Abstract
Particle adhesion in vivo is highly dependent on the microvascular environment comprising of unique anatomical, geometrical, physiological fluid flow conditions and cell-particle and cell-cell interactions. Hence, proper design of vascular-targeted drug carriers that efficiently deliver therapeutics to the targeted cells or tissue at effective concentrations must account for these complex conditions observed in vivo. In this study, we build upon our previous results with the goal of characterizing the effects of bifurcations and their corresponding angle on adhesion of functionalized particles and neutrophils to activated endothelium. Our hypothesis is that adhesion is significantly affected by the type of biochemical interactions between particles and vessel wall as well as the presence of bifurcations and their corresponding angle. Here, we investigate adhesion of functionalized particles (2 µm and 7 µm microparticles) to protein coated channels as well as adhesion of human neutrophils to human endothelial cells under various physiological flow conditions in microfluidic bifurcating channels comprising of different contained angles (30°, 60°, 90°, or 120°). Our findings indicate that both functionalized particle and neutrophil adhesion propensity increases with a larger bifurcation angle. Moreover, the difference in adhesion patterns of neutrophils and rigid, similar sized (7 µm) particles is more apparent in the junction regions with a larger contained angle. By selecting the right particle size range, enhanced targeted binding of vascular drug carriers can be achieved along with a higher efficacy at optimal drug dosage. Hence, vascular drug particle design needs to be tailored to account for higher binding propensity at larger bifurcation angles.
Keywords: Bifurcation, adhesion, functionalized particle, neutrophil, microfluidic
Introduction
Particle adhesion in vivo is highly dependent on the microvascular environment comprising of unique anatomical, geometrical, physiological fluid flow conditions and cell-particle and cell-cell interactions (Cho et al., 1985; Ishikawa et al., 2011; Ku, 1997; Lee and Lee, 2009; Mayrovitz et al., 1977; Prabhakarpandian et al., 2011a). The biochemical processes involved in cell–cell and particle–cell interactions have been traditionally characterized using in vitro static assays consisting of tissue culture dishes coated with either protein matrices or adherent cells, in which the number of adherent particles/cells is quantified after a period of incubation (Amoozgar et al., 2013; Baker-Groberg et al., 2014; Irino et al., 2014; Neal et al., 2011). However, these static assays lack the in vivo complex microcirculation environment, in which the presence of physiological fluid flow and geometric features can determine spatiotemporal changes in hemodynamic conditions and factors impacting adhesion (Fahim, 2003). Therefore, in recent years in vitro flow chambers characterized by a simple geometry and defined flow conditions have been used to study the adhesive interactions between particles/cells and adhesion molecules of the endothelium (Decuzzi et al., 2007; Haun and Hammer, 2008; Jutila et al., 2007; Sang et al., 2007; Sperandio et al., 2006). With the advancement of MEMS-based microfluidic systems during the last few years, micro-scale flow chambers have been developed to accurately reproduce the in vivo conditions (e.g. stenosis, bifurcations). Using these devices comprised of fluidic channels with dimensions ranging from few micrometers to hundred micrometers, several investigators were able to characterize leukocyte adhesion (Dixit et al., 2012; Rouleau et al., 2010; Schaff et al., 2007) and platelet adhesion (Ku et al., 2008; Sarvepalli et al., 2009; Tovar-Lopez et al., 2010) in an in vivo mimicking fluidic microenvironment.
Different types of targeting moieties on carrier’s surface have been investigated for achieving a higher efficiency of drug carrier interaction and adhesion to the endothelium (Burch et al., 2002). In prior studies, we showed that adhesion efficiency of functionalized particles and leukocytes in vitro using synthetic microvascular networks (SMNs) mimicking the in vivo microvasculature and fluid flow conditions (Lamberti et al., 2014; Prabhakarpandian et al., 2011b) and leukocytes in in vivo vessels (Tousi et al., 2010) is significantly affected by geometric features of the vessels. Doshi et al. (2010) showed that a simple bifurcating microfluidic flow chamber can be used to characterize the transport and adhesion dynamics of drug carrying particles. Moreover, the junction region of the microfluidic flow chamber was able to select the best particle shape for optimal adhesion over the traditionally used linear microchannels. In a recent study (Lamberti et al., 2013), we showed that adhesive interactions of functionalized particles with the endothelium are significantly higher in junction regions than straight sections of in vitro systems. These studies showed that, regardless of size, shape and biochemical interactions between particles and endothelium, significantly higher adhesion was observed at the junctions compared to the straight sections of the channels. The importance of geometrical features and hemodynamic forces in the vasculature has also been widely investigated in the pathobiology of atherosclerosis (Gimbrone and García-Cardeña, 2013). It has been shown that straight regions of arteries are exposed to steady laminar blood flow and are protected from atherosclerosis, while regions of bifurcations are characterized by disturbed blood flow that predisposes to atherosclerosis (Malek et al., 1999; Nigro et al., 2011; Wootton and Ku, 1999). Therefore, in order to characterize the efficiency of targeted drug carriers in vitro, it is important to consider the geometry of the microvasculature, in particular the bifurcations and junctions of the microvascular network.
