Abstract
In the present study, we investigate the inhibitory effect of novel H2S donors, AP67 and AP72 on isolated bovine posterior ciliary arteries (PCAs) under conditions of tone induced by an adrenoceptor agonist. Furthermore, we examined the possible mechanisms underlying the AP67- and AP72-induced relaxations. Isolated bovine PCA were set up for measurement of isometric tension in organ baths containing oxygenated Krebs solution. The relaxant action of H2S donors was studied on phenylephrine-induced tone in the absence or presence of enzyme inhibitors for the following pathways: cyclooxygenase (COX); H2S; nitric oxide and the ATP-sensitive K+ (KATP) channel. The H2S donors, NaHS (1 nM - 10 μM), AP67 (1 nM - 10 μM) and AP72 (10 nM -1 μM) elicited a concentration-dependent relaxation of phenylephrine-induced tone in isolated bovine PCA. While the COX inhibitor, flurbiprofen (3 μM) blocked significantly (p < 0.05) the inhibitory response elicited by AP67, it had no effect on relaxations induced by NaHS and AP72. Both aminooxyacetic acid (30 μM) and propargylglycine (1 mM), enzyme inhibitors of H2S biosynthesis caused significant (p < 0.05) rightward shifts in the concentration-response curve to AP67 and AP72. Furthermore, the KATP channel antagonist, glibenclamide (300 μM) and the NO synthase inhibitor, L-NAME (100 μM) significantly attenuated (p < 0.05) the relaxation effect induced by AP67 and AP72 on PCA. We conclude that H2S donors can relax pre-contracted isolated bovine PCA, an effect dependent on endogenous production of H2S. The inhibitory action of only AP67 on pre-contracted PCA may involve the production of inhibitory endogenous prostanoids. Furthermore, the observed inhibitory action of H2S donors on PCA may depend on the endogenous biosynthesis of NO and by an action of KATP channels.
Keywords: Hydrogen sulfide, posterior ciliary artery, Ocular blood flow, cystathionine β-synthase, cystathionine γ-lyase, KATP channel, Nitric oxide
Introduction
For decades, hydrogen sulfide (H2S) was best known as toxic environmental pollutant until recent studies provided evidence that this gas can serve as a key regulator of several physiologic and pathophysiological processes in animals. H2S is endogenously produced in mammalian tissues from amino acids, L-cysteine and homocysteine in the presence of enzymes of the sulfur metabolic network i.e. cystathionine β-synthase (CBS), cystathionine λ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (3MST) along with cysteine aminotransferase (CAT). The biological roles of H2S as a gaseous transmitter are multiple and rapidly expanding in most organ systems including the central and peripheral nervous systems, and the cardiovascular, gastrointestinal and respiratory systems. H2S was recently described as a vasodilator and an endothelium-hyperpolarizing factor (EDHF) candidate produced in the vascular tissues, mesenteric arteries, hepatic arteries and aorta of various species like rat, mouse and rabbits (Kimura, 2010). This vasodilatory response can be due to a variety of mechanisms like activation of phospholipase A2, inhibition of phosphodiesterase, secretion of adipocyte-derived relaxing factor (that opens voltage-dependent Kþ (Kv) channels), activation of large-conductance Ca2+-activated K+ (BKCa) channels and activation of ATP-sensitive K+ (KATP) channels (Mustafa et al., 2009a; Mustafa et al., 2009b).
In the eye, studies from our laboratory showed that H2S can be endogenously produced in ocular tissues using H2S- releasing compounds and that this gas can induce relaxation of pre-contracted porcine irides, alter sympathetic and excitatory amino acid transmission and regulate signal transduction processes in mammalian retina (Chitnis et al., 2013; Kulkarni et al., 2009; Kulkarni et al., 2011; Monjok et al., 2008; Njie-Mbye et al., 2010; Njie-Mbye et al., 2012; Ohia et al., 2010; Opere et al., 2009). In a recent study, we found that both slow releasing- (GYY 4137) and fast-releasing (NaHS) H2S donors can relax the precontracted isolated long posterior ciliary artery (PCA) (Chitnis et al., 2013). Furthermore, we showed that the GYY4137-mediated relaxation of pre-contracted PCA was dependent upon the production of excitatory endogenous prostanoids and that this response was due, at least in part, on the endogenous biosynthesis of H2S by CBS and CSE and by an action of the release gas on KATP channels (Chitnis et al., 2013). There is evidence that H2S can interact with other gaseous molecules such as nitric oxide (NO) and carbon monoxide (CO) in exerting physiological and pharmacological actions in ocular tissues (Bucolo & Drago, 2011; Pong & Eldred, 2009; Salomone et al., 2014). In the rabbit ophthalmic artery, Salomone et al.(2014) reported that CO can synergize with NO to induce relaxations whereas H2S counteracted the actions of endogenous NO. Taken together, it appears that the pharmacological actions induced by H2S donors in the ocular vasculature may involve a cross talk between H2S, NO and CO.
