Abstract
The epithelial basement membrane (BM) is a specialized extracellular matrix that has been shown to have a critical role in corneal development, wound healing, and disease. Although the epithelial BM contributes to corneal homeostasis, relatively little is know about non-epithelial production of its components that may be important in defective regeneration of the epithelial basement membrane associated with opacity after photorefractive keratectomy. The purpose of the current study was to investigate stromal production of corneal epithelial BM proteins in wounded human corneas using immunohistochemistry. A total of five unwounded control eyes and five 30-minute epithelial-wounded corneas were obtained from fresh corneoscleral buttons removed from human eyes enucleated due to choroidal melanoma with normal anterior segments. In the wounded corneas, an eight mm patch of central corneal epithelium and epithelial BM was removed with a Beaver blade when the patient was under general anesthesia. Immunohistochemical analyses were performed to detect perlecan and nidogen-2 proteins–important components of the epithelial BM lamina lucida and lamina densa zones. Perlecan and nidogen-2 proteins were detected in the BM itself and at low levels in keratocytes in all unwounded corneas. After epithelial injury, both perlecan and nidogen-2 was expressed at high levels in stromal keratocytes, including superficial keratocytes in the early phases of apoptosis. Thus, after epithelial and epithelial BM injury, stromal keratocytes contribute important perlecan and nidogen-2 components to the regenerating epithelial BM.
Keywords: Cornea, perlecan, nidogen-2, epithelial basement membrane, wound healing, myofibroblast, keratocyte, stroma, haze, apoptosis
Basement membranes (BM) are specialized forms of extracellular matrix that regulate biological events, including local concentrations of growth factors and cytokines (Kruegel and Miosge, 2010). The formation of BM is a prerequisite for normal tissue development and function (Smyth et al., 1999; Yurchenco et al., 2004).
In corneal wound healing, the epithelial BM has been shown to have a critical role in regulating the generation of severe late corneal opacity after corneal surgeries such as photorefractive keratectomy (PRK) (Fini and Stramer, 2005; Fujikawa et al., 1984; Netto et al., 2006; Sta Iglesia and Stepp, 2000; Torricelli et al., 2013a and b; Torricelli and Wilson, 2014; Wilson et al., 1999). Prior studies have shown that structural integrity of the regenerated BM after stromal injury is an important determinant of whether a particular cornea develops haze—likely by limiting access of epithelium-derived growth factors, such as transforming growth factor beta (TGFβ), that modulate myofibroblast development from precursors cells, and persistence of these cells, in the subepithelial stroma (Fini and Stramer, 2005; Netto et al., 2006; Stramer et al., 2003; Torricelli, et al., 2013a and b).
Epithelial BMs are composed of diverse extracellular matrix molecules and the specific composition varies somewhat in different tissues (Kruegel and Miosge, 2010). In general terms, the corneal epithelial BM is assembled from four primary components: laminins, collagens, heparan sulfate proteoglycans, such as perlecan, and nidogens (Torricelli et al., 2013b). The laminia lucida and lamina densa layers that are not regenerated in rabbit corneas with haze (Torricelli, et al., 2013a) are thought to be composed of specific components that include perlecan, nidogen-1, nidogen-2 and laminin 332 (Yurchenco, Amenta, and Patton, 2004: Kruegel and Miosge, 2010). Perlecan has an important function in maintaining cell adhesion and integrity of the corneal matrix (Vittitow and Borras, 2004) and also functions to maintain the selective basement membrane barrier (Rossi et al., 2003; Smith and Hassell, 2006). There are two different nidogen genes, nidogen-1, also called entactin (Timpl, et al., 1983), and nidogen-2, also called osteonidogen or entactin-2 (Kohfeldt, et al., 1998). Nidogen-1 and nidogen-2 have similar structure and affinity for other extracellular matrix proteins (Fox, et al., 1991; Salmivirta, et al., 2002 Hohenester and Engel, 2002), and have been shown to compensate for each other in single-gene knockouts (Schymeinsky, et al., 2002). Double nidogen knockouts have numerous defects and perinatal lethality (Bader, et al., 2005). Nidogens act as bridging molecules linking different components of the BM, including perlecan (Schittny, Timpl and Engel, 1988; Kvansakul, et al., 2001; Hopf, et al., 2001) and also have strong affinity for laminins and collagen type IV Ho et al., 2008). Both nidogen-2 and perlecan contribute to the lamina lucida and lamina densa that show defective regeneration in corneas with severe haze (Torricelli, et al., 2013a and b).