In this study, we build upon our previous results with the goal of characterizing the effects of bifurcations and their contained angle on adhesion of functionalized particles and neutrophils to activated endothelium. Our hypothesis is that adhesion is significantly affected by the presence of bifurcations and their corresponding angle as well as the type of biochemical interactions between particles and vessel wall. In order to study the effects of the presence of bifurcations in the vasculature, we investigated adhesion of functionalized particles to protein coated channels and adhesion of human neutrophils to human endothelial cells in microfluidic channels comprising of different bifurcation angles. To model drug particles, we used polystyrene microspheres, which although not biodegradable, have been widely used as model drug carriers for medical research and biological laboratory experiments (Gentile et al., 2008; Kendall et al., 2009; Namdee et al., 2013; Rodgers et al., 2000). A comparison of neutrophil/particle adhesion density between the junction and the straight regions of the channel was performed to examine the non-uniform distribution of neutrophils/particles at vessel bifurcations. Finally, we compared functionalized particle adhesion patterns with neutrophil adhesion in cellularized bifurcating channels to examine neutrophil-endothelium interactions at the junction and in the straight section of the system.
Materials and Methods
Fabrication of Bifurcating Synthetic Microvascular Network (SMN)
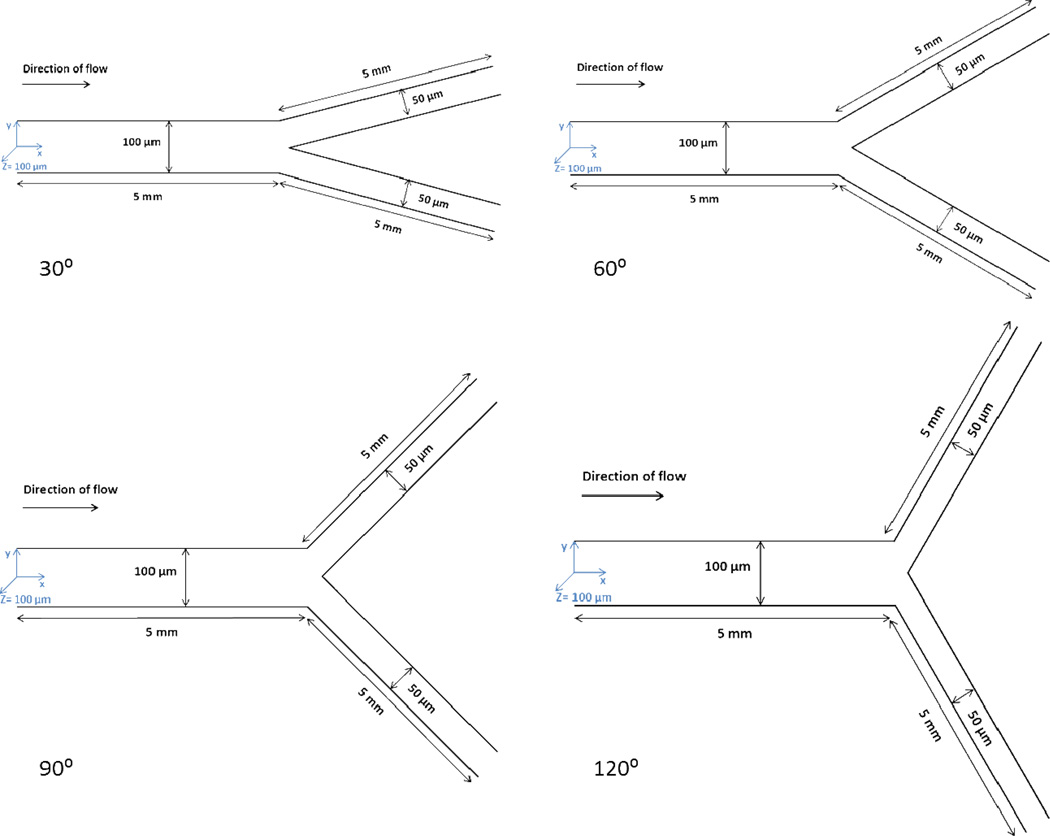
Bifurcating SMN (Fig. 1) was fabricated using soft lithography processes (Doshi et al., 2010). Briefly, Sylgard 184 PDMS was poured over the developed masters in a 150 mm Petri dish, and degassed for 15 min. The polymer was then allowed to cure overnight in an oven at 65 °C. The bonding surfaces of the PDMS and 1×3 in. glass slide were plasma treated (200 mTorr, 18 W, 30 s) in a plasma generator (Harrick Scientific, Ithaca, NY). Tygon Microbore tubing (0.06 inch OD and 0.02 inch ID) was used to connect inlet and outlet ports punched on PDMS using a biopsy punch. The networks used in these studies comprised of channels of rectangular cross-section with a parent channel of 100 µm width and 100 µm depth and two daughter channels of 50 µm width and 100 µm depth and bifurcation contained angles of 30°, 60°, 90°, 120° respectively. The bifurcation is symmetric about the parent channel.
Figure 1.
Schematics of bifurcating SMNs with an angle of 30°, 60°, 90° or 120°.
Coating of Bifurcating SMN with Avidin
The inlet port of the microfluidic device was connected to a 1 ml syringe filled with avidin (Invitrogen, Carlsbad, CA) at a concentration of 20 µg/ml mounted on a programmable syringe pump (PHD 2000, Harvard Apparatus, Holliston, MA). Avidin was introduced into the channel at a flow rate of 1 µl/min for 10 min. The flow was then stopped and the entire device was placed at 4 °C for 4–6 h. The device coated with avidin was allowed to come to room temperature (~10 min) before the experiments.
Seeding of human umbilical endothelial cells into the SMN
Using our established protocol (Lamberti et al., 2013; Rosano et al., 2009), the fabricated device was degassed and then washed with sterile DI water. The device was then incubated with fibronectin (100 µg/ml) for 1 h. Human umbilical vein endothelial cells (HUVEC) (Lonza, Walkersville, MD) were introduced and incubated at 37 °C and 5% CO2. Media was replaced every 24 h until the cells were confluent in the device. Confluent endothelial cells were activated with 10 U/ml of TNF-α for 4 h before the experiments.
