SUMMARY
The MLL gene is a common target of chromosomal translocations found in human leukemia. MLL-fusion leukemia has a consistently poor outcome. One of the most common translocation partners is AF9 (MLLT3). MLL-AF9 recruits DOT1L, a histone 3 lysine 79 methyltransferase (H3K79me1/me2/me3), leading to aberrant gene transcription. We show that DOT1L has three AF9 binding sites, and present the NMR solution structure of a DOT1L-AF9 complex. We generate structure-guided point mutations and find they have graded effects on recruitment of DOT1L to MLL-AF9. ChIP-Seq analyses of H3K79me2 and H3K79me3 show that graded reduction of the DOT1L interaction with MLL-AF9 results in differential loss of H3K79me2 and me3 at MLL-AF9 target genes. Furthermore, the degree of DOT1L recruitment is linked to the level of MLL-AF9 hematopoietic transformation.
INTRODUCTION
The mixed lineage leukemia (MLL) protein is a histone 3 lysine 4 methyltransferase that positively regulates gene expression during development. The MLL gene is a common target of chromosomal translocations found in acute leukemias where an N-terminal fragment of MLL is fused to over 70 different nuclear, cytoplasmic, or membrane partners (Meyer et al., 2013). MLL leukemia accounts for up to 10% of acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL), but is over-represented in infants and adults treated with drugs that target DNA topoisomerase II (Muntean and Hess, 2012).
MLL-fusion partners AF9, ENL and AF4 account for over two-thirds of MLL rearrangements (Krivtsov and Armstrong, 2007) and have been shown to associate with each other as members of transcriptional elongation complexes, suggesting that misregulation of transcriptional elongation is a common mechanism in MLL-dependent leukemogenesis (Biswas et al., 2011; Lin et al., 2010; Mohan et al., 2010; Mueller et al., 2007; Yokoyama et al., 2010). The N-terminal portions of these fusion partner proteins are lost in MLL-rearrangements whereas C-terminal domains are retained; in AF9 this domain is referred to as the ANC1 homology domain (AHD). This enables the MLL-fusion protein to constitutively interact with members of these transcriptional elongation complexes. In particular, the AF9 AHD can recruit AF4 family members leading to the subsequent phosphorylation of RNA polymerase II via P-TEFb (Bitoun et al., 2007; Erfurth et al., 2004), as well as DOT1L, a methyltransferase responsible for histone 3 lysine 79 (H3K79) methylation, a mark associated with active transcription (Steger et al., 2008; Zhang et al., 2006). The constitutive recruitment of these proteins by MLL-AF9, combined with the gene-specific recognition binding domains of the N-terminal portion of MLL, leads to dysregulated expression of MLL target genes such as HOXA9 and MEIS1, decreased differentiation, and increased self-renewal (Muntean and Hess, 2012).
A number of in vitro, in vivo, and small molecule inhibitor studies have recently shown that DOT1L is essential for MLL-rearranged leukemia. DOT1L is the only known enzyme to catalyze the mono-, di-, and tri- methylation of the globular domain of histone H3 at lysine 79 (H3K79me1, H3K79me2, H3K79me3). This enzyme plays a role in many different cellular processes from cell cycle regulation to differentiation and has been implicated in leukemogenesis, kidney injury, and cardiac disorders (reviewed in Nguyen and Zhang, 2011). DOT1L-mediated H3K79 methylation marks are enriched in the gene body and are coupled with gene transcription (Steger et al., 2008). Occupancy of the MLL-AF9 fusion protein is correlated with elevated H3K79me2 levels at target genes (Bernt et al., 2011; Nguyen et al., 2011). A small-molecule inhibitor, EPZ-5676, that inhibits the enzymatic activity of DOT1L leads to loss of H3K79me2 at MLL-fusion loci and has shown some efficacy in mouse models of MLL-fusion leukemia (Daigle et al., 2013).
The protein-protein interaction between AF9 and DOT1L has been roughly mapped (Biswas et al., 2011; Shen et al., 2013; Yokoyama et al., 2010; Zhang et al., 2006), but there is a lack of structural characterization of this interaction and functional effects of the direct recruitment of DOT1L. To that end, we now show that there are three separate regions in DOT1L that interact with AF9 and fold into structurally similar complexes. We also present the first structure of a DOT1L-AF9 complex. Using structure-guided mutagenesis, we develop point mutations that reduce DOT1L binding to AF9 in a graded manner. Functional characterization of these point mutations in the context of MLL-AF9 shows that the degree of DOT1L recruitment to the MLL-AF9 fusion protein differentially affects H3K79me2 and H3K79me3 levels at specific target genes and that direct recruitment of DOT1L is essential for the transforming potential of MLL-AF9.
RESULTS
DOT1L has three separate motifs for binding AF9 that form structurally similar complexes
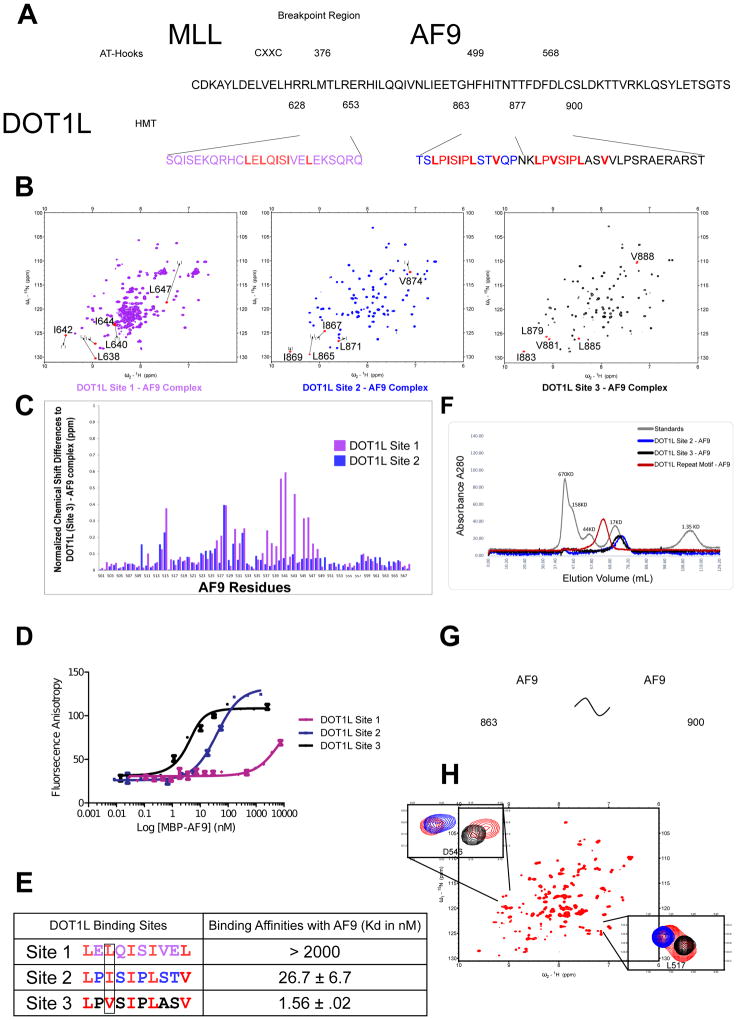
Previous biochemical studies have broadly determined regions of both DOT1L (aa. 479–659 and aa. 828–1095) as well as AF9 (aa. 495–568) which mediate their interaction (Biswas et al., 2011; Yokoyama et al., 2010; Zhang et al., 2006). The region of AF9 defined, referred to as the ANC1 homology domain or AHD, is the same region for which we have recently determined the structure of an AF4 peptide-AF9 complex (Leach et al., 2013). Co-expression of the previously delineated regions of AF9 and DOT1L with one another resulted in stable complexes but very poor NMR spectra. To define appropriate regions to utilize for structural studies, we expressed deletion constructs and analyzed them using heteronuclear triple resonance NMR to assign resonances and {15N}-1H heteronuclear NOE measurements to assess the dynamic behavior of the residues, i.e. identify what residues were flexible and remove them. This process led not only to high quality 15N-1H HSQC NMR spectra but also the identification of three separate DOT1L motifs for binding with AF9: Site 1 (aa. 628–653), Site 2 (aa. 863–878), and Site 3 (aa. 877–900) (Figures 1A and 1B). For AF9, we identified a functional domain (aa. 499–568) that produces optimal NMR spectra with all three DOT1L motifs. The 15N-1H HSQC spectra of the three complexes of AF9 with the different DOT1L motifs show a strong similarity (Figure 1B). Indeed, comparison of the individual AF9 amide chemical shifts among the three complexes shows relatively small changes (Figure 1C). As chemical shifts are highly dependent on protein structure and local environment, this indicates that AF9 folds in a very similar manner with each of the separate DOT1L binding motifs.