Despite the importance of the epithelial BM in corneal wound healing, relatively little is known about non-epithelial biosynthesis of these components; with many studies concluding exclusively the epithelial cells themselves produce them. However, there has been data published suggesting underlying stromal cells produce some of the components (Kohfeldt et al., 1998; Maguen et al., 2008). The present study aimed to investigate stromal production of corneal epithelial BM proteins that are components of the defective zones noted with TEM after PRK—perlecan and nidogens—in wounded and unwounded human corneas. Several commercially available antibodies for nidogen-1 were tested but did not yield adequate staining in human corneas. Therefore, this study was limited to immunohistochemistry for nidogen-2 and perlecan proteins.
Informed consent for tissue donation and use in research was obtained and the study adhered to the tenets of the Declaration of Helsinki for experiments involving human tissues. Approval to perform this study was also obtained from the Institutional Review Board at the Cleveland Clinic.
Five unwounded control corneas (donors age: 60 ± 15, range 34–71) and five wounded corneas (donors age: 61 ± 19, range 47–85) were obtained from fresh corneoscleral buttons of human eyes with normal anterior segments enucleated for choroidal melanoma. In wounded corneas, the central epithelium was scraped to remove an eight mm patch of central corneal epithelium and BM when the patients were under general anesthesia. The corneas were collected from the eyes 30 minutes after injury using a 9.0 mm trephine. Specimens were immediately embedded in liquid optimal cutting temperature (OCT) compound (Sakura FineTek, Torrance, CA, USA) within a 24 mm × 24 mm × 5 mm mould (Fisher Scientific, Pittsburgh, PA, USA). Corneas were centered within the mould so that the block could be bisected and transverse sections cut from the center of the cornea. Frozen tissue blocks were stored at −80° C until sectioning was performed.
Central corneal sections (seven μm thick) were cut with a cryostat (HM 505M, Micron GmbH, Walldorf, Germany). Sections were placed on 25 mm × 75 mm × 1 mm microscope slides (Superfrost Plus, Fisher) and maintained at −80°C until staining was performed.
Cryostat-cut sections were dried for 20 minutes at room temperature, washed in phosphate-buffered saline (PBS) and blocked in PBS with 5% donkey serum for 60 minutes. The sections were incubated in primary antibodies against perlecan at 1:50 (Santa Cruz Biotechnologies; Santa Cruz, CA, cat# sc-27449) or nidogen-2 at 1:50 (20 mg/ml) (Abcam, Cambridge, MA, cat#, ab14513) in 5% donkey serum overnight at 4°C. Sections were washed with PBS and then incubated at room temperature for 60 minutes in donkey anti-goat IgG-FITC (Santa Cruz Bio, cat# sc-2024) secondary antibody diluted at 1:200 in PBS for perlecan or goat anti-rabbit IgG (H+L) (Jackson ImmunoResearch, West Grove, PA, cat# 111-545-144) secondary antibody diluted 1:75 in PBS for nidogen-2. In negative control slides, a non-specific isotypic control antibody was used for nidogen-2 staining (no preabsorption antigen available). Pre-absorption with blocking peptide (Santa Cruz Bio. cat# sc-27449 P) at 10X the antibody concentration was performed in controls for perlecan staining. Coverslips were mounted with Vectashield containing DAPI (Vector Laboratories Inc., Burlingame, CA) to allow visualization of all nuclei in the tissue sections. The sections were viewed and photographed with a Leica DM5000 microscope equipped with Q-Imaging Retiga 4000RV (Surrey, BC, Canada) camera and ImagePro software. Immunoshistochemistry was performed at least three times on sections from each cornea to confirm consistent results.