Neutrophil Isolation and Labeling
Human blood was obtained via venipuncture from healthy adult donors and collected into a sterile tube containing sodium heparin (BD Biosciences) after informed consent was obtained as approved by the Institutional Review Board of Temple University. Neutrophils were then isolated using a one-step Ficoll-Plaque gradient (GE Healthcare, Piscataway, NJ). After isolation, neutrophils were counted and resuspended in HBSS (5×105 cells/ml). Neutrophils were labeled in suspension using CFDA SE probe (Molecular Probes, Carlsbad, CA) for 10 min at room temperature. The labeled neutrophil solution was washed with Hanks Balanced Salt Solution (HBSS) and resuspended in pre-warmed endothelial cell media containing 10 ng/ml of TNF-α and kept in the incubator for 10 min prior the experiments.
Adhesion measurements in bifurcating SMN
Adhesion studies were conducted using biotin-conjugated microspheres of 2 µm or 7 µm diameter (Polysciences, Inc., Warrington, PA) and human neutrophils, prepared at a concentration of 5×105/ml in PBS. A programmable syringe pump (PHD 2000, Harvard Apparatus, MA) was used to inject the particles and the flow was set for a shear rate of 240 1/sec. Every 3 min, the flow was reduced by half reaching a final shear rate of 7.5 1/sec, similar to our previous studies (Doshi et al., 2010; Kolhar et al., 2013; Lamberti et al., 2013; Prabhakarpandian et al., 2011c, 2001; Sakhalkar et al., 2003). The new shear rate was maintained for 3 min to allow sufficient time for a statistically significant number of adhesion events to occur (Doshi et al., 2010; Kolhar et al., 2013; Lamberti et al., 2013; Prabhakarpandian et al., 2011c; Smith et al., 2014). A cooled CCD camera, Retiga Exi (Qimaging, Surrey, BC, Canada) was used for acquiring images. The entire process was automated using the NIKON Elements software (NIKON, Melville, NY). Particle/Neutrophil adhesion patterns were analyzed in both the junction area (defined as a distance of 200 um from the bifurcation) and the straight section (defined as a distance of 1000 µm from the junction area) as described before (Lamberti et al., 2013). Particles and neutrophils that did not move for 30 sec during the 3 min acquisitions period were considered adherent and counted using a 4× objective.
Statistical Analysis
Unless otherwise noted, data are presented as Mean ± Standard Error of the Mean (SEM). Analysis of variance (ANOVA) was used to determine significant differences between different bifurcation angles, shear rate values and junction vs. straight sections.
Results
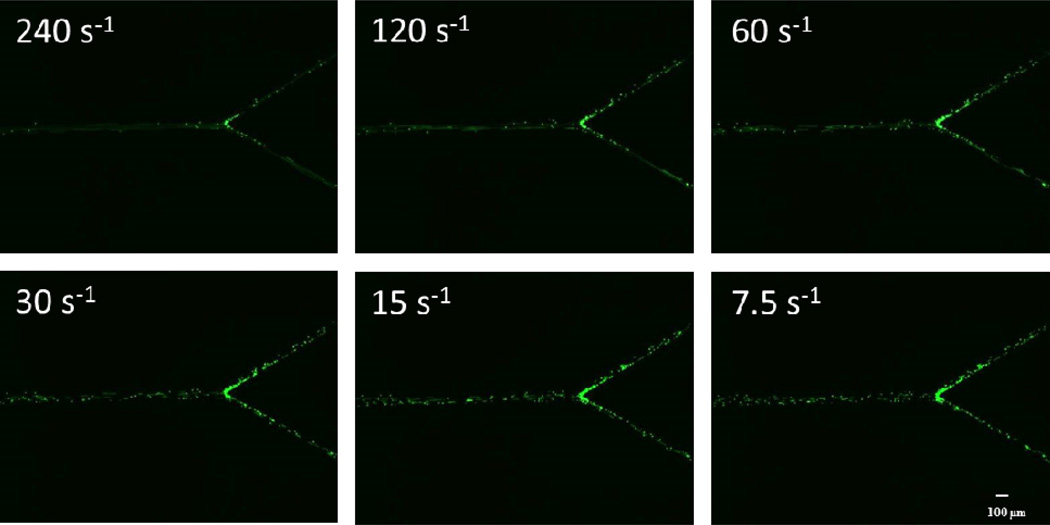
Images of the different shapes of bifurcating channels are presented in Figure 1. Adhesion profiles of biotinylated particles were quantified in different contained angles in bifurcating SMNs coated with avidin. Different sized functionalized particles (2 µm or 7 µm) or neutrophils were perfused through the bifurcating channels at shear rates ranging from 240.0 to 7.5 1/sec, which are the typical physiological shears observed in the microcirculation (Kim and Sarelius, 2003). Fluorescent images of straight sections and the junction regions were taken at the end of a fixed time interval of 3 min (Fig. 2). The adhesion propensity was quantified in terms of the number of particles and neutrophils attached per unit area of the bifurcating SMNs.
Figure 2.
Images show adhesion of 2 µm biotin-coated particles to avidin-coated bifurcating SMN with an angle of 60° at different shear values. The difference in the adhesion propensities can be clearly observed between high and low shear rates.