Figure 1. DOT1L has three separate sites of interaction with AF9.
A) The minimal interacting sites of DOT1L, Site 1 (Purple), Site 2 (Blue) and Site 3 (Black) with MLL-AF9 (aa. 499–568). Identified in red are the similar motifs making up each of the binding sites. B) 15N-1H HSQC NMR spectra of the co-expressed minimal interacting sites of DOT1L with AF9. Labeled in red are the chemical shifts of hydrophobic residues within the separate DOT1L motifs compared to that of the same residues within DOT1L Site 3 (displayed as brackets). C) Chemical shift difference between AF9 amide NH resonances from the DOT1L (Site 1)-AF9 complex (Purple) and DOT1L (Site 2)-AF9 complex (Blue) compared to DOT1L (Site 3)-AF9. D) Results of fluorescence polarization assay for determination of the Kd values for binding of MBP-AF9 AHD to each of the three DOT1L binding motifs. E) Table of Kd values for the three DOT1L sites with their respective primary sequences. Shown in red are hydrophobic residues of these DOT1L motifs. Highlighted is the third position of this motif that differs between each of the binding sites. F) Size exclusion profile of complexes of AF9 with DOT1L Site 2 (blue), DOT1L Site 3 (black), and with the repeat element (aa. 863–900; red) bound to AF9 overlaid with protein standards (grey). The size of AF9 bound to the DOT1L repeat motif is consistent with that of two AF9 proteins bound to the repeat motif. G) Cartoon depicting that two separate AF9 proteins can bind simultaneously to both high affinity DOT1L binding sites. H) 15N-1H HSQC NMR spectrum of DOT1L repeat motif (aa. 863–900; red) bound to AF9. Shown are examples of two AF9 resonance peaks, overlaid with the same AF9 amide peak from DOT1L Site 2 AF9 (blue) and DOT1L Site 3 AF9 (black).
Comparison of the DOT1L motifs across species shows that they are conserved (Figures S1A and S1B) and exhibit a periodic pattern of hydrophobic residues consistent with that of a β strand (Figures 1A and 1E). The DOT1L (Site 2) and (Site 3) motifs are nearly identical, with the exception of a bulkier Isoleucine residue in the third position of the DOT1L (Site 2) binding site as opposed to a Valine in DOT1L (Site 3) (Figure 1E). Fluorescence polarization based binding studies revealed a 17 fold weaker affinity for DOT1L (Site 2) with AF9 (Figures 1D and 1E). In the same position, DOT1L (Site 1) has a bulkier Leucine residue as well as a Leucine in the 10th position, as opposed to a Valine, as seen in the other two binding sites (Figures 1A and 1E). Our binding studies show weak affinity for this binding site (Kd > 2000 nM) (Figure 1D and 1E). As ENL, another fusion partner with MLL, is highly homologous to AF9, we tested binding of each of these DOT1L peptides with ENL which yielded similar binding affinities for ENL as seen for AF9 (Figure S2A and S2B).
Intriguingly, DOT1L (Site 2) and DOT1L (Site 3) are high affinity binding motifs, which are only separated by a 4 amino acid spacer sequence, i.e. they form a repeat motif in DOT1L (Figure 1A). To test whether it was sterically feasible for two AF9 AHDs to bind simultaneously to both of these DOT1L sites, we co-expressed the entire repeat motif consisting of both DOT1L (Sites 2 and 3) (aa. 863–900) with the AF9 AHD. Size-exclusion chromatography shows the formation of a complex consistent with two AF9 AHDs bound (Figure 1F & 1G). The 15N-1H HSQC NMR spectrum of this complex shows that there are duplicate AF9 peaks for each AF9 residue which overlay very closely with the AF9 amide resonances from the DOT1L (Site 2) - AF9 and DOT1L (Site 3) - AF9 individual complexes (Figure 1H). Additionally, the DOT1L resonances in this entire repeat motif overlap with the resonances from each of the individual DOT1L (Site 2) and (Site 3) complexes, indicating that the complexes on the repeat motif closely resemble those of the individual complexes (Figure 1H).
We have previously shown that the C-terminal domain of AF9 is an intrinsically disordered protein that folds into structured complexes upon binding partner proteins (Leach et al., 2013). Each of the DOT1L sites interacting with AF9 is predicted to be disordered (Figure S1C), suggesting that there is a mutual synergistic folding between AF9 and DOT1L at each of these binding sites. Our binding measurements of DOT1L (Site 2) with AF9 yielded Kd values significantly different from those reported in a recent publication that biochemically mapped the interaction of this particular DOT1L site with AF9, but did not identify the interactions of the other DOT1L sites (Shen et al., 2013). As is the case with many intrinsically disordered proteins, the AF9 AHD has a propensity to aggregate, requiring significant care in the concentrations and conditions employed for binding measurements, perhaps suggesting a rationale for the difference in the measured binding affinities.
NMR Solution Structure of the DOT1L-AF9 Complex
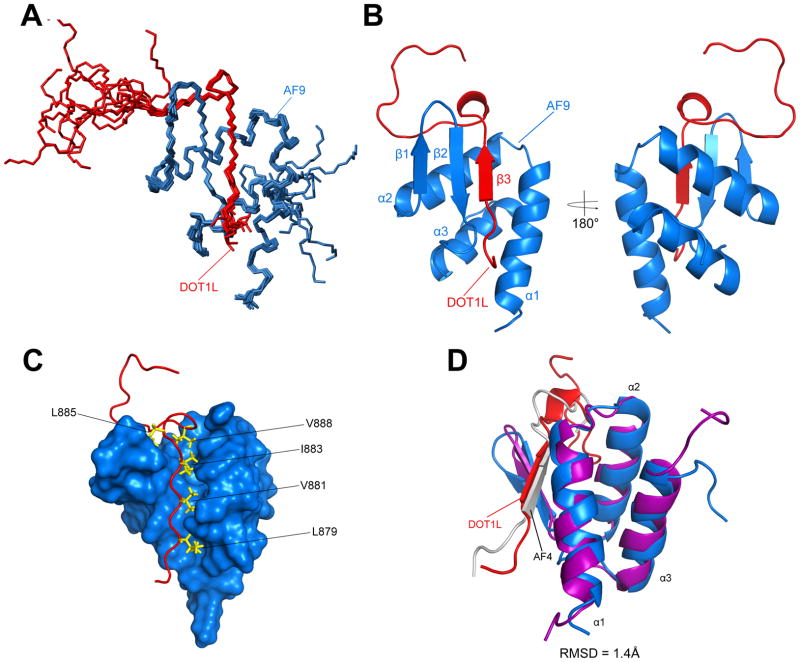
We solved the NMR solution structure of the highest affinity DOT1L-AF9 complex, (DOT1L Site 3 877–900) (PDB ID: 2MV7) using dihedral angle, NOE, and residual dipolar coupling (RDC) restraints without any significant constraint violations (Table S1). The NMR ensemble of the 10 lowest energy structures of DOT1L-AF9 shows a well-formed complex (Figure 2A). The DOT1L-AF9 complex forms a mixed alpha-beta structure and the DOT1L residues (879–884) form a β strand followed by a β turn (aa. 885–888). Immediately C-terminal, residues 889–895 make contacts with AF9, but not as significant as the preceding DOT1L residues and the following C-terminal amino acids, 896–900, are unstructured (Figure 2B). The interface between the two proteins is largely hydrophobic, as DOT1L L879, V881, I883, L885, and V888 are critical hydrophobic residues that are buried within the DOT1L-AF9 interface (Figure 2C). AF9 forms three helices around the DOT1L peptide (α1, α2, α3) and a β hairpin (β1 and β2), which forms a three-stranded antiparallel β sheet with the β strand from DOT1L; the C-terminus of AF9 (aa. 563–568) is unstructured (Figure 2B). While the chemical shift differences are minimal between the three DOT1L-AF9 complexes, larger chemical shift changes are seen around AF9 residues adjacent to the DOT1L peptide (aa. 537–547) (Figure 1C and S1D). Our NMR data and chemical shift mapping results suggest that the three sites of DOT1L all form the same mixed alpha-beta structure. The structure of the DOT1L-AF9 complex is very similar to our previously solved AF4-AF9 complex (PDB ID: 2LM0). Both DOT1L and AF4 share a similar consensus hydrophobic motif (Figure S3A) and superposition of the backbone residues yields an RMSD of 1.4 Å (Figure 2D). According to the Dali server, the BRD4 ET domain (PDB ID: 2JNS) has a similar helical fold to the helical portion of AF9 (RMSD = 3.98Å) (Figure S3B).
Figure 2. Structure of the DOT1L-AF9 complex.