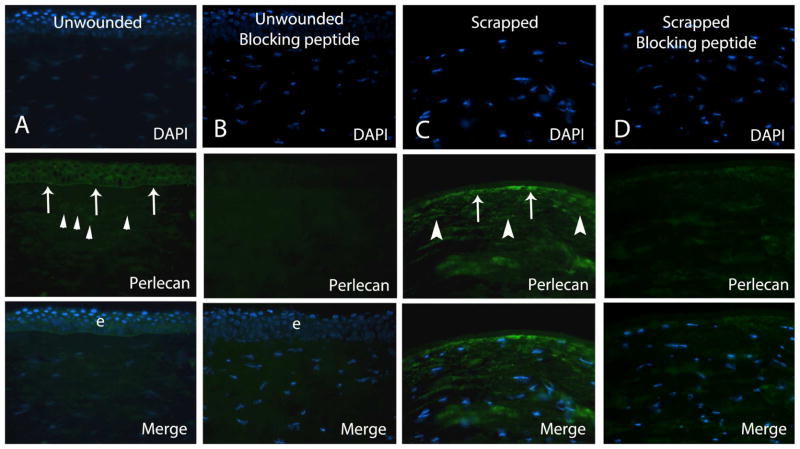
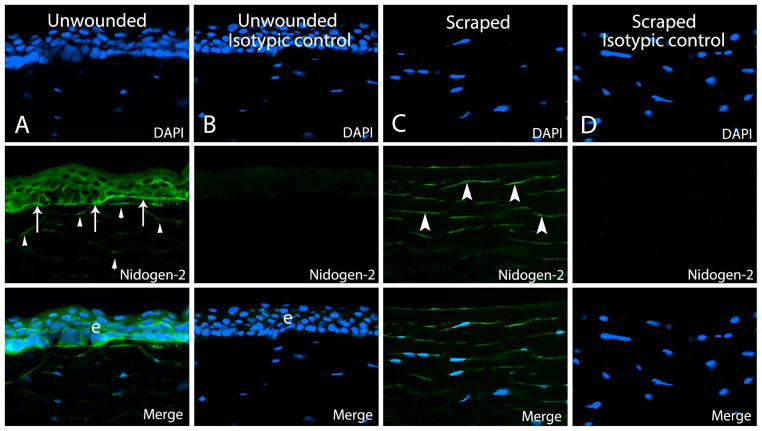
In human control corneas, immunohistochemical staining for both perlecan and nidogen-2 revealed a sharp and continuous reactivity in the BM and diffuse staining in all layers of the epithelium (Fig. 1 and Fig. 2) in each cornea. Faint immunoreactivity was detected in the stroma for both BM components in unwounded corneas (Fig. 1 and 2).
Figure 1.
Representative immunohistochemistry for perlecan in human corneas. Column A. Perlecan protein was noted in the epithelium and epithelial basement membrane (arrows) and at low levels in keratocytes (arrowheads) in unwounded control corneas. Column B. Negative control in unwounded cornea with pre-absorption with blocking peptide shows specific blocking of antigen-antibody interaction. Column C. In all corneas at 30 minutes after epithelial scrape, strong perlecan immunoreactivity was noted in keratocytes (arrowheads), as shown in this representative section from one cornea, including in keratocytes in the early stages of apoptosis that label with the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) assay (not shown). Staining near the stromal surface (arrows) could represent BM that was not completely removed by scrape or expression in keratocytes undergoing apoptosis—which cannot be distinguished at this level of magnification. Column D. Pre-absorption with blocking peptide shows specific blocking of antigen-antibody interaction in the negative control cornea after epithelial scrape. Blue is DAPI staining of cell nuclei. e is epithelium. Magnification 400x.
Figure 2.