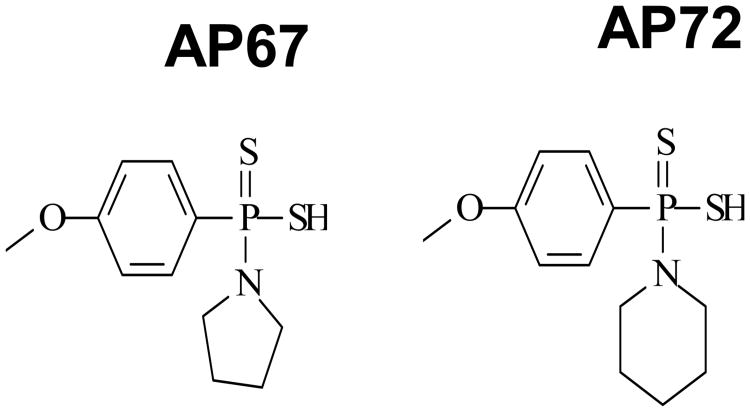
The overall objective of the present study was to investigate the pharmacological actions of other slow releasing H2S donors, AP67 {(4-methoxyphenyl)pyrrolidin-1-ylphosphinodithioc acid} and AP72 {(4-methoxyphenyl)piperidin-1-ylphosphinodithioc acid)} on isolated bovine long posterior ciliary arteries (PCAs). We also compared the pharmacological actions of AP67 and AP72 with that of the fast releasing H2S donor, NaHS. Furthermore, we studied the mechanism of action of AP67 and AP72 in eliciting relaxations of isolated pre-contracted bovine PCAs. The chemical structures of AP67 and AP72 are illustrated in Figure 1. Parts of the work presented in this paper has been communicated in an abstract form (,Ohia et al., 2014).
Figure 1.
Chemical Structure of AP67 {(4-methoxyphenyl)pyrrolidin-1-ylphosphinodithioc acid} and AP72 {(4-methoxyphenyl)piperidin-1-ylphosphinodithioc acid)}
Methods
Chemicals
AP67 and AP72 were kind gifts from Dr. Whiteman's laboratory. Propargylglycine (PAG), aminooxyacetic acid (AOAA), NaHS, S-adenosyl-L-methionine (SAM), glibenclamide and L-nitroarginine methyl ester (L-NAME) were all purchased from Sigma Aldrich, St. Louis, MO. Flurbiprofen was procured from Cayman Chemicals, Ann Arbor, MI. All test agents were freshly prepared immediately before use on the day of the study. Stock solution of NaHS, AP67 and AP72 were prepared in distilled water whilst all other compounds were prepared in 70% ethanol. Vehicle controls were prepared using 0.1% saline.
Tissue preparation
Bovine eyes were obtained from Vision Tech, Dallas, TX within 24h following enucleation and transported to the laboratory (shipped on ice). The medial or lateral long posterior ciliary arteries were dissected free from the optic nerve at a distance of 2 cm from the posterior aspect of the globe of each eye. The fatty and connective tissues were removed and the vessel with attached endothelium was cut into strips of 2 mm length and placed in oxygenated Krebs solution maintained at 37 °C as described by Dalske, 1974).
Measurement of isometric tension
Posterior ciliary artery rings (2 mm long) were prepared for muscle contraction/relaxation studies using a 25 ml organ bath containing oxygenated Krebs solution (pH 7.4) at 37 °C. Two vessel rings were prepared from each eyeball. In brief, vascular rings were mounted on two tungsten wires (with one wire immobilized and the other connected to a Grass FT03 transducer) for measurement of isometric tension using a Grass Polyview Software. The Krebs solution had the following composition (mM): potassium chloride, 4.8; sodium chloride, 118; calcium chloride, 2.5; potassium dihydrogen phosphate, 1.2; sodium bicarbonate, 25; magnesium sulfate, 2.0; and dextrose, 10. Each ciliary artery ring preparation was mechanically stretched to achieve a basal tension of 5.0 g and then allowed to equilibrate for 45 minutes before the start of the experiment. Ciliary artery rings were pre-contracted with sub-maximal concentrations of the adrenoceptor receptor agonist, phenylephrine to induce tone, and then exposed to different concentration of H2S-donors for 2-5 minutes or until the development of a stable tone. When used, enzyme inhibitors, activator or ion channel blockers were incubated with the vascular segments for 30 minutes before exposure to H2S-donors.