Adhesion patterns of 2 µm particles is dependent on the angle of bifurcation
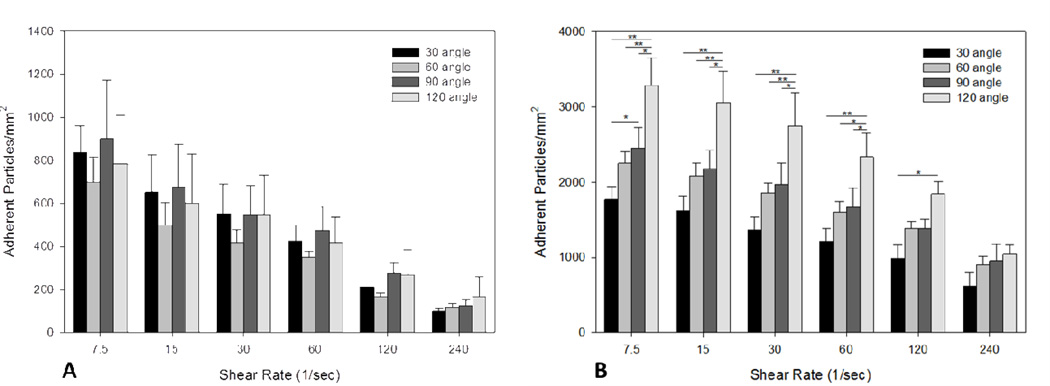
Adhesion of functionalized particles decreased with increasing shear rate in both junctions and straight regions of all bifurcating SMNs (bifurcation angles of 30°, 60°, 90° and 120°), indicating an inverse relationship between levels of shear and particle adhesion (Fig. 3A and 3B). In addition, our results showed a significantly higher particle adhesion (1.5 times higher or more) in junctions compared to straight sections.
Figure 3.
A) Adhesion of 2 µm functionalized particles to straight sections significantly increases with decreasing shear values ranging from 240 to 7.5 1/sec. There was no statistic significant difference in the level of adhesion of particles between different angles at all shear values. B) Adhesion of 2 µm functionalized particles to junction regions significantly increases with the angle of bifurcations (p<0.05) for shear values ranging from 7.5 to 120 1/sec. *p< 0.05; **p< 0.01. Please note that the scales for the Y-axis in panels A and B are different and that there was significantly higher particle adhesion in junctions as compared to straight sections.
Furthermore, particle adhesion at junctions increased with an increase in the angle of bifurcation (Fig. 3B). In particular, at all shear values the bifurcation angle of 120° resulted in the highest level of adhered particles as compared to the smaller bifurcation angles (30°, 60°, and 90°). This dependence is not seen in the adhesion data in the straight sections, suggesting that this phenomenon is due to the geometry of the bifurcation region.
The difference in adhesion patterns of neutrophils and 7 µm particles is more apparent in the junction regions
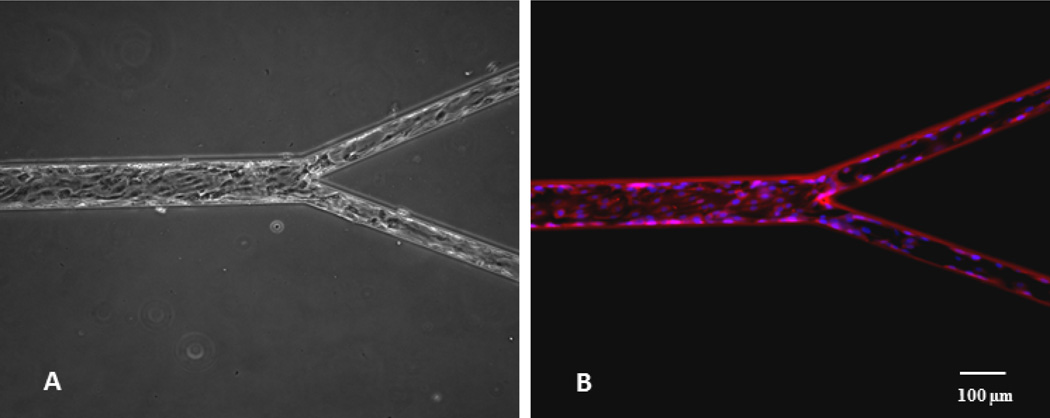
In order to investigate whether the effects of the geometry observed in the in vitro systems correspond to biological particles with different biophysical characteristics, we examined neutrophil-endothelium adhesive interactions in bifurcating SMNs with different bifurcation angles. To better approximate the in vivo environment, endothelial cells were seeded in the bifurcating SMNs and then exposed to flow overnight (Fig. 4A and 4B).
Figure 4.
A) Endothelial cells cultured in bifurcating SMN reach confluence under shear flow conditions; B) Endothelial cells stained with Hoechst (blue, nuclei) and MitoTracker Red CMXRos (red, mitochondria) show elongation in the direction of flow.