Representative immunohistochemistry for nidogen-2 in human corneas. Column A. Nidogen-2 protein was noted in the epithelial basement membrane (arrows) and epithelium (e), as well as at low levels in keratocytes (arrowheads) in unwounded control corneas. Column B. Negative control in unwounded cornea with isotypic control antibody showed no non-specific immunostaining for nidogen-2. Column C. In a cornea at 30 minutes after epithelial scrape, nidogen-2 protein was up-regulated in stromal keratocytes (arrowheads), including anterior stromal keratocytes in the early stages of apoptosis detected with the TUNEL assay (not shown). Staining near the stromal surface (arrows) could represent BM that was not completely removed by scrape or expression in keratocytes undergoing apoptosis. Column D. Isotypic control shows specific antigen-antibody interaction in the control cornea after epithelial scrape. Blue is DAPI staining of cell nuclei. e is epithelium. Magnification 400x.
Immunostaining for perlecan was up-regulated in anterior to mid-stromal keratocytes, compared to control unwounded corneas, at 30 minutes after epithelial and epithelial BM scrape in human corneas (Fig. 1), including in cells in the early stages of apoptosis (not shown). Perlecan was also detected at higher levels in remnants of the epithelial BM or BM that had already begun to regenerate, compared to the unwounded control corneas. Nidogen-2 was up-regulated in keratocytes in the anterior to mid-stroma and stromal connective tissue in all wounded corneas, including keratocytes in the early stages of apoptosis (not shown), as well as in remnants of the epithelial BM or BM that had already begun to regenerate (Fig. 2).
This study reveals that stromal keratocytes up-regulate production of perlecan and nidogen-2 proteins after epithelial and BM injury in human corneas. Thus, keratocytes likely contribute to epithelial BM regeneration after epithelial-basement membrane scrape injury in humans. Thus, both the epithelium and underlying keratocytes contribute components to regenerate the corneal epithelial BM (Marinkovich et al., 1993).
Interestingly, it was noted that both perlecan and nidogen-2 were up-regulated in keratocytes that were undergoing apoptosis detected by the TUNEL assay at 30 minutes after epithelial scrape. Prior studies on keratocyte apoptosis showed that the dying cells and apoptotic body remnants persist for more than 4 hours after epithelial injury (Wilson, et al., 1996; Ambrósio, Kara-José and Wilson, 2009), even though anterior stromal keratocytes are found to have ultrastructural changes associated with apoptosis detectible by transmission electron microscopy immediately after epithelial scrape injury. The findings here suggest those cell remnants can continue to generate at least some proteins until apoptosis is completed and they disappear.
The importance of normal regeneration of epithelial BM blocking myofibroblast development and maintenance of corneal stromal opacity has been demonstrated by several studies (Fini and Stramer, 2005; Gipson et al., 1989; Netto et al., 2006; Stramer et al., 2003, Torricelli, et al., 2013a and b). Normal BM barrier function likely modulates myofibroblast development from precursors and persistence in the anterior stroma by limiting penetration of epithelial-derived growth factor such as TGFβ and platelet-derived growth factor (PDGF) (Torricelli et al., 2013b). Mature myofibroblasts are themselves opaque and excrete a large amount of disorganized extracellular matrix that causes a loss of corneal transparency (Jester et al., 1999; Wilson, 2012). Transmission electron microscopic analyses in rabbits showed there was abnormal regeneration of epithelial BM (no regeneration of lamina lucida and lamina densa of which nidogens and perlecans are components) in corneas with opacity at one month after PRK for high myopia (Torricelli et al., 2013a). Conversely, there was full regeneration of the epithelial BM, undistinguishable to that found in unwounded control corneas, in corneas that had PRK for moderate myopia that healed with normal transparency (Torricelli et al., 2013a).