A) Ensemble of the 10 lowest energy conformers. DOT1L is shown in red and AF9 in blue. B) Cartoon representation of the lowest energy conformer. DOT1L (red) forms a β strand, along with the β hairpin from AF9 (blue), while AF9 additionally forms three α helices. C) Surface representation of the DOT1L-AF9 complex. Shown are hydrophobic residues from DOT1L that are buried within the protein-protein interface and are critical for the interaction. D) Superposition of DOT1L-AF9 with our previously solved AF4-AF9 complex (PDB code: 2LM0; AF9 is purple and AF4 is white). RMSD = 1.4Å.
Point mutations in AF9 and DOT1L differentially attenuate the multiple DOT1L-AF9 interactions
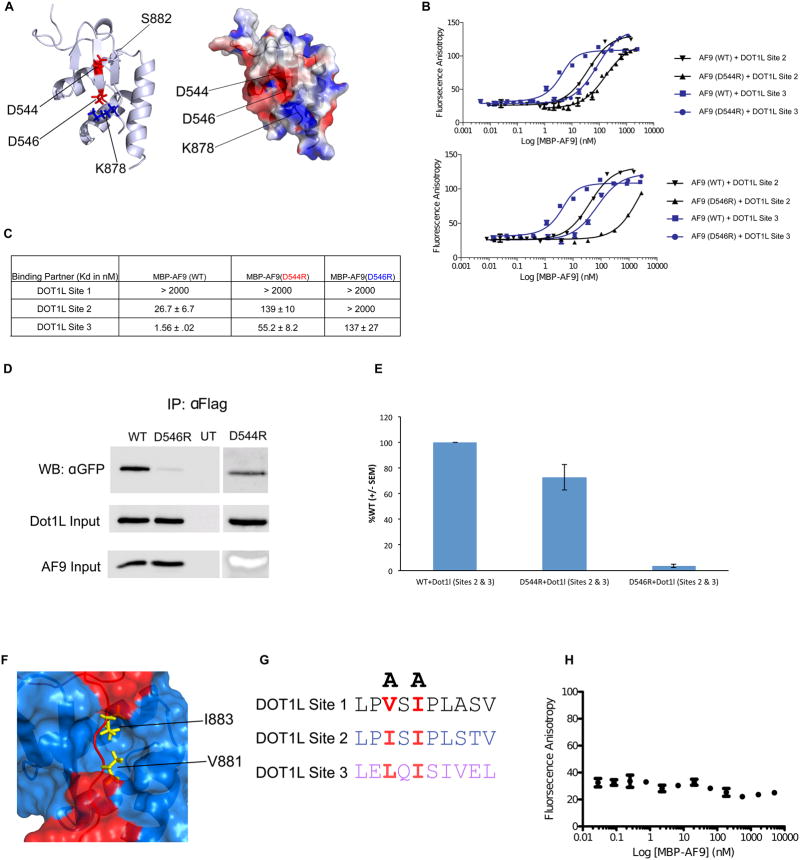
The DOT1L-AF9 structure was used as a basis to rationally design point mutations within both proteins to disrupt this protein-protein interaction. We identified an AF9 residue, D546, which is part of the AF9 β-hairpin (β2) and is in position to make an electrostatic interaction with DOT1L K878 (Figure 3A). We mutated this residue to arginine (denoted as D546R) to create charge repulsion. The D546R AF9 mutant significantly reduces binding to both DOT1L high affinity sites (Figures 3B and 3C), cannot pull down a DOT1L construct comprised of the high affinity DOT1L binding sites (Figure 3D), and has significantly decreased binding to full length DOT1L (Figure S4A). Interestingly, the AF9 D546R mutant protein is still capable of immunoprecipitating the low affinity Dot1L (Site 1) (Figure S4A). We also employed a previously described AF9 D544R mutation (Lokken et al., 2014). D544 is also a part of the AF9 β-hairpin (β2) and makes direct contacts with DOT1L S882 (Figure 3A). Interestingly, the D544R mutation shows a pronounced effect on binding to DOT1L (Site 3) and a limited effect on binding to DOT1L (Site 2) (Figure 3B and 3C). Consistent with this, the D544R mutant protein has been shown to still pull down DOT1L (Site 1) (Lokken et al., 2014), has a slightly diminished ability to pull down DOT1L (Sites 2 and 3) (Figure 3D and 3E), and still interacts with full length Dot1L (Figure S4B). As the binding of wildtype and mutant AF9 proteins with the DOT1L (Site 1) is extremely weak, we were unable to quantify any differences in binding to this site using our FP assays (Figure 3C).
Figure 3. Identification of mutations that disrupt the DOT1L-AF9 interactions.
A) Left: Cartoon representation of the DOT1L-AF9 structure showing D544 and D546, located on the AF9 β hairpin, that make direct interactions with DOT1L. Right: Electrostatic surface representation depicting that D546 (AF9) and K878 (DOT1L) make a charge-charge interaction. B) Results of fluorescence polarization assays for determination of the Kd values for binding of MBP-AF9 AHD (WT), MBP-AF9 AHD (D544R) (Top), and MBP-AF9 AHD (D546R) (Bottom), to DOT1L binding motifs 2 and 3. C) Table of Kd values for binding of MBP-AF9 AHD (WT), MBP-AF9 AHD (D544R), and MBP-AF9 AHD (D546R) to the DOT1L binding motifs. MBP-AF9 (D546R) significantly affects binding of both DOT1L high binding sites 2 and 3, whereas MBP-AF9 (D544R) has a more significant effect only on Site 3. D) and E) Co-IP data of D546R and D544R with the high affinity sites of DOT1L. F) Surface representation of the DOT1L (red) – AF9 (blue) complex. Sidechains of two buried hydrophobic residues form DOT1L, V881 and I883, are shown in yellow. G) Two alanine mutations of similarly positioned hydrophobic residues within each DOT1L binding site used to disrupt AF9 binding. H) Results of fluorescence polarization assay for MBP-AF9 AHD titrated into fluorescently tagged DOT1L peptide with (V881A, I883A), showing no binding.
The structure of the DOT1L-AF9 complex that we have determined, and our previously described AF4-AF9 structure (Leach et al., 2013), show that both AF4 and DOT1L bind in the same site on AF9, consistent with previous biochemical studies showing that the binding of the two is mutually exclusive (Biswas et al., 2011; Yokoyama et al., 2010). Based on the similarity of the NMR spectra of AF9 complexes with BCoR and CBX8 (Leach et al., 2013), it is likely these partners also bind in the same site. Due to this, it is challenging to identify point mutations in AF9 that can selectively inhibit the binding of specific partner proteins. Both of our AF9 mutants indeed have effects on the binding of other AF9 interacting partners (Figures S4C and S4D), and notably we see an increase in full-length CBX8 binding with our D546R mutant (Figure S4D). As binding to DOT1L and AF4 are presumed to be most critical for gene activation, it is important to note that the effects of the D544R and D546R mutations on AF4 binding are similar, so a comparison of the biological effects of these two mutations in the context of MLL-AF9 should give meaningful insights into the role of recruitment of DOT1L in particular.
In order to complement the biological readouts we obtained with the AF9 D544R and D546R mutations, we have also characterized mutations in DOT1L that selectively inhibit binding to each of the three AF9 binding motifs. Mutation of two DOT1L hydrophobic residues, V881 and I883, that are buried within the protein-protein interface (Figure 3F), to alanine (Figure 3G), denoted as V881A, I883A, completely abrogates binding of DOT1L (Site 3) to AF9 (Figure 3H). To disrupt the binding to each of the remaining DOT1L binding sites we made similar mutations of the corresponding DOT1L hydrophobic residues at Site 1 (L640A, I642A) and Site 2 (I867A, I869A) (Figures S5A and S5B). To this end, we created a series of DOT1L mutants that disrupted the three DOT1L-AF9 interactions alone as well as in various combinations.