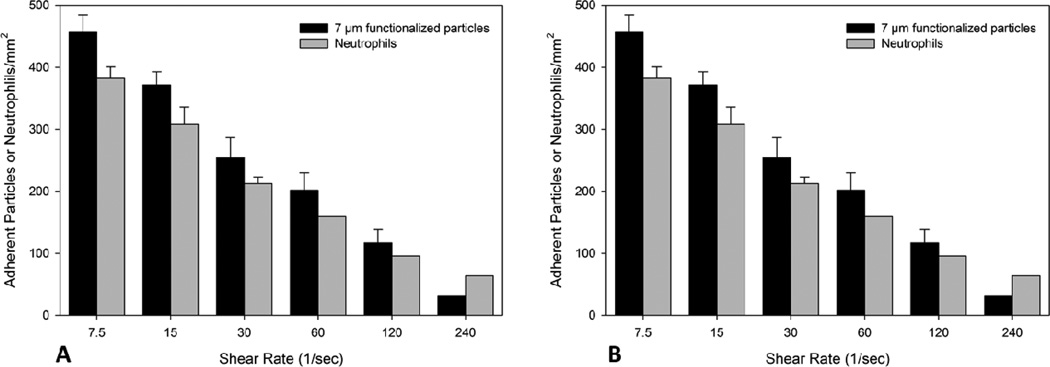
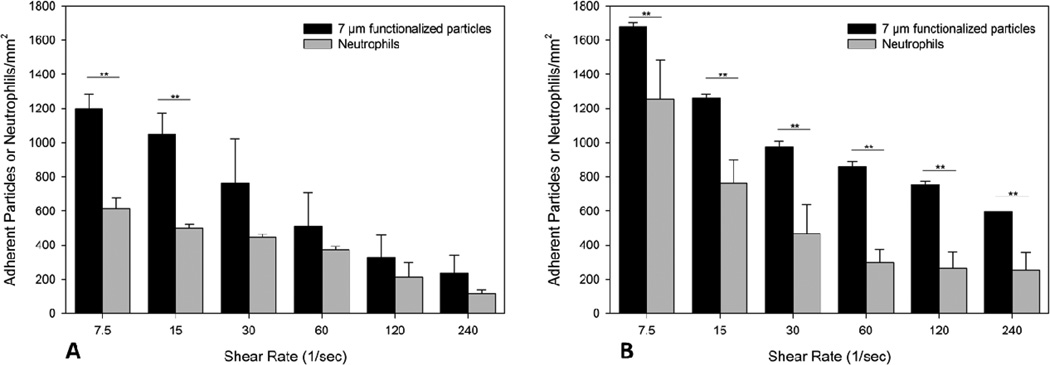
Endothelial cells were then activated with TNF-α in the SMN, while human neutrophils were isolated and then perfused on activated cells under the same shear conditions used for the functionalized particles. In these experiments, we used SMNs with a bifurcation angle of 30° and 120° which represent the narrowest and the widest angles studied, respectively. We also studied the adhesion level of 7 µm functionalized particles which have similarly sized as the neutrophils but are rigid. Similar to the results obtained for both 2 µm and 7 µm functionalized particles, adhesion level of neutrophils on endothelial cells in the bifurcation SMNs decreased with an increase of shear rate (p < 0.01) at both straight and junction regions (Fig. 5 and 6). As a negative control experiment, adhesion of 7 µm biotin particles on non-activated surface of the bifurcating SMNs with an angle of 30° coated with 0.1% BSA solution was investigated. The results showed no adhesion at all shear conditions (data not shown).
Figure 5.
In the straight sections of the bifurcating SMN with an angle of 30° (A) and 120° (B) there is no significant difference in the level of adhesion between 7 µm particles and neutrophils at all shear conditions.
Figure 6.
A) In the junction regions of the bifurcating SMN with an angle of 30° there is significant difference in the level of adhesion between 7 µm particles and neutrophils at low shear conditions. B) In the junction regions of the bifurcating SMN with an angle of 120° there is significant difference in the level of adhesion between 7 µm particles and neutrophils at all shear conditions. The adhesion of particles and neutrophils is higher (~ 2 times) than in the bifurcation angle of 30°. *p< 0.05; **p< 0.01.
When we examined the level of adhesion of both 7 µm particles and neutrophils in the junction regions of bifurcating SMNs with an angle of 30°, there was a significant difference in the level of adhesion between particles and neutrophils at low shear conditions (p< 0.01) (Fig. 6A). On the other hand, junction region with an angle of 120° showed a significant difference between 7 µm particles and neutrophils at all shear conditions (p< 0.01) (Fig. 6B). Furthermore, the adhesion of particles and neutrophils in the larger bifurcation angle of 120° was higher (~ 2 times) than that in the 30° bifurcation angle.
Discussion
In this study we have used synthetic microvascular networks (SMNs) with different bifurcation angles (30°, 60°, 90°, 120°) to study the adhesion patterns of functionalized particles and neutrophils under different geometric and flow conditions. 2 µm biotinylated particles interacting with avidin coated channels were used for all the bifurcation angles and under various shear conditions, while neutrophil-endothelial interactions were studied using human neutrophils flowing on HUVECs pretreated with TNF-α for 4 h in SMNs with bifurcation angles of 30° and 120° under various physiological shear conditions. Adhesion patterns of neutrophils to activated endothelial cells were compared to that of similar size 7 µm, but rigid, functionalized particles.