The relaxant actions of NaHS, AP67 and AP72 on phenylephrine-induced tone were studied in the absence or presence of an inhibitor of cyclooxygenase (flurbiprofen (3 μM). Furthermore, effects of AP67 and AP72 were studied in the absence or presence of inhibitors and activators of enzymes for the biosynthetic pathways of H2S, and nitric oxide production and ion channels.: inhibitors of cystathionine γ-lyase, PAG (1 mM) and cystathionine β-synthase, AOAA (30 μM); an activator of cystathionine (3-synthase, SAM (100 μM); an inhibitor of nitric oxide synthase, L-NAME (100 μM) and the inhibitor of KATP channel, glibenclamide (300 μM). The concentrations of PAG, AOAA, SAM, L-NAME and glibenclamide employed in these assays are based on published reports in literature (Chitnis et al., 2013; Dawe et al., 2007; Ghasemi et al., 2012; Monjok et al., 2008; Teague et al., 2002).
Data analysis
The response of each tissue is expressed as percentage of relaxation of the phenylephrine-induced tone. Cumulative concentration-response curves were constructed, analyzed and IC50 values (i.e., agonist concentrations that produced 50% inhibition of the observed maximum response) were determined using Graph Pad Prism 5.0 software (San Diego, CA). 0% relaxation depicts conditions whereby agonists failed to cause relaxation of the phenylephrine-induced tone. Comparision of controls and treatment groups (with inhibitor/activator agents) were performed on the same sets of tissues from different eyeballs. Values given are arithmetic means ± S.E.M. Significance of differences between control and test values were evaluated using one-way analysis of variance (ANOVA) followed by Dunnett's test (Graph Pad Prism software San Diego, CA). P values <0.05 were accepted as statistically significant.
Results
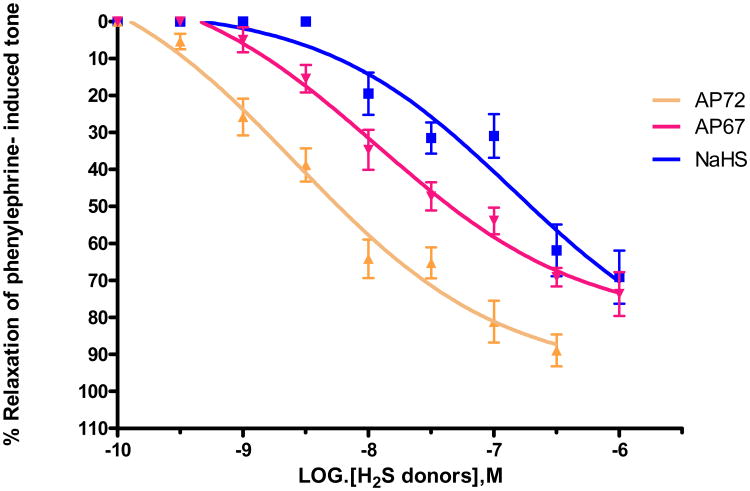
In the present study, we studied the inhibitory effects of slow-releasing H2S donors, AP67 and AP72 in the presence of tone induced by submaximal concentrations of the adrenoceptor agonist, phenylephrine. A submaximal concentration of phenylephrine was established in each preparation and it generally corresponded to doses that elicited 60-80% of the maximum contractile response. We also compared the inhibitory actions of the slow-releasing H2S donors with that of its fast-releasing counterpart, NaHS in bovine PCAs. NaHS (1 nM - 10 μM), AP67 (1 nM - 10 μM) and AP72 (10 nM - 1 μM) produced a concentration-dependant relaxation of phenylephrine-induced tone with IC50 values of 0.16 ± 0.02 μM (n = 6), 0.08 ± 0.04 μM (n = 8) and 4.3 ± 0.9 nM (n = 8), respectively (Figure 2).
Figure 2.
Concentration-dependent relaxation of phenylephrine-induced tone in isolated bovine ciliary artery by H2S donors AP67, AP72 and NaHS. Vertical bars represent means ± S.E.M.; n= 6-36.