Disruption of DOT1L recruitment via high affinity binding sites to MLL-AF9 leads to dramatic losses in hematopoietic transformation
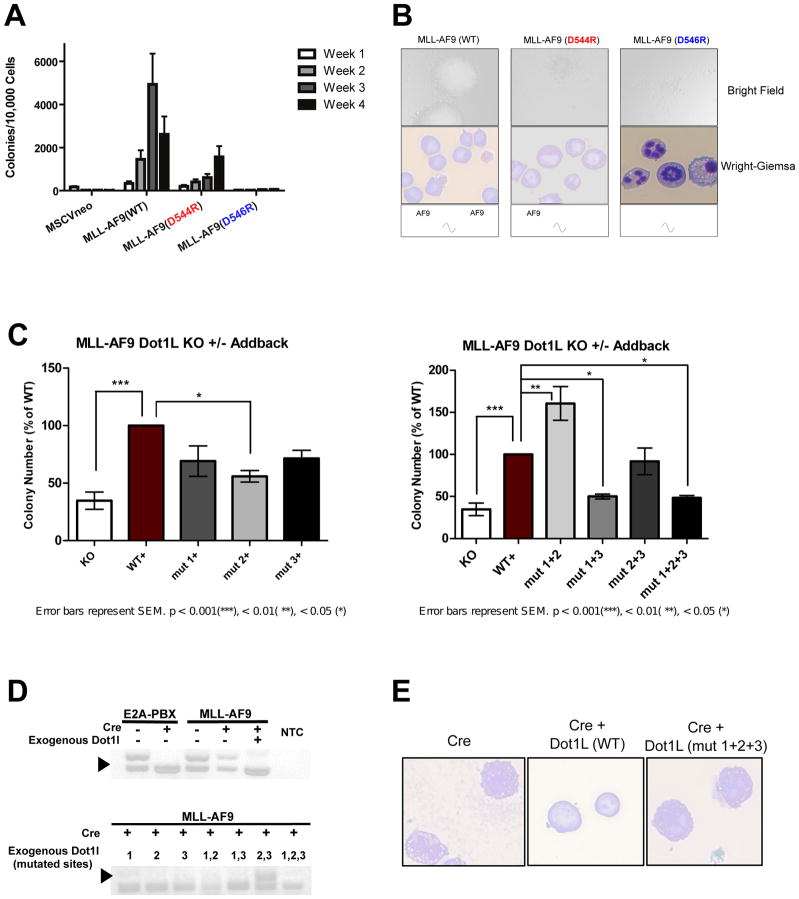
We used serial colony formation assays to assess the biological impact of differentially disrupting the DOT1L interactions with MLL-AF9. Briefly, hematopoietic progenitor cells isolated from mouse bone marrow were transduced with retrovirus expressing either wildtype MLL-AF9 (WT), or the mutants, MLL-AF9 (D544R) and MLL-AF9 (D546R). Cells were then serially replated on a weekly basis over a period of four weeks. Colony forming ability shows a gradient between cells expressing MLL-AF9 (WT), which efficiently replated for four weeks, the MLL-AF9 (D544R) mutant, which exhibited reduced colony formation in agreement with our recent publication (Lokken et al., 2014), and MLL-AF9 (D546R), which showed a more dramatic loss in colony formation (Figure 4A). Both of the mutants showed more diffuse colonies comprised of fewer cells compared to the dense, compact colonies formed by MLL-AF9 (WT) cells (Figure 4B). Cytospin and Wright-Giemsa staining indicate that MLL-AF9 (WT) expressing cells exhibit a blast-like morphology and cells expressing MLL-AF9 (D546R) appear to be differentiated (Figure 4B).
Figure 4. The level of DOT1L recruitment to MLL-AF9 defines the degree of serial replating capability.
A) Results of serial replating assays for MLL-AF9 (wt), MLL-AF9 (D544R), and MLL-AF9 (D546R). Cells expressing MLL-AF9 (WT) consistently replated over a period of 4 weeks. Cells expressing MLL-AF9 (D544R) show a reduction in serial replating ability. Cells expressing MLL-AF9 (D546R) show a complete abrogation of serial replating ability B) Bright field and Wright-Giemsa images of MLL-AF9 (WT) colonies show large, tight colonies (top) containing mostly cells with a blast-like morphology (bottom), whereas MLL-AF9(D544R) have more dispersed colonies, some with tight centers, and more differentiated cells. Strikingly, MLL-AF9(D546R) colonies are completely diffuse, with almost exclusively differentiated cells. C) Results of methylcellulose colony assays from MLL-AF9-transformed Dot1lfl/Δ cells, co-transduced with wild type or mutant Dot1l, either with or without Cre recombinase. D) Dot1l genomic status was examined by PCR at day 7 after methylcellulose culture of MLL-AF9- or E2A-PBX-transformed Dot1lfl/Δ cells, expressing the indicated exogenous proteins. Dark arrowhead, floxed allele (510 bp); open arrowhead, deleted allele (378 bp). NTC, non-template control. E) Wright-Giemsa images of cells from Dot1l complementation methylcellulose colony experiment, showing ability of exogenous Dot1l (WT), but not Dot1l (mut 1+2+3) to rescue blast-like morphology of MLL-AF9-transformed Dot1lfl/Δ cells.
A complementary experiment was performed to determine whether there were functional differences between the individual AF9-binding sites of DOT1L. Bone marrow progenitor cells isolated from conditional Dot1l deletion mice transformed with MLL-AF9 (Chang et al., 2010) were co-transduced with retroviruses expressing GFP-wildtype DOT1L or GFP-mutant DOT1L plus either mCherry-Cre or mCherry alone. GFP/mCherry double positive cells were sorted and assessed for colony forming ability. Deletion of endogenous Dot1l significantly decreased colony forming ability (Figure 4C, KO+ vs WT+), as we have shown previously (Chang et al., 2010). Remaining colonies result from expansion of cells that escaped Cre-mediated deletion (Figure 4D). Because there is such strong selective pressure for MLL-AF9-transformed cells to retain functional Dot1l expression, unless exogenous functional DOT1L is provided, only cells that retain endogenous Dot1l grow and expand, as demonstrated by the presence of the undeleted Dot1l allele (Figure 4D, upper gel). This dependence was not true for E2A-PBX-transformed cells, as we have shown previously (Figure 4D and S5C).
To further determine the functional significance of each AF9-binding site in DOT1L, mutant versions of DOT1L were exogenously provided in combination with deletion of the endogenous Dot1l. Mutations that disrupt each AF9-binding site in DOT1L individually cause reduced colony formation, but only the mutant DOT1L (Site 2) rises to statistical significance. With each single site mutant, there is no selection bias for cells retaining the endogenous Dot1l allele (Figure 4D). Simultaneous mutation of two sites proved to be particularly interesting. Mutation of Sites 1+2 showed no decrease in colony forming ability compared to wildtype DOT1L, but rather an increase. Mutation of Sites 1+3 was no different than when no exogenous DOT1L was added. Although mutation of Sites 2+3 did not demonstrate a statistically different colony-forming ability, remaining colonies were due to expansion of cells retaining the endogenous Dot1l allele (Figure 4D). Thus, DOT1L with both Sites 2+3 mutated does not confer colony-forming capacity to MLL-AF9-transformed cells. Simultaneously blocking all three DOT1L binding sites with alanine mutations shows colonies with differentiated morphology (Figure 4E) and a loss in colony formation similar to that when no exogenous DOT1L was provided (Figure 4C). This demonstrates that high affinity DOT1L binding to AF9 is necessary for the colony forming ability of MLL-AF9, and that the multiple binding sites act in concert with one another.
MLL-AF9 (D544R) and MLL-AF9 (D546R) display distinct patterns of loss of H3K79me2 and H3K79me3 on a select set of genes
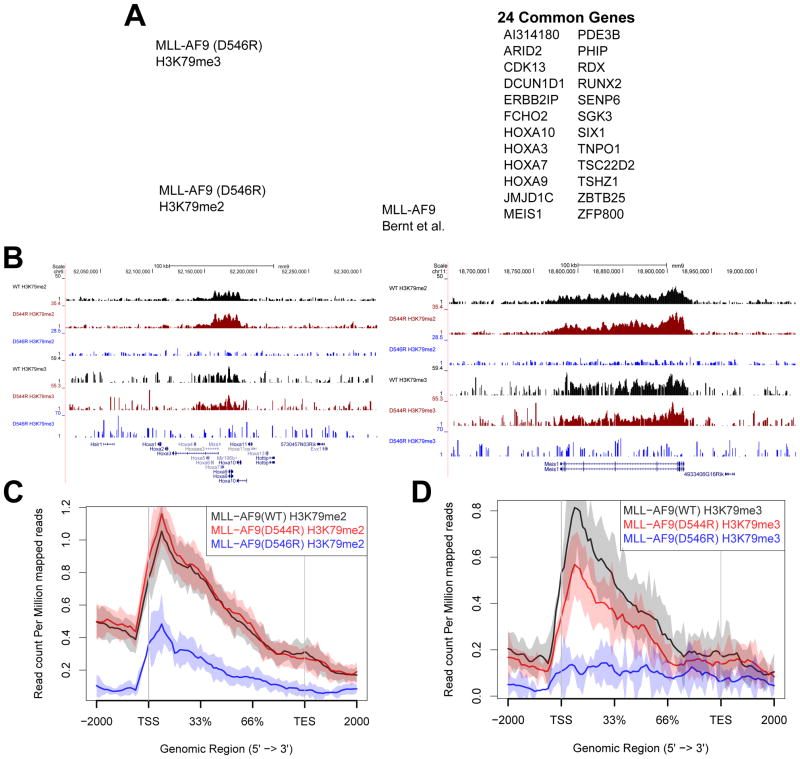
To assess the epigenetic effects of disrupting the multiple DOT1L and MLL-AF9 interactions, we used chromatin immunoprecipitation followed by next-generation sequencing (ChIP-Seq) to identify the genome-wide localization of H3K79me2 and H3K79me3 in primary hematopoietic progenitor cells expressing wildtype or mutant MLL-AF9. We analyzed H3K79me2 and H3K79me3 profiles and compared both MLL-AF9 (D544R) and MLL-AF9 (D546R) mutants to wildtype MLL-AF9 (WT). There were modest changes in individual genes for H3K79me2 and H3K79me3 marks when comparing the MLL-AF9 (D544R) mutant to MLL-AF9 (WT), but none rising to the level of significance (FDR < 0.1). In contrast, our MLL-AF9 (D546R) data show that with complete disruption of the high affinity DOT1L interaction to MLL-AF9, 44 genes display a significant loss of the H3K79me2 mark, and 42 genes show a reduction of the H3K79me3 mark (FDR < 0.1) compared to MLL-AF9 (WT) data (Figure 5A and Figure S6). A majority (31 out of 44 for H3K79me2 and 30 out of 42 for H3K79me3) of these genes are direct targets of MLL-AF9, as defined by a previous MLL-AF9 ChIP-Seq study (Figure 5A) (Bernt et al., 2011). A number of the identified genes with both decreased H3K79me2 and H3K79me3 have been shown to play a role in MLL-rearranged leukemia such as Hoxa9, Meis1, Runx2, and Jmjd1C (Figures 5A, 5B, S6A, S6B, and Table S2). There are not many genes with differentially affected changes in only H3K79me2 or H3K79me3, but not both, with the MLL-AF9(D546R) mutant. Of note is Cdk6, which shows a significant decrease in the H3K79me3 mark, but not H3K79me2, and has recently been shown to be important for MLL-rearranged leukemia (Figure S6D) (Placke et al., 2014). In contrast, Eya1 shows decreased H3K79me2 without significant change in H3K79me3, to the level of detection (Figure S6C), and is overexpressed in MLL leukemia (Wang et al., 2011).