Nidogens are produced by mesenchymal cells and are deposited into epithelial BM during development (Dziadek, 1995). In skin tissue, fibroblasts have been described to be the source of nidogens (Fleischmajer et al., 1995; Kohfeldt et al., 1998). Maguen and coworkers (2008) reported nidogen-2 accumulation around intrastromal corneal rings implanted for post-laser keratectasia and keratoconus. In the current study, both epithelial cells and keratocytes produce nidogen-2 protein. Perlecan has been shown to participate in many biological processes in different tissues, including regulation of wound healing, cell proliferation, and cell survival (Rossi et al., 2003; Sher et al., 2006; Zhou et al., 2004). Inomata et al (2012) found a thin and poorly-differentiated corneal epithelium in perlecan-deficient mice and suggested that BM perlecan was critical for normal epithelium formation and terminal differentiation. The current study found that both corneal epithelial cells and keratocytes produce perlecan protein.
We hypothesize, based on our work and the work of other investigators (Fini and Stramer, 2005; Stramer et al., 2003; Torricelli et al., 2013a; Torricelli et al., 2013b), that 1) after epithelial-BM injury the epithelium begins the process of BM regeneration through the production of BM components such as laminin that self-assemble, and that once this nascent BM is laid down, more posterior components, such as nidogens and perlecan, which contribute to layers such as the lamina lucida and lamina densa, must be provided, at least in part, by stromal keratocytes. 2) In corneas with haze, or in an opacified zone within a transparent cornea, a deficiency in stromal cell BM component contributions leads to defective regeneration of the epithelial BM and ongoing penetration of TGFβ from the epithelium that drives myofibroblast generation. 3) Once the myofibroblasts become established in the subepithelial stroma, they prevent repopulation of the anterior stroma with keratocytes, or even produce alternatively-spliced components that interfere with BM regeneration, and thereby block contributions of the keratocytes to regenerate the normal epithelial BM—leading to persistence of the myofibroblasts and associated opacity. 4) Over time, measured in months or years, transparent areas, called lacunae, appear in the haze where the normal epithelial BM has been restored, stromal TGFβ levels have fallen, myofibroblasts have undergone apoptosis and keratocytes repopulate the subepithelial stroma and reabsorb disorganized extracellular matrix to reestablish corneal transparency.
Ongoing studies are aimed at better characterizing cellular production of each corneal epithelial BM component during different stages of corneal wound healing and during homeostasis in the unwounded cornea. However, this study in humans confirms the important role that stromal cells likely have in regeneration of the normal epithelial basement membrane after injury.
Highlights.
Keratocytes up-regulate nidogen-2 protein after corneal injury
Keratocytes up-regulate perlecan protein after corneal injury
Stromal cells produced epithelial basement membrane components
Acknowledgments
Supported in part by US Public Health Service grants EY10056 and EY015638 from the National Eye Institute, National Institutes of Health, Bethesda, MD and Research to Prevent Blindness, New York, NY.
Footnotes
Proprietary interest statement: None of the authors have any proprietary or financial interests in the topics discussed in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrósio R, Jr, Kara-José N, Wilson SE. Early keratocyte apoptosis after epithelial scrape injury in the human cornea. Exp Eye Res. 2009;89:597–9. doi: 10.1016/j.exer.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader BL, Smyth N, Nedbal S, Miosge N, Baranowsky A, Mokkapati S, Murshed M, Nischt R. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol Cell Biol. 2005;25:6846–56. doi: 10.1128/MCB.25.15.6846-6856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziadek M. Role of laminin-nidogen complexes in basement membrane formation during embryonic development. Experientia. 1995;51:901–913. doi: 10.1007/BF01921740. [DOI] [PubMed] [Google Scholar]