Vascular endothelium has been shown to sense different flow patterns and to have different, flow-specific responses both at the molecular and at the cellular levels (Nigro et al., 2011). Previously, our group has shown that the presence or absence of endothelial cells and/or receptor concentrations on their surfaces, are not the driving forces behind the preferential adhesion patterns of neutrophils near bifurcations (Prabhakarpandian et al., 2011c; Rosano et al., 2009; Tousi et al., 2010). Similar to our previous results (Lamberti et al., 2013), the number of adherent particles/neutrophils in junction regions is statistically higher than in straight sections depending on local shear stress. In this study, we tested the hypothesis that adhesion is significantly affected by the type of biochemical interactions between particles and vessel wall as well as the presence of bifurcations and their corresponding angle. We estimated the influence of the bifurcation angle on particle adhesion by using bifurcating SMNs with various angles and we then compared the results with those obtained from neutrophil adhesion to endothelial cells in the same bifurcating geometries. Our findings indicate that both functionalized particle and neutrophil adhesion increases with a larger bifurcation angle. The higher adhesion of particles/neutrophils at the larger bifurcation angle observed in SMNs is likely due to altered flow patterns, which lead to a higher interaction of particles/neutrophils with the walls (Prabhakarpandian et al., 2011c). Given the fact that neutrophil-endothelial cell interactions involve a much larger number of biochemical interactions compared to biotin-avidin interactions of the 7 µm rigid particles, it is interesting to note that as the bifurcation angle increases, differences between particle adhesion and neutrophil adhesion profiles becomes more pronounced. The interaction between biotinylated particles and avidin coated vascular walls is a simplification of the more complex interaction of neutrophil-endothelial cells. The differences in neutrophil and particle adhesion could stem from a variety of factors e.g. neutrophil deformation vs. particle rigidity, endothelial cell signaling in neutrophil-endothelial interactions vs. the absence of signaling for particle adhesion, and differences in binding affinity between neutrophil-leukocyte interactions and avidin-biotin interaction, among others. Additional controlled studies would be needed to develop a detailed mechanistic understanding of the differences attributable to these factors, and could be pursued in future efforts.
Several computational studies have shown the important effects of the presence of bifurcations on cell margination and adhesion; for example the distribution of red blood cells in the microvasculature resulted to be uneven in the downstream channels of a bifurcation vessel (Chesnutt and Marshall, 2009). In our studies, we did not see any differences in particle/neutrophil adhesion between the upstream and downstream channels following the junction region, but believe that the presence of red blood cells may enhance this phenomenon. In this study the experiments were performed using neutrophils or particles suspended in cell culture medium or buffer and were designed to study the effects of shear stress and the in vitro geometry of bifurcating SMNs on cell/particle adhesion. However, we did not completely reproduce the in vivo blood rheology, in which the presence of erythrocytes enhance neutrophil/particle rolling and arrest by displacing them from the bulk flow toward the wall and increase binding of neutrophil/particle to endothelium (Migliorini et al., 2002). Future studies may be performed by including erythrocytes in the neutrophil suspension and study the overall efficiency of neutrophil capture in bifurcating SMNs by increasing the frequency of collision as neutrophils interact with the endothelium.
Atherosclerotic lesions are preferentially located at the outer walls of the arterial branches and curvatures, where the local flow is disturbed (e.g., non-uniform, irregular oscillation, and recirculation) (Asakura and Karino, 1990; Caro et al., 1971; Zarins et al., 1983). Since the margination of microspheres to the vessel wall, and therefore the probability to their adhesion to the endothelium, has been shown to be a combined function of particle size, blood flow rate and the wall shear rate within the vessel (Charoenphol et al., 2010), the results obtained in this study may help to characterize the behavior of drug carriers in conditions of disturbed blood flow typical of many clinical disorders, including atherosclerosis, arterial aneurysm, aortic valve calcification, postsurgical intimal hyperplasia, in-stent restenosis, venous varicosity, chronic venous insufficiency, ischemia/reperfusion injury, and transplant vasculopathy (Chiu and Chien, 2011).
In conclusion, drug carrier particle adhesion is significantly affected by the presence of bifurcations and their corresponding angle as well as the type of biochemical interactions between particle and vessel wall. By choosing the right particle size range and the right vascular geometry, enhanced targeted binding of vascular drug carriers can be achieved along with a higher efficacy and minimal drug dosage. Hence, drug particle design needs to be tailored to account for higher binding propensity at larger bifurcation angles.
Highlights.
Bifurcation angle influences interaction of particles with vessel wall
Cell particle adhesion propensity increases with a larger bifurcation angle
Bifurcation angle enhances differences between rigid particle and neutrophil adhesion
Acknowledgments