Figure 5. MLL-AF9 (D544R) and MLL-AF9 (D546R) display distinct patterns of loss of H3K79me2 and H3K79me3 on a select set of genes.
A) Venn diagram overlaying gene sets showing significant loss of H3K79me2 and H3K79me3 marks upon comparison of MLL-AF9 (D546R) with MLL-AF9 (WT) (FDR < 0.1). Additionally overlaid is the set of genes identified previously by ChIP-Seq as direct MLL-AF9 targets (Bernt et al., 2011). Listed to the right are genes that overlap between the H3K79me2, H3K79me3, and MLL-AF9 direct target datasets. B) Chip-seq profiles of the HOXA cluster and Meis1 both show a loss in the H3K79me2 and H3K79me3 marks with the D546R mutation. C) H3K79me2 genomic profile at genes we identified to be significant in Figure 5A shows that MLL-AF9 (D546R) significantly reduces H3K79me2 marks whereas there is no difference in the profile between MLL-AF9 (D544R) and MLL-AF9 (WT). D) H3K79me3 genomic profile at genes we identified to be significant in Figure 5A shows that MLL-AF9 (D546R) reduces H3K79me3 to background levels, even lower than observed for the H3K79me2 profile of this same mutant. There is also a significant difference in MLL-AF9 (D544R) and MLL-AF9 (WT) profiles (Wilcoxon test p value = 0.02).
Mapping the genome-wide distribution of H3K79me2 and H3K79me3 marks across the set of genes that we found to be changed as a result of disrupting the DOT1L interaction with MLL-AF9 demonstrated no significant decrease in H3K79me2 levels for MLL-AF9 (D544R), but a substantial reduction for MLL-AF9 (D546R) (Figure 5C). Interestingly, a different pattern of effects on H3K79me3 are observed. The H3K79me3 data show that MLL-AF9 (D544R) levels significantly decrease in the H3K79me3 profile plot compared to wildtype (Wilcoxon test p-value = 0.02) (Figure 5D) and a complete loss of H3K79me3 is observed for MLL-AF9 (D546R). Thus, there is a differential effect of the loss of binding of MLL-AF9 to one or two high affinity DOT1L binding motifs on H3K79me2 and H3K79me3 levels at target genes.
Selected H3K79me2 and H3K79me3 genes display distinct patterns of gene expression in MLL-AF9 (D544R) and MLL-AF9 (D546R) mutant cells
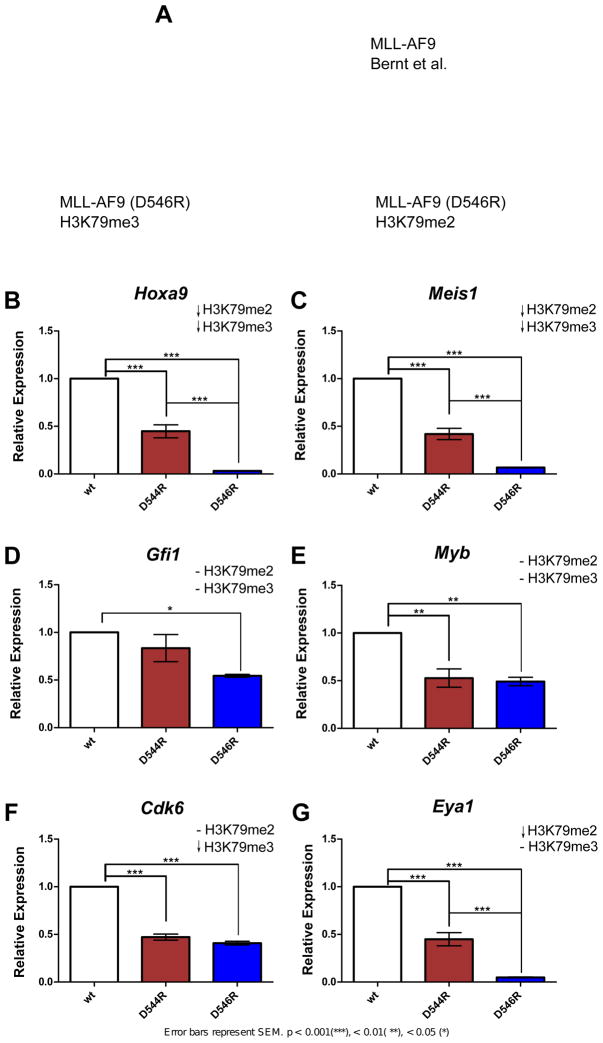
We next investigated whether the differential recruitment of Dot1l to MLL-AF9 and observed changes in H3K79 di- and tri-methylation correlated to changes in gene expression levels in selected genes. To assess this, gene expression levels were determined by quantitative RT-PCR (qRT-PCR) in primary bone marrow cells expressing MLL-AF9, and the MLL-AF9 mutants. We selected genes that represented a decrease in both H3K79me2 and me3 (Hoxa9 and Meis1), a decrease in only H3K79me2 or H3K79me3 (Eya1 and Cdk6, respectively), or no change in either (Myb and Gfi1) (Figures S6E and S6F), with our MLL-AF9 (D546R) mutant (Figure 6A). All of these genes are MLL-AF9 targets (Bernt et al., 2011) and have previously been shown to be involved in leukemogenesis (Khandanpour et al., 2013; Placke et al., 2014; Wang et al., 2011).
Figure 6. Gene expression data using MLL-AF9 (WT), MLL-AF9 (D544R), and MLL-AF9 (D546R).
A) Venn diagram depicting genes that were selected for gene expression analyses. Gene expression data of B) Hoxa9 C) Meis1 D) Gfi1 E) Myb F) Cdk6 G) Eya1 from murine bone marrow progenitor cells expressing MLL-AF9 (WT), MLL-AF9(D544R) or MLL-AF9(D546R) after one week in methylcellulose culture.
Interestingly, we observe different patterns of gene expression for these different selected gene classes (Figure 6). Hoxa9 and Meis1 both show losses in H3K79me2 and H3K79me3 (Figure 5) and we observe a graded decrease in expression going from wildtype MLL-AF9 to MLL-AF9(D544R) to MLL-AF9(D546R) (Figures 6B and 6C). Gfi1 and Myb show no change in H3K79me2 and H3K79me3, however they show different patterns of expression with the mutations. Gfi1 shows no effect with D544R but decreased expression with D546R (Figure 6D) whereas Myb shows similar reductions in expression with both mutations (Figure 6E), suggesting additional mechanisms of regulation at these genes. Cdk6 only shows losses in H3K79me3 and shows a similar decrease in expression for both D544R and D546R mutations (Figure 6F), consistent with a role for only H3K79me3 at this gene. Interestingly, Eya1 shows only decreased H3K79me2 but displays the same graded reduction in expression with the mutations as observed for Hoxa9 and Meis1 (Figure 6G).
