS cohort (Table 3). In fact, 228 cases (18%) were grade 4 or less, and FECD grade distribution was not influenced by whether one or both eyes were enrolled. The grade 0 cases, confirmed with the investigators at these sites, are possibly explained by an unusual, previously described clinical presentation of corneal edema in phakic eyes with no history of trauma or inflammation and without guttae.43 This earlier intervention in the disease, motivated by the desire to prevent or limit stromal scarring from edema,23 and the many cases combined with cataract surgery, provides evidence explaining in part the 34% growth of DSAEK in the United States between 2008 and 2013 from 17,468 to 23,465 cases, respectively.5
In conclusion, the CPTS offers the prospect of improving donor cornea distribution and supply by expanding options for use of the corneas that for logistical and other reasons cannot be distributed within 7 days, by strengthening the evidence base for increasing PT up to the FDA-approved 14 days. CPTS data may also provide important mitigation against risks to availability of donor tissue, which include the need to screen broadly for emerging infectious agents, as well as short-term disruptions because of factors such as inclement weather. The study will also yield information on factors that affect DSAEK graft failure and endothelial cell loss after the procedure, guiding the best approach for future DSAEK surgeries. The newly emerging EK procedure, DMEK, may also benefit from these insights, particularly as donor and donor cornea risk factors may be common to both procedures.
Supplementary Material
Footnotes
Supported by cooperative agreements with the National Eye Institute, National Institutes of Health, Department of Health and Human Services EY20797 and EY20798. Additional support was provided by Eye Bank Association of America, The Cornea Society, Vision Share, Inc, Alabama Eye Bank, Cleveland Eye Bank, Eye Bank for Sight Restoration, Iowa Lions Eye Bank, Lions Eye Bank of Albany, Midwest Eye-Banks, San Diego Eye Bank, and SightLife.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.corneajrnl.com).
Participating CPTS clinical sites, investigators, and coordinators; eye bank investigators; members of the Operations, Executive, Eye Bank Advisory, Data Safety and Monitoring Committee; Coordinating Center, Cornea Image Analysis Reading Center (CIARC), and Data Management and Analysis Center Staff; and the National Eye Institute staff are provided in Supplemental Digital Content 1 (http://links.lww.com/ICO/A266).
Eye banks that provided data on donor cornea preservation time between 2010 and 2011 are provided in Supplemental Digital Content 2 (http://links.lww.com/ICO/A266).
REFERENCES
- 1.Gal RL, Dontchev M, Beck RW, et al. The effect of donor age on corneal transplantation outcome results of the Cornea Donor Study. Ophthalmology. 2008;115:620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lass JH, Gal RL, Dontchev M, et al. Donor age and corneal endothelial cell loss 5 years after successful corneal transplantation. Specular Microscopy Ancillary Study results. Ophthalmology. 2008;115:627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lass JH, Benetz BA, Gal RL, et al. Donor age and factors related to endothelial cell loss 10 years after penetrating keratoplasty: Specular Microscopy Ancillary Study. Ophthalmology. 2013;120:2428–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannis MJ, Holland EJ, Gal RL, et al. The effect of donor age on penetrating keratoplasty for endothelial disease: graft survival after 10 years in the cornea donor study. Ophthalmology. 2013;120:2419–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eye Bank Association of America. 2013 Eye Banking Statistical Report. 2014. [Google Scholar]
- 6.Van Meter W, Sheeth PH. Potential adverse effects on the cornea donor pool in 2031. Int J Eye Banking. 2013;1:1–9. [Google Scholar]
- 7.Centers for Disease Control and Prevention. Data Reports. 2014. Available at: http://www.cdc.gov/sepsis/datareports/index.html. Accessed November 24, 2014. [Google Scholar]
- 8.Sugar A, Gal RL, Beck W, et al. Baseline donor characteristics in the Cornea Donor Study. Cornea. 2005;24:389–396. [DOI] [PubMed] [Google Scholar]
- 9.Lindstrom RL, Kaufman HE, Skelnik DL, et al. Optisol corneal storage medium. Am J Ophthalmol. 1992;114:345–356. [DOI] [PubMed] [Google Scholar]
- 10.Walkenbach RJ, Boney F, Ye GS. Corneal function after storage in dexsol or optisol. Invest Ophthalmol Vis Sci. 1992;33:2454–2458. [PubMed] [Google Scholar]
- 11.Lindstrom RL. Advances in corneal preservation. Trans Am Ophthalmol Soc. 1990;88:555–648. [PMC free article] [PubMed] [Google Scholar]
- 12.Means TL, Geroski DH, Hadley A, et al. Viability of human corneal endothelium following Optisol-GS storage. Arch Ophthalmol. 1995;113:805–809. [DOI] [PubMed] [Google Scholar]
- 13.Chang SD, Pecego JG, Zadnik K, et al. Factors influencing graft clarity. Cornea. 1996;15:577–581. [PubMed] [Google Scholar]
- 14.Doganay S, Hepsen IF, Yologlu S, et al. Effect of the preservation-to-surgery interval on corneal allograft survival in low-risk patients. Ophthalmic Surg Lasers Imaging. 2007;38:457–461. [DOI] [PubMed] [Google Scholar]
- 15.Wagoner MD, Gonnah el-S. Corneal graft survival after prolonged storage in Optisol-GS. Cornea. 2005;24:976–979. [DOI] [PubMed] [Google Scholar]
- 16.Chen ES, Terry MA, Shamie N, et al. Precut tissue in Descemet's stripping automated endothelial keratoplasty donor characteristics and early postoperative complications. Ophthalmology. 2008;115:497–502. [DOI] [PubMed] [Google Scholar]