Giuseppina Lamberti is a pre-doctoral fellow of the American Heart Association. We thank Dr. Satya P. Kunapuli (Temple University School of Medicine, Philadelphia, PA) and members of his laboratory for their technical assistance. This project was supported by grants from the National Institutes of Health and Shriners Hospitals for Children.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amoozgar Z, Park J, Lin Q, Weidle JH, Yeo Y. Development of quinic acid-conjugated nanoparticles as a drug carrier to solid tumors. Biomacromolecules. 2013;14:2389–2395. doi: 10.1021/bm400512g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura T, Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ. Res. 1990;66:1045–1066. doi: 10.1161/01.res.66.4.1045. [DOI] [PubMed] [Google Scholar]
- Baker-Groberg SM, Cianchetti FA, Phillips KG, McCarty OJT. Development of a method to quantify platelet adhesion and aggregation under static conditions. Cell. Mol. Bioeng. 2014;7:285–290. doi: 10.1007/s12195-014-0328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch EE, Shinde Patil VR, Camphausen RT, Kiani MF, Goetz DJ. The N-terminal peptide of PSGL-1 can mediate adhesion to trauma-activated endothelium via Pselectin in vivo. Blood. 2002;100:531–538. doi: 10.1182/blood.v100.2.531. [DOI] [PubMed] [Google Scholar]
- Caro CG, Fitz-Gerald JM, Schroter RC. Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. ProcR. Soc. LondB. Biol. Sci. 1971;177:109–159. doi: 10.1098/rspb.1971.0019. [DOI] [PubMed] [Google Scholar]
- Charoenphol P, Huang RB, Eniola-Adefeso O. Potential role of size and hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials. 2010;31:1392–1402. doi: 10.1016/j.biomaterials.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Chesnutt JKW, Marshall JS. Effect of particle collisions and aggregation on red blood cell passage through a bifurcation. Microvasc. Res. 2009;78:301–313. doi: 10.1016/j.mvr.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Chiu J-J, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YI, Back LH, Crawford DW. Experimental investigation of branch flow ratio, angle, and Reynolds number effects on the pressure and flow fields in arterial branch models. J. Biomech. Eng. 1985;107:257–267. doi: 10.1115/1.3138551. [DOI] [PubMed] [Google Scholar]
- Decuzzi P, Gentile F, Granaldi a, Curcio a, Causa F, Indolfi C, Netti P, Ferrari M. Flow chamber analysis of size effects in the adhesion of spherical particles. Int. J. Nanomedicine. 2007;2:689–696. [PMC free article] [PubMed] [Google Scholar]
- Dixit N, Kim M-H, Rossaint J, Yamayoshi I, Zarbock A, Simon SI. Leukocyte function antigen-1, kindlin-3, and calcium flux orchestrate neutrophil recruitment during inflammation. J. Immunol. 2012;189:5954–5964. doi: 10.4049/jimmunol.1201638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi N, Prabhakarpandian B, Rea-Ramsey A, Pant K, Sundaram S, Mitragotri S. Flow and adhesion of drug carriers in blood vessels depend on their shape: a study using model synthetic microvascular networks. J. Control. Release. 2010;146:196–200. doi: 10.1016/j.jconrel.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim M. Cardiovascular sensory receptors and their regulatory mechanisms. Indian. J. Physiol. Pharmacol. 2003;47:124–146. [PubMed] [Google Scholar]
- Gentile F, Curcio A, Indolfi C, Ferrari M, Decuzzi P. The margination propensity of spherical particles for vascular targeting in the microcirculation. J. Nanobiotechnology. 2008;6:9. doi: 10.1186/1477-3155-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone Ma, García-Cardeña G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc. Pathol. 2013;22:9–15. doi: 10.1016/j.carpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun JB, Hammer DA. Quantifying Nanoparticle Adhesion Mediated by Specific Molecular Interactions 8821–8832. 2008 doi: 10.1021/la8005844. [DOI] [PubMed] [Google Scholar]
- Irino T, Takeuchi H, Matsuda S, Saikawa Y, Kawakubo H, Wada N, Takahashi T, Nakamura R, Fukuda K, Omori T, Kitagawa Y. CC-Chemokine receptor CCR7: a key molecule for lymph node metastasis in esophageal squamous cell carcinoma. BMC Cancer. 2014;14:291. doi: 10.1186/1471-2407-14-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Fujiwara H, Matsuki N. Asymmetry of blood flow and cancer cell adhesion in a microchannel with symmetric bifurcation and confluence 159–167. 2011 doi: 10.1007/s10544-010-9481-7. [DOI] [PubMed] [Google Scholar]
- Jutila Ma, Walcheck B, Bargatze R, Palecanda A. Measurement of neutrophil adhesion under conditions mimicking blood flow. Methods Mol. Biol. 2007;412:239–256. doi: 10.1007/978-1-59745-467-4_16. [DOI] [PubMed] [Google Scholar]
- Kendall Ra, Alhnan Ma, Nilkumhang S, Murdan S, Basit AW. Fabrication and in vivo evaluation of highly pH-responsive acrylic microparticles for targeted gastrointestinal delivery. Eur. J. Pharm. Sci. 2009;37:284–290. doi: 10.1016/j.ejps.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Kim MB, Sarelius IH. Distributions of wall shear stress in venular convergences of mouse cremaster muscle. Microcirculation. 2003;10:167–178. doi: 10.1038/sj.mn.7800182. [DOI] [PubMed] [Google Scholar]
- Kolhar P, Anselmo AC, Gupta V, Pant K, Prabhakarpandian B, Ruoslahti E, Mitragotri S. Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc. Natl. Acad. Sci. U. S. A. 2013;110:10753–10758. doi: 10.1073/pnas.1308345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku C-J, D’Amico Oblak T, Spence DM. Interactions between multiple cell types in parallel microfluidic channels: monitoring platelet adhesion to an endothelium in the presence of an anti-adhesion drug. Anal. Chem. 2008;80:7543–7548. doi: 10.1021/ac801114j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku DN. BLOOD FLOW IN ARTERIES. Annu. Rev. Fluid Mech. 1997;29:399–434. [Google Scholar]