DISCUSSION
It has been established that DOT1L is required for MLL-AF9 leukemogenesis, however the role of the direct recruitment of DOT1L by MLL-AF9 has not been clearly delineated. Our study provides important insights into the detailed mechanism and functional role of the interactions of DOT1L with MLL-AF9. We have found that DOT1L has three separate AF9 binding sites and that each separate DOT1L interacting site forms a similarly structured DOT1L-AF9 complex. Our NMR solution structure of the highest affinity DOT1L-AF9 complex is very similar to that of AF4-AF9, as both form nearly identical mixed α/β structures. Indeed, our results provide a structural basis for findings that interactions of AF9 are mutually exclusive with its different binding partners (Biswas et al., 2011; Leach et al., 2013; Yokoyama et al., 2010).
The AF9 AHD is intrinsically disordered, a characteristic seen in numerous signaling hubs, potentially allowing the hub protein to gain kinetic advantages from the binding of one interacting partner and its exchange for another (Dyson and Wright, 2005). Each of the AF9 binding proteins contains a similar binding motif, consistent with the observation that many proteins are enriched with short linear motifs, or eukaryotic linear motifs, which are small intrinsically disordered regions of functional modules from 3 to 11 amino acid residues (Tompa et al., 2014). These play a large role in many hub proteins that regulate diverse cellular processes. Eukaryotic linear motifs are enriched with post-translational modification sites that serve as a means of dynamic regulation and control of their activity (Van Roey et al., 2014). Indeed, we have previously shown that phosphorylation of AF4 at a site that has been shown to be phosphorylated in cells (Beausoleil et al., 2004) reduces its affinity for AF9 (Leach et al., 2013). Phosphorylation of serines within each of the DOT1L binding motifs (aa. 643, 868, 882) could also lead to reduction in binding, but only S882 has been shown to be phosphorylated in vivo (Hornbeck et al., 2012). DOT1L is unique among the AF9 binding partners in that there are multiple DOT1L motifs that bind to AF9, and each is predicted to be disordered. Even though a large number of eukaryotic motifs in IDPs have been characterized in various diseases, the biological function and necessity of multiple eukaryotic linear motifs within one protein is only beginning to be unraveled (Tompa et al., 2014).
While we were successful in identifying two separate AF9 mutations with differential effects on the DOT1L interactions with AF9, neither is completely specific for the DOT1L-AF9 interaction and both have varying effects on other AF9 binding partners. As the structures of the different AF9 complexes are very similar, it is challenging to make such specific mutations. It is important to note that co-immunoprecipitation experiments alone do not necessarily provide sufficient information to determine lack of effect of a particular mutation on protein interaction. Measurements of the binding affinities of these AF9 mutant proteins with binding partners show that these and other previously described AF9 (or ENL) mutations (Biswas et al., 2011; Lokken et al., 2014; Maethner et al., 2013; Tan et al., 2011) are not completely specific for a single protein partner (Figures S4C and S7). While it is definitely the case that the full-length proteins may behave differently, our results point out the importance of having the appropriate quantitative binding data to meaningfully interpret the biological effects of these mutations.
We clearly observe that the transforming properties of MLL-AF9, as measured by colony formation, are affected by the degree of direct recruitment of DOT1L to MLL-AF9. Partial disruption of high affinity DOT1L binding (D544R) reduces colony formation substantially but complete disruption (D546R) results in an even greater decrease in colony formation ability. Individually blocking each DOT1L binding site leads to similar losses in colony formation suggesting that individual DOT1L binding sites can have distinct functional roles. However, we see the most dramatic reduction in serial replating ability upon simultaneously blocking multiple DOT1L interactions with MLL-AF9. Mutation of either all three sites (1+2+3) or sites (1+3) function similarly to cells without any Dot1l. This suggests an additive function of the binding sites. Indeed, morphological data show that both our MLL-AF9 (D546R) and our Dot1L Site (1+2+3) mutants lead to differentiation of hematopoietic progenitors. Interestingly, mutation of sites (1+2) results in significantly higher colony number than wildtype DOT1L addback. In this context, it is important to remember that the mutations in DOT1L will affect not only binding to MLL-AF9 but also binding to wildtype AF9 and ENL, so the phenotypic output will be the sum of effects on all three targets, i.e. it is not possible to ascribe the observed effects only to the MLL-AF9 interaction.
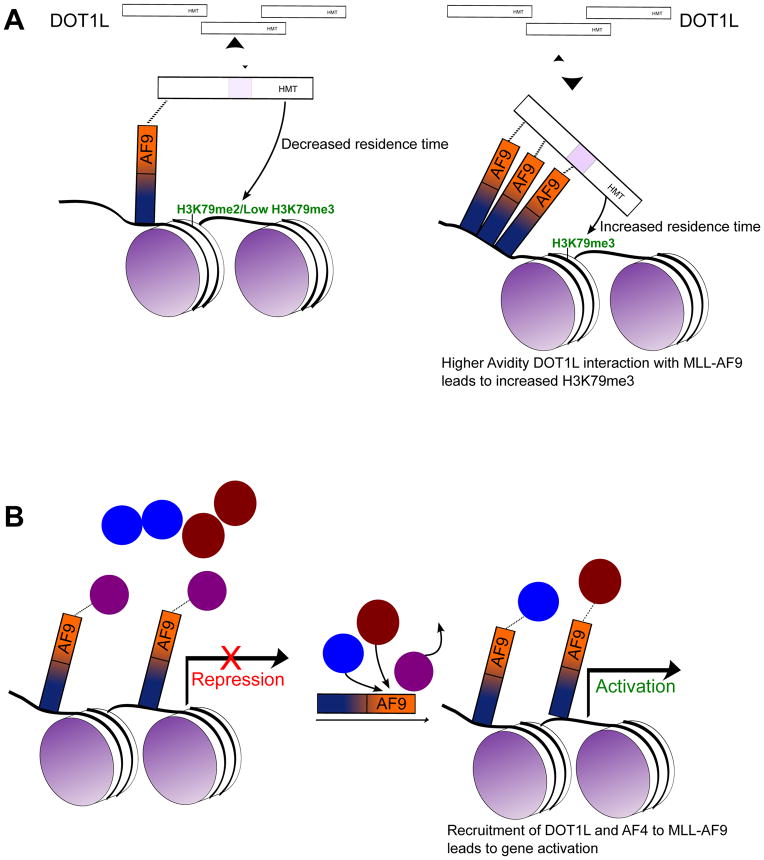
DOT1L is a non-processive, or distributive, enzyme, meaning that at most one round of methylation can take place in each encounter with its substrate before the enzyme must dissociate and re-associate to achieve subsequent rounds of methylation. As a result of this distributive nature of DOT1L, it has been suggested that there could be a functional redundancy between the H3K79 methylation marks (Frederiks et al., 2008). In contrast, several studies have suggested that different methylation states at H3K79 may have different functions in gene regulation (Nguyen and Zhang, 2011). H3K79me2 and H3K79me3 in yeast do not have overlapping chromatin patterns, and unlike H3K79me3 levels, which do not vary over the cell cycle, H3K79me2 levels change (Schulze et al., 2009). We show that blocking the high affinity DOT1L interactions with MLL-AF9, via our MLL-AF9 (D546R) mutant, results in significant losses of H3K79me2 and H3K79me3 at only a select number of genes, many of which have already been defined as MLL-AF9 targets. Additionally, we observe significant losses in the methylation pattern across the gene body at these genes, and the losses are more pronounced for the H3K79me3 mark. With the MLL-AF9 (D544R) mutation, we do observe losses in the H3K79me3 mark that are significant across the gene body despite changes in individual genes not rising to the level of statistical significance, a decrease not seen in the H3K79me2 mark. We hypothesize that losses of the H3K79me3 mark are directly linked to the presence of multiple sites for binding of AF9 to DOT1L, particularly the high affinity repeat motif in DOT1L. Binding of two (or three) sites on DOT1L to MLL-AF9 would significantly increase the residence time of DOT1L at a specific site on the chromatin. As the enzyme is distributive, a longer residence time increases the probability of proceeding all the way to the H3K79 tri-methylated state (see Figure 7A). With a reduction in contacts between MLL-AF9 and DOT1L (D544R mutant), the residence time would be decreased and the level of H3K79 tri-methylation would be reduced. This may also explain the more pronounced effect of the D546R mutation on H3K79me3 versus H3K79me2 across the gene body. Thus, there is a plausible mechanism for the cell to selectively di- or tri-methylate genomic sites by using mono- versus di- (or tri-) valent interaction of DOT1L with AF9 (or ENL) at particular sites in the genome. As DOT1L is the only known H3K79 methyltransferase, H3K79 methylation levels reflect DOT1L occupancy, however our model will likely require further experimental validation using techniques such as cross-linking kinetic analyses (Poorey et al., 2013) to directly assess DOT1L residence time at specific sites in the genome. However, the lack of a ChIP validated antibody for native DOT1L will likely require the use of tagged DOT1L for such experiments.