- Fini ME, Stramer BM. How the cornea heals: cornea-specific repair mechanisms affecting surgical outcomes. Cornea. 2005;24:S2–S11. doi: 10.1097/01.ico.0000178743.06340.2c. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R, Schechter A, Bruns M, Perlish JS, Macdonald ED, Pan TC, Timpl R, Chu ML. Skin fibroblasts are the only source of nidogen during early basal lamina formation in vitro. J Invest Derm. 1995;105:597–601. doi: 10.1111/1523-1747.ep12323604. [DOI] [PubMed] [Google Scholar]
- Fox JW, Mayer U, Nischt R, Aumailley M, Reinhardt D, Wiedemann H, Mann K, Timpl R, Krieg T, Engel J. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J. 1991;10:3137–46. doi: 10.1002/j.1460-2075.1991.tb04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa LS, Foster CS, Gipson IK, Colvin RB. Basement membrane components in healing rabbit corneal epithelial wounds: immunofluorescence and ultrastructural studies. J Cell Bio. 1984;98:128–138. doi: 10.1083/jcb.98.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Tisdale A, Keough M. Reassembly of the anchoring structures of the corneal epithelium during wound repair in the rabbit. Invest Ophthalmol Vis Sci. 1989;30:425–434. [PubMed] [Google Scholar]
- Ho MS, Bose K, Mokkapati S, Nischt R, Smyth N. Nidogens-Extracellular matrix linker molecules. Microscopy research and technique. 2008;71:387–395. doi: 10.1002/jemt.20567. [DOI] [PubMed] [Google Scholar]
- Hohenester E, Engel J. Domain structure and organisation in extracellular matrix proteins. Matrix Biol. 2002;21:115–28. doi: 10.1016/s0945-053x(01)00191-3. [DOI] [PubMed] [Google Scholar]
- Hopf M, Göhring W, Ries A, Timpl R, Hohenester E. Crystal structure and mutational analysis of a perlecan-binding fragment of nidogen-1. Nat Struct Biol. 2001;8:634–40. doi: 10.1038/89683. [DOI] [PubMed] [Google Scholar]
- Inomata T, Ebihara N, Funaki T, Matsuda A, Watanabe Y, Ning L, Xu Z, Murakami A, Arikawa-Hirasawa E. Perlecan-deficient mutation impairs corneal epithelial structure. Investigative ophthalmology & visual science. 2012;53:1277–1284. doi: 10.1167/iovs.11-8742. [DOI] [PubMed] [Google Scholar]
- Jester JV, Moller-Pedersen T, Huang J, Sax CM, Kays WT, Cavangh HD, Petroll WM, Piatigorsky J. The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. Journal of cell science. 1999;112 (Pt 5):613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- Kvansakul M, Hopf M, Ries A, Timpl R, Hohenester E. Structural basis for the high-affinity interaction of nidogen-1 with immunoglobulin-like domain 3 of perlecan. EMBO J. 2001;20:5342–6. doi: 10.1093/emboj/20.19.5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohfeldt E, Sasaki T, Gohring W, Timpl R. Nidogen-2: a new basement membrane protein with diverse binding properties. J Mol Biol. 1998;282:99–109. doi: 10.1006/jmbi.1998.2004. [DOI] [PubMed] [Google Scholar]
- Kruegel J, Miosge N. Basement membrane components are key players in specialized extracellular matrices. Cellular and molecular life sciences : CMLS. 2010;67:2879–2895. doi: 10.1007/s00018-010-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguen E, Rabinowitz YS, Regev L, Saghizadeh M, Sasaki T, Ljubimov AV. Alterations of extracellular matrix components and proteinases in human corneal buttons with INTACS for post-laser in situ keratomileusis keratectasia and keratoconus. Cornea. 2008;27:565–573. doi: 10.1097/ICO.0b013e318165b1cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovich MP, Keene DR, Rimberg CS, Burgeson RE. Cellular origin of the dermal-epidermal basement membrane. Developmental dynamics: an official publication of the Am Assoc Anatomists. 1993;197:255–267. doi: 10.1002/aja.1001970404. [DOI] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006;82:788–797. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Morita H, Sormunen R, Airenne S, Kreivi M, Wang L, Fukai N, Olsen BR, Tryggvason K, Soininen R. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 2003;22:236–245. doi: 10.1093/emboj/cdg019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmivirta K, Talts JF, Olsson M, Sasaki T, Timpl R, Ekblom P. Binding of mouse nidogen-2 to basement membrane components and cells and its expression in embryonic and adult tissues suggest complementary functions of the two nidogens. Exp Cell Res. 2002;279:188–201. doi: 10.1006/excr.2002.5611. [DOI] [PubMed] [Google Scholar]