- Lamberti G, Prabhakarpandian B, Garson C, Smith A, Pant K, Wang B, Kiani MF. Bioinspired microfluidic assay for in vitro modeling of leukocyte-endothelium interactions. Anal. Chem. 2014;86:8344–8351. doi: 10.1021/ac5018716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberti G, Tang Y, Prabhakarpandian B, Wang Y, Pant K, Kiani MF, Wang B. Adhesive interaction of functionalized particles and endothelium in idealized microvascular networks. Microvasc. Res. 2013;89:107–114. doi: 10.1016/j.mvr.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Lee JY, Lee SJ. Murray’s law and the bifurcation angle in the arterial micro-circulation system and their application to the design of microfluidics. Microfluid. Nanofluidics. 2009;8:85–95. [Google Scholar]
- Malek A, Alper S, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. Jama. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- Mayrovitz HN, Tuma RF, Wiedeman MP, Tuma F, Mary P. Relationship between microvascular and pressure distribution blood velocity 400–405. 1977 doi: 10.1152/ajpheart.1977.232.4.H400. [DOI] [PubMed] [Google Scholar]
- Migliorini C, Qian Y, Chen H, Brown EB, Jain RK, Munn LL. Red blood cells augment leukocyte rolling in a virtual blood vessel. Biophys. J. 2002;83:1834–1841. doi: 10.1016/S0006-3495(02)73948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namdee K, Thompson AJ, Charoenphol P, Eniola-Adefeso O. Margination Propensity of Vascular-Targeted Spheres from Blood Flow in a Microfluidic Model of Human Microvessels. Langmuir. 2013 doi: 10.1021/la304746p. [DOI] [PubMed] [Google Scholar]
- Neal CL, Mckeithen D, Odero-Marah Va. Snail negatively regulates cell adhesion to extracellular matrix and integrin expression via the MAPK pathway in prostate cancer cells. Cell Adh. Migr. 2011;5:249–257. doi: 10.4161/cam.5.3.15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro P, Abe J-I, Berk BC. Flow shear stress and atherosclerosis: a matter of site specificity. Antioxid. Redox Signal. 2011;15:1405–1414. doi: 10.1089/ars.2010.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakarpandian B, Goetz DJ, Swerlick Ra, Chen X, Kiani MF. Expression and functional significance of adhesion molecules on cultured endothelial cells in response to ionizing radiation. Microcirculation. 2001;8:355–364. doi: 10.1038/sj/mn/7800105. [DOI] [PubMed] [Google Scholar]
- Prabhakarpandian B, Shen M-C, Pant K, Kiani MF. Microfluidic devices for modeling cell-cell and particle-cell interactions in the microvasculature. Microvasc. Res. 2011a doi: 10.1016/j.mvr.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakarpandian B, Wang Y, Rea-Ramsey A, Sundaram S, Kiani MF, Pant K. Bifurcations: focal points of particle adhesion in microvascular networks. Microcirculation. 2011b;18:380–389. doi: 10.1111/j.1549-8719.2011.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakarpandian B, Wang Y, Rea-Ramsey A, Sundaram S, Kiani MF, Pant K. Bifurcations: focal points of particle adhesion in microvascular networks. Microcirculation. 2011c;18:380–389. doi: 10.1111/j.1549-8719.2011.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers SD, Camphausen RT, Hammer Da. Sialyl Lewis(x)-mediated, PSGL-1-independent rolling adhesion on P-selectin. Biophys. J. 2000;79:694–706. doi: 10.1016/S0006-3495(00)76328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano JM, Tousi N, Scott RC, Krynska B, Rizzo V, Prabhakarpandian B, Pant K, Sundaram S, Kiani MF. A physiologically realistic in vitro model of microvascular networks. Biomed. Microdevices. 2009;11:1051–1057. doi: 10.1007/s10544-009-9322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau L, Copland IB, Tardif J-C, Mongrain R, Leask RL. Neutrophil adhesion on endothelial cells in a novel asymmetric stenosis model: effect of wall shear stress gradients. Ann. Biomed. Eng. 2010;38:2791–2804. doi: 10.1007/s10439-010-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhalkar HS, Dalal MK, Salem AK, Ansari R, Fu J, Kiani MF, Kurjiaka DT, Hanes J, Shakesheff KM, Goetz DJ. Leukocyte-inspired biodegradable particles that selectively and avidly adhere to inflamed endothelium in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 2003;100:15895–15900. doi: 10.1073/pnas.2631433100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang A, Ham W, Goetz DJ, Klibanov AL, Lawrence MB. Microparticle Adhesive Dynamics and Rolling Mediated by Selectin-Specific Antibodies Under Flow. 2007;96:596–607. doi: 10.1002/bit.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvepalli DP, Schmidtke DW, Nollert MU. Design considerations for a microfluidic device to quantify the platelet adhesion to collagen at physiological shear rates. Ann. Biomed. Eng. 2009;37:1331–1341. doi: 10.1007/s10439-009-9708-z. [DOI] [PubMed] [Google Scholar]
- Schaff UY, Xing MMQ, Lin KK, Pan N, Jeon NL, Simon SI. Vascular mimetics based on microfluidics for imaging the leukocyte--endothelial inflammatory response. Lab Chip. 2007;7:448–456. doi: 10.1039/b617915k. [DOI] [PubMed] [Google Scholar]
- Smith AM, Prabhakarpandian B, Pant K. Generation of shear adhesion map using SynVivo synthetic microvascular networks. J. Vis. Exp. 2014 doi: 10.3791/51025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio M, Pickard J, Unnikrishnan S, Acton ST, Ley K. Analysis of leukocyte rolling in vivo and in vitro. Methods Enzymol. 2006;416:346–371. doi: 10.1016/S0076-6879(06)16023-1. [DOI] [PubMed] [Google Scholar]
- Tousi N, Wang B, Pant K, Kiani MF, Prabhakarpandian B. Preferential adhesion of leukocytes near bifurcations is endothelium independent. Microvasc. Res. 2010;80:384–388. doi: 10.1016/j.mvr.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Lopez FJ, Rosengarten G, Westein E, Khoshmanesh K, Jackson SP, Mitchell A, Nesbitt WS. A microfluidics device to monitor platelet aggregation dynamics in response to strain rate micro-gradients in flowing blood. Lab Chip. 2010;10:291–302. doi: 10.1039/b916757a. [DOI] [PubMed] [Google Scholar]
- Wootton DM, Ku DN. Fluid mechanics of vascular systems, diseases, and thrombosis. Annu. Rev. Biomed. Eng. 1999;1:299–329. doi: 10.1146/annurev.bioeng.1.1.299. [DOI] [PubMed] [Google Scholar]
- Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ. Res. 1983;53:502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]