- 17.Guttman C. Donor death-surgery interval may affect DSAEK outcomes. Ophthamology Times. 15April2009. [Google Scholar]
- 18.Price MO, Price FW., Jr Endothelial cell loss after Descemet stripping with endothelial keratoplasty influencing factors and 2-year trend. Ophthalmology. 2008;115:857–865. [DOI] [PubMed] [Google Scholar]
- 19.Terry MA. Precut tissue for Descemet stripping automated endothelial keratoplasty: complications are from technique, not tissue. Cornea. 2008;27:627–629. [DOI] [PubMed] [Google Scholar]
- 20.Sugar A, Montoya MM, Beck R, et al. Impact of the Cornea Donor Study on acceptance of corneas from older donors. Cornea. 2012;31:1441–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eye Bank Association of America. Medical Standards. Washington, DC, 2014. [Google Scholar]
- 22.Louttit MD, Kopplin LJ, Igo RP, Jr, et al. A multicenter study to map genes for Fuchs endothelial corneal dystrophy: baseline characteristics and heritability. Cornea. 2012;31:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price MO, Price FW. Descemet's stripping endothelial keratoplasty. Curr Opin Ophthalmol. 2007;18:290–294. [DOI] [PubMed] [Google Scholar]
- 24.Benetz B, Yee R, Bidros M, et al. Specular Microscopy. In: Krachmer JH, Mannis JJ, Holland E, eds. Cornea: Fundamentals, Diagnosis, Management. 3rd ed St. Louis, MO: Mosby; 2011:177–203. [Google Scholar]
- 25.Benetz BA, Gal RL, Rice C, et al. Dual grading methods by a central reading center for corneal endothelial image quality assessment and cell density determination in the Specular Microscopy Ancillary Study of the Cornea Donor Study. Curr Eye Res. 2006;31:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eye Bank Association of America. 2011 Eye Banking Statistical Report. 2012. [Google Scholar]
- 27.Price MO, Fairchild KM, Price DA, et al. Descemet's stripping endothelial keratoplasty five-year graft survival and endothelial cell loss. Ophthalmology. 2011;118:725–729. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman HE, Beuerman RW, Steinemann TL, et al. Optisol corneal storage medium. Arch Ophthalmol. 1991;109:864–868. [DOI] [PubMed] [Google Scholar]
- 29.Price MO, Knight O, Benetz BA, et al. Randomized, prospective, single-masked clinical trial of endothelial keratoplasty performance with two donor cornea 4° storage solutions and associated chambers. Cornea. 2015;34:253–256. [DOI] [PubMed] [Google Scholar]
- 30.Sugar J, Montoya M, Dontchev M, et al. Donor risk factors for graft failure in the Cornea Donor Study. Cornea. 2009;28:981–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lass JH, Beck RW, Benetz BA, et al. Baseline factors related to endothelial cell loss following penetrating keratoplasty. Arch Ophthalmol. 2011;129:1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stulting RD, Sugar A, Beck R, et al. Effect of donor and recipient factors on corneal graft rejection. Cornea. 2012;31:1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lass JH, Riddlesworth TD, Gal RL, et al. The effect of donor diabetes history on graft failure and endothelial cell density 10 years after penetrating keratoplasty. Ophthalmology. 2015;122:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugar A, Tanner JP, Dontchev M, et al. Recipient risk factors for graft failure in the cornea donor study. Ophthalmology. 2009;116:1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price MO, Gorovoy M, Price FW, Jr, et al. Descemet's stripping automated endothelial keratoplasty: three-year graft and endothelial cell survival compared with penetrating keratoplasty. Ophthalmology. 2012;120:246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terry MA, Saad HA, Shamie N, et al. Endothelial keratoplasty: the influence of insertion techniques and incision size on donor endothelial survival. Cornea. 2009;28:24–31. [DOI] [PubMed] [Google Scholar]
- 37.Terry MA, Shamie N, Chen ES, et al. Endothelial keratoplasty: the influence of preoperative donor endothelial cell densities on dislocation, primary graft failure, and 1-year cell counts. Cornea. 2008;27:1131–1137. [DOI] [PubMed] [Google Scholar]
- 38.Ang M, Mehta JS, Lim F, et al. Endothelial cell loss and graft survival after Descemet's stripping automated endothelial keratoplasty and penetrating keratoplasty. Ophthalmology. 2012;119:2239–2244. [DOI] [PubMed] [Google Scholar]
- 39.Anshu A, Price MO, Price FW., Jr Descemet stripping automated endothelial keratoplasty for Fuchs endothelial dystrophy-influence of graft diameter on endothelial cell loss. Cornea. 2012;32:5–8. [DOI] [PubMed] [Google Scholar]
- 40.Elbaz U, Yeung SN, Lichtinger A, et al. EndoGlide versus EndoSerter for the insertion of donor graft in Descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2014;158:257–262. [DOI] [PubMed] [Google Scholar]
- 41.Phillips PM, Phillips LJ, Much JW, et al. Descemet stripping endothelial keratoplasty: six-month results of the first 100 consecutive surgeries performed solo by a surgeon using 1 technique with 100% follow-up. Cornea. 2012;31:1361–1364. [DOI] [PubMed] [Google Scholar]
- 42.Terry MA, Shamie N, Chen ES, et al. Endothelial keratoplasty for Fuchs' dystrophy with cataract: complications and clinical results with the new triple procedure. Ophthalmology. 2009;116:631–639. [DOI] [PubMed] [Google Scholar]
- 43.Abbott RL, Fine BS, Webster RG, Jr, et al. Specular microscopic and histologic observations in nonguttate corneal endothelial degeneration. Ophthalmology. 1981;88:788–800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