Figure 7. Proposed model of protein recruitment to MLL-AF9.
A) One binding event of DOT1L to MLL-AF9 leads to H3K79 di-methylation, but binding of two (or three) sites on DOT1L to MLL-AF9 would significantly increase the residence time of DOT1L at a specific site on the chromatin. As the number of binding events of DOT1L to MLL-AF9 increase from one to two (or three), this increases the residence time of DOT1L at a specific site on the chromatin increasing the probability of proceeding to the H3K79 tri-methylated state. B) CBX8 is initially bound to MLL-AF9 leading to a default transcriptionally repressed state. Recruitment of activators such as DOT1L or AF4 leads to the displacement of CBX8 from MLL-AF9 leading to gene activation.
As shown in Figures 5 and 6, we observe different patterns of changes in H3K79 methylation marks and in gene expression for various MLL-AF9 target genes, suggesting an underlying complexity in the regulation, which has not been fully elucidated. Indeed, a recent study by Armstrong and co-workers has shown that the DOT1L mediated conversion of H3K79me1 to H3K79me2 is regulated by AF10 (Deshpande et al., 2014). They showed that AF10 knockout leads to a profound reduction in H3K79me3 levels and to a lesser but significant decrease in H3K79me2 at specific Hoxa genes, with concomitant changes in gene expression. Interestingly, it has been reported that AF10 directly binds to DOT1L at a site that is in close proximity to the lowest affinity AF9 binding site (Site 1) (Okada et al., 2005) as well as in proximity to the site where DOT1L has been reported to bind to the phosphorylated CTD of RNA polymerase II (Kim et al., 2012).
As AF9 binds both activators (AF4 and DOT1L) and repressors (CBX8 and BCoR) of gene expression, it suggests that it functions as a signaling hub that provides different outputs depending on the binding partner. It is not clear, however, how this “dance” of binding partners is orchestrated. Interestingly, the introduction of the D546R mutation into MLL-AF9 results in an increase in CBX8 binding, as measured by co-immunoprecipitation (Figure S4D). This is not observed with the D544R mutation, where only partial loss of DOT1L binding is induced. Apparently, loss of binding of the co-activators, particularly DOT1L, results in increased repressor (CBX8) binding. While the exact mechanism is not yet clear, we would speculate that CBX8 binding to MLL-AF9 (or AF9) leading to stable transcriptional silencing is the default state in the absence of activators; the binding of DOT1L and AF4, in some sequence, relieves this default repressive state leading to activation of gene expression (Figure 7B). It has been reported that direct CBX8 binding is essential for MLL-AF9 leukemogenesis (Tan et al., 2011). Based on our structure and binding measurements, the point mutations used in that study (T542A and T554A) have additional impact on DOT1L and AF4 binding that was not appreciated at the time (Figure S7B). Further detailed studies are needed to determine the mechanism of CBX8-dependence of MLL leukemia.
We have shown that the degree of DOT1L recruitment to MLL-AF9 defines the level of hematopoietic transformation. Further studies are necessary to understand similar interactions in other MLL-fusion leukemias, but it is reasonable to expect that MLL-ENL fusions will behave in a similar manner. MLL-AF9 and MLL-ENL leukemias are highly aggressive and patients often suffer from early relapse after treatment (Krivtsov and Armstrong, 2007). The most promising current therapeutic for MLL-fusion leukemias, EPZ-5676, targets the enzymatic activity of DOT1L. Not surprisingly, due to its genome wide role in regulation of transcription, DOT1L inhibition has an effect on many genes, the long-term effects of which is not yet clear (Daigle et al., 2013). We propose that the DOT1L interactions with either AF9 or ENL would be excellent therapeutic targets for MLL-AF9 and MLL-ENL leukemias. Blocking this protein-protein interaction is likely to have distinct advantages over inhibiting DOT1L enzymatic activity, as only a very limited number of MLL-AF9 target genes show significant losses in H3K79 methylation marks, thus the scale of the effects on gene expression would be limited to these genes and those which are targets of wildtype AF9 and ENL regulation.
EXPERIMENTAL PROCEDURES
DOT1L-AF9 Assignments, Structure Determination and Refinement
All proteins were cloned, expressed and purified according to standard methods and constructs were optimized as described previously (Leach et al., 2013). For all NMR experiments, assignments and calculations were made using standard methods. Details are provided in Supplementary Procedures.
Protein Binding Measurements, Co-Immunoprecipitation and Western Blots
Fluorescence polarization based binding measurements were performed as previously described (Leach et al., 2013). Co-Immunoprecipitation experiments were conducted using HEK293 cells that were cotransfected with 3xFLAG-AF9 (WT, D544R or D546R mutant) C-terminal amino acid fragments 376–568 or 470–568, and GFP-tagged full length Dot1l, DOT1L(828–1095) encompassing both Sites 2 and 3, DOT1L(479–659) including Site 1, full length CBX8, or AF4 (647–871) encompassing the AF9 interaction domain. Details are provided in Supplementary Procedures.
Serial replating assays
MLL-AF9 wildtype and MLL-AF9 mutant serial replating assays were conducted with murine bone marrow c-kit+ cells transduced with MSCVneo, MSCVneo-MLL-AF9 (WT), MSCVneo-MLL-AF9 (D544R) or MSCVneo-MLL-AF9 (D546R) retroviruses. For DOT1L mutant serial replating assays, full length DOT1L, either wild type or containing alanine mutations, were cloned into an MSCV-mCherry retroviral vector. See Supplementary Procedures for further details and for primer sequences.
Quantitative RT-PCR
Cells were harvested from methylcellulose colony assays after one week, RNA was isolated and cDNA was synthesized. Quantitative Real-Time PCR was performed using TaqMan probes for Hoxa9, Meis1, Gfi1, Myb, Cdk6 and Eya1 (Applied Biosystems), and data were analyzed using the 2−ΔΔ Ct method. Expression was normalized to Gapdh expression and was performed in triplicate.
ChIP-Seq Experiments
Small scale ChIP experiments for Histone H3 Lysine 79 dimethylation (H3K79me2, Abcam ab3594) and trimethylation marks (H3K79me3, Diagenode cat# pAb-068-050) were carried out using previously published mini ChIP protocol (Adli and Bernstein, 2011). ChIP-Seq data can be accessed under accession number GSE64365 in the Gene Expression Omnibus. Details are provided in the Supplementary Procedures.