Effect of cyclooxygenase inhibition
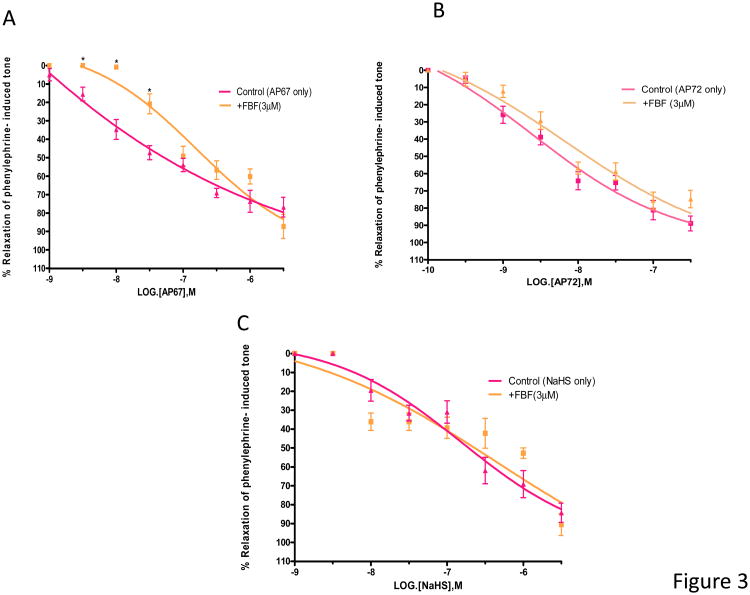
We next examined the role of the COX inhibitor, flurbiprofen on relaxation induced by NaHS, AP67 and AP72. On its own, flurbiprofen (3 μM) had no significant action on the tone induced by phenylephrine. In the presence of flurbiprofen (3 μM), concentration-response curves to AP67 was shifted to the right and IC50 values were increased significantly (p < 0.05) from 0.08 ± 0.04 μM (n = 8) to 200 ± 64 nM (n = 6) (Figure 3A). In contrast, flurbiprofen (3 μM) had no significant effect (p > 0.05) on relaxations induced by AP72 and NaHS (Figures 3B, 3C).
Figure 3.
Concentration-dependant relaxation of phenylephrine-induced tone in isolated bovine ciliary artery by H2S donors (A) AP67, (B) AP72 and (C) NaHS: control, and in the presence of flurbiprofen (FBF, 3 μM). FBF blocked relaxations induced by lower concentrations of AP67 but not those elicited by AP72 and NaHS. Vertical bars represent means ± S.E.M.; n=6--36. *p<0.05, significantly different from control.
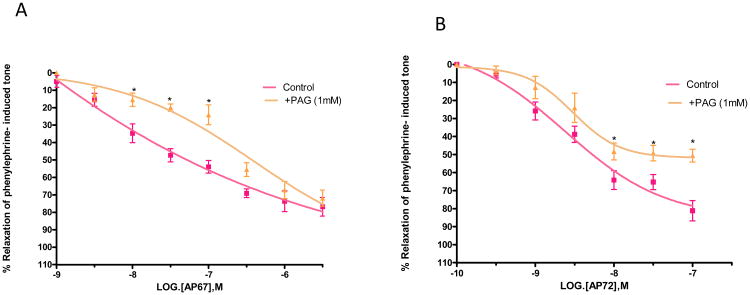
Role of cystathionine β-synthase and cystathionine γ-lyase
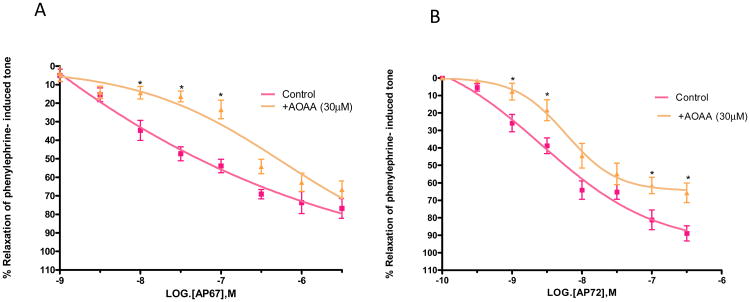
To determine the role of biosynthetic enzymes of H2S pathway in the pharmacological responses elicited by AP67 and AP72, we studied the relaxations produced by the slow-releasing gas donors in the absence and presence of enzyme inhibitors, aminooxyacetic acid (AOAA) and proparglyglycine (PAG). Both AOAA (30 μM) and PAG (1 mM) had no significant effect on tone induced by phenylephrine. As shown in Figures 4A and 4B, an inhibitor of cystathionine β-synthase, AOAA (30 μM) caused significant (p < 0.05) rightward shifts in the concentration-response curve to AP67 and AP72. IC50 values were increased from 0.08 ± 0.04 μM (n = 8) to 0.6 ± 0.012 μM (n = 5) for AP67 and from 4.3 ± 0.9 nM (n=8) to 20 ± 2 nM (n=6) for AP72 