- Schittny JC, Timpl R, Engel J. High resolution immunoelectron microscopic localization of functional domains of laminin, nidogen, and heparan sulfate proteoglycan in epithelial basement membrane of mouse cornea reveals different topological orientations. J Cell Biol. 1988;107:1599–610. doi: 10.1083/jcb.107.4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schymeinsky J, Nedbal S, Miosge N, Pöschl E, Rao C, Beier DR, Skarnes WC, Timpl R, Bader BL. Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol Cell Biol. 2002;22:6820–30. doi: 10.1128/MCB.22.19.6820-6830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher I, Zisman-Rozen S, Eliahu L, Whitelock JM, Maas-Szabowski N, Yamada Y, Breitkreutz D, Fusenig NE, Arikawa-Hirasawa E, Iozzo RV, Bergman R, Ron D. Targeting perlecan in human keratinocytes reveals novel roles for perlecan in epidermal formation. J Biol Chem. 2006;281:5178–5187. doi: 10.1074/jbc.M509500200. [DOI] [PubMed] [Google Scholar]
- Smith S, Hassell JR. Focus on molecules: perlecan (HSPG2) Exp Eye Res. 2006;83:471–2. doi: 10.1016/j.exer.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144:151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sta Iglesia DD, Stepp MA. Disruption of the basement membrane after corneal debridement. Invest Ophthalmol Vis Sci. 2000;41:1045–1053. [PubMed] [Google Scholar]
- Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- Timpl R, Dziadek M, Fujiwara S, Nowack H, Wick G. Nidogen: a new, self-aggregating basement membrane protein. Eur J Biochem. 1983;137:455–65. doi: 10.1111/j.1432-1033.1983.tb07849.x. [DOI] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Agrawal V, Santhiago MR, Wilson SE. Transmission electron microscopy analysis of epithelial basement membrane repair in rabbit corneas with haze. Invest Ophthalmol Vis Sci. 2013a;54:4026–4033. doi: 10.1167/iovs.13-12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AA, Singh V, Santhiago MR, Wilson SE. The corneal epithelial basement membrane: structure, function, and disease. Invest Ophthalmol Vis Sci. 2013b;54:6390–6400. doi: 10.1167/iovs.13-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torricelli AA, Wilson SE. Cellular and extracellular matrix modulation of corneal stromal opacity. Exp Eye Res. 2014;129:151–60. doi: 10.1016/j.exer.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittitow J, Borras T. Genes expressed in the human trabecular meshwork during pressure-induced homeostatic response. J Cellular Phys. 2004;201:126–137. doi: 10.1002/jcp.20030. [DOI] [PubMed] [Google Scholar]
- Wilson SE. Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency. Experimental eye research. 2012;99:78–88. doi: 10.1016/j.exer.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, He Y-G, Weng J, Li Q, McDowall AW, Vital M, Chwang EL. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996;62:325–8. doi: 10.1006/exer.1996.0038. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Liu JJ, Mohan RR. Stromal-epithelial interactions in the cornea. Prog Ret Eye Res. 1999;18:293–309. doi: 10.1016/s1350-9462(98)00017-2. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22:521–38. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Cheng YS, Campbell K, Li S. Loss of basement membrane, receptor and cytoskeletal lattices in a laminin-deficient muscular dystrophy. J Cell Sci. 2004;117:735–742. doi: 10.1242/jcs.00911. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Wang J, Cao R, Morita H, Soininen R, Chan KM, Liu B, Cao Y, Tryggvason K. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res. 2004;64:4699–4702. doi: 10.1158/0008-5472.CAN-04-0810. [DOI] [PubMed] [Google Scholar]