Supplementary Material
Acknowledgments
This work was supported by an American Heart Association Predoctoral Fellowship to A.K, an NIH T32 GM080186 Predoctoral Fellowship to A.K., and a grant from the National Cancer Institute (R01 CA155328) to J.H.B., C.S.H and N.J.Z-L. This work was also supported by NMR equipment purchased with an NIH High End Instrumentation grant (S10 RR023035) and housed in the Biomolecular Magnetic Resonance Facility at the University of Virginia. We acknowledge expert technical assistance of Shubin Zhang and thank Stefan Bekiranov for helpful advice and discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adli M, Bernstein BE. Whole-genome chromatin profiling from limited numbers of cells using nano-ChIP-seq. Nat Protoc. 2011;6:1656–1668. doi: 10.1038/nprot.2011.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, Villén J, Li J, Cohn MA, Cantley LC, Gygi SP. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci U S A. 2004;101:12130–12135. doi: 10.1073/pnas.0404720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernt KM, Zhu N, Sinha AU, Vempati S, Faber J, Krivtsov AV, Feng Z, Punt N, Daigle A, Bullinger L, et al. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell. 2011;20:66–78. doi: 10.1016/j.ccr.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas D, Milne Ta, Basrur V, Kim J, Elenitoba-Johnson KSJ, Allis CD, Roeder RG. Function of leukemogenic mixed lineage leukemia 1 (MLL) fusion proteins through distinct partner protein complexes. Proc Natl Acad Sci U S A. 2011;108:15751–15756. doi: 10.1073/pnas.1111498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitoun E, Oliver PL, Davies KE. The mixed-lineage leukemia fusion partner AF4 stimulates RNA polymerase II transcriptional elongation and mediates coordinated chromatin remodeling. Hum Mol Genet. 2007;16:92–106. doi: 10.1093/hmg/ddl444. [DOI] [PubMed] [Google Scholar]
- Chang M-J, Wu H, Achille NJ, Reisenauer MR, Chou C-W, Zeleznik-Le NJ, Hemenway CS, Zhang W. Histone H3 lysine 79 methyltransferase Dot1 is required for immortalization by MLL oncogenes. Cancer Res. 2010;70:10234–10242. doi: 10.1158/0008-5472.CAN-10-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle SR, Olhava EJ, Therkelsen CA, Basavapathruni A, Jin L, Boriack-Sjodin PA, Allain CJ, Klaus CR, Raimondi A, Scott MP, et al. Potent inhibition of DOT1L as treatment of MLL-fusion leukemia. Blood. 2013;122:1017–1025. doi: 10.1182/blood-2013-04-497644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande AJ, Deshpande A, Sinha AU, Chen L, Chang J, Cihan A, Fazio M, Chen C, Zhu N, Koche R, et al. AF10 Regulates Progressive H3K79 Methylation and HOX Gene Expression in Diverse AML Subtypes. Cancer Cell. 2014 doi: 10.1016/j.ccell.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- Erfurth F, Hemenway CS, de Erkenez AC, Domer PH. MLL fusion partners AF4 and AF9 interact at subnuclear foci. Leukemia. 2004;18:92–102. doi: 10.1038/sj.leu.2403200. [DOI] [PubMed] [Google Scholar]
- Frederiks F, Tzouros M, Oudgenoeg G, van Welsem T, Fornerod M, Krijgsveld J, van Leeuwen F. Nonprocessive methylation by Dot1 leads to functional redundancy of histone H3K79 methylation states. Nat Struct Mol Biol. 2008;15:550–557. doi: 10.1038/nsmb.1432. [DOI] [PubMed] [Google Scholar]
- Hornbeck PV, Kornhauser JM, Tkachev S, Zhang B, Skrzypek E, Murray B, Latham V, Sullivan M. PhosphoSitePlus: a comprehensive resource for investigating the structure and function of experimentally determined post-translational modifications in man and mouse. Nucleic Acids Res. 2012;40:D261–70. doi: 10.1093/nar/gkr1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandanpour C, Phelan JD, Vassen L, Schütte J, Chen R, Horman SR, Gaudreau MC, Krongold J, Zhu J, Paul WE, et al. Growth factor independence 1 antagonizes a p53-induced DNA damage response pathway in lymphoblastic leukemia. Cancer Cell. 2013;23:200–214. doi: 10.1016/j.ccr.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-K, Jung I, Lee H, Kang K, Kim M, Jeong K, Kwon CS, Han Y-M, Kim YS, Kim D, et al. Human histone H3K79 methyltransferase DOT1L protein [corrected] binds actively transcribing RNA polymerase II to regulate gene expression. J Biol Chem. 2012;287:39698–39709. doi: 10.1074/jbc.M112.384057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7:823–833. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- Leach BI, Kuntimaddi A, Schmidt CR, Cierpicki T, Johnson Sa, Bushweller JH. Leukemia fusion target AF9 is an intrinsically disordered transcriptional regulator that recruits multiple partners via coupled folding and binding. Structure. 2013;21:176–183. doi: 10.1016/j.str.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Smith ER, Takahashi H, Lai KC, Martin-Brown S, Florens L, Washburn MP, Conaway JW, Conaway RC, Shilatifard A. AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Mol Cell. 2010;37:429–437. doi: 10.1016/j.molcel.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokken AA, Achille NJ, Chang M, Lin JJ, Kuntimaddi A, Leach BI, Malik B, Nesbit JB, Zhang S, Bushweller JH, et al. Importance of a Specific Amino Acid Pairing for Murine MLL Leukemias Driven by MLLT1/3 or AFF1/4. Leuk Res. 2014 doi: 10.1016/j.leukres.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maethner E, Garcia-Cuellar M-P, Breitinger C, Takacova S, Divoky V, Hess JL, Slany RK. MLL-ENL inhibits polycomb repressive complex 1 to achieve efficient transformation of hematopoietic cells. Cell Rep. 2013;3:1553–1566. doi: 10.1016/j.celrep.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Hofmann J, Burmeister T, Gröger D, Park TS, Emerenciano M, Pombo de Oliveira M, Renneville A, Villarese P, Macintyre E, et al. The MLL recombinome of acute leukemias in 2013. Leukemia. 2013;27:2165–2176. doi: 10.1038/leu.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan M, Herz H-M, Takahashi Y-H, Lin C, Lai KC, Zhang Y, Washburn MP, Florens L, Shilatifard A. Linking H3K79 trimethylation to Wnt signaling through a novel Dot1-containing complex (DotCom) Genes Dev. 2010;24:574–589. doi: 10.1101/gad.1898410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Bach C, Zeisig D, Garcia-Cuellar MP, Monroe S, Sreekumar A, Zhou R, Nesvizhskii A, Chinnaiyan A, Hess JL, et al. A role for the MLL fusion partner ENL in transcriptional elongation and chromatin modification. Blood. 2007;110:4445–4454. doi: 10.1182/blood-2007-05-090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntean AG, Hess JL. The pathogenesis of mixed-lineage leukemia. Annu Rev Pathol. 2012;7:283–301. doi: 10.1146/annurev-pathol-011811-132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AT, Zhang Y. The diverse functions of Dot1 and H3K79 methylation. Genes Dev. 2011;25:1345–1358. doi: 10.1101/gad.2057811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AT, Taranova O, He J, Zhang Y. DOT1L, the H3K79 methyltransferase, is required for MLL-AF9-mediated leukemogenesis. Blood. 2011;117:6912–6922. doi: 10.1182/blood-2011-02-334359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Feng Q, Lin Y, Jiang Q, Li Y, Coffield VM, Su L, Xu G, Zhang Y. hDOT1L links histone methylation to leukemogenesis. Cell. 2005;121:167–178. doi: 10.1016/j.cell.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Placke T, Faber K, Nonami A, Putwain SL, Salih HR, Heidel FH, Krämer A, Root DE, Barbie DA, Krivtsov AV, et al. Requirement for CDK6 in MLL-rearranged acute myeloid leukemia. Blood. 2014;124:13–23. doi: 10.1182/blood-2014-02-558114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorey K, Viswanathan R, Carver MN, Karpova TS, Cirimotich SM, McNally JG, Bekiranov S, Auble DT. Measuring chromatin interaction dynamics on the second time scale at single-copy genes. Science. 2013;342:369–372. doi: 10.1126/science.1242369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Roey K, Uyar B, Weatheritt RJ, Dinkel H, Seiler M, Budd A, Gibson TJ, Davey NE. Short linear motifs: ubiquitous and functionally diverse protein interaction modules directing cell regulation. Chem Rev. 2014;114:6733–6778. doi: 10.1021/cr400585q. [DOI] [PubMed] [Google Scholar]
- Shen C, Jo SY, Liao C, Hess JL, Nikolovska-Coleska Z. Targeting recruitment of disruptor of telomeric silencing 1-like (DOT1L): characterizing the interactions between DOT1L and mixed lineage leukemia (MLL) fusion proteins. J Biol Chem. 2013;288:30585–30596. doi: 10.1074/jbc.M113.457135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger DJ, Lefterova MI, Ying L, Stonestrom AJ, Schupp M, Zhuo D, Vakoc AL, Kim J-E, Chen J, Lazar MA, et al. DOT1L/KMT4 recruitment and H3K79 methylation are ubiquitously coupled with gene transcription in mammalian cells. Mol Cell Biol. 2008;28:2825–2839. doi: 10.1128/MCB.02076-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Jones M, Koseki H, Nakayama M, Muntean AG, Maillard I, Hess JL. CBX8, a Polycomb Group Protein, Is Essential for MLL-AF9-Induced Leukemogenesis. Cancer Cell. 2011;20:563–575. doi: 10.1016/j.ccr.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompa P, Davey NE, Gibson TJ, Babu MM. A Million Peptide Motifs for the Molecular Biologist. Mol Cell. 2014;55:161–169. doi: 10.1016/j.molcel.2014.05.032. [DOI] [PubMed] [Google Scholar]
- Wang QF, Wu G, Mi S, He F, Wu J, Dong J, Luo RT, Mattison R, Kaberlein JJ, Prabhakar S, et al. MLL fusion proteins preferentially regulate a subset of wild-type MLL target genes in the leukemic genome. Blood. 2011;117:6895–6905. doi: 10.1182/blood-2010-12-324699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A, Lin M, Naresh A, Kitabayashi I, Cleary ML. A higher-order complex containing AF4 and ENL family proteins with P-TEFb facilitates oncogenic and physiologic MLL-dependent transcription. Cancer Cell. 2010;17:198–212. doi: 10.1016/j.ccr.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Xia X, Reisenauer MR, Hemenway CS, Kone BC. Dot1a-AF9 complex mediates histone H3 Lys-79 hypermethylation and repression of ENaCalpha in an aldosterone-sensitive manner. J Biol Chem. 2006;281:18059–18068. doi: 10.1074/jbc.M601903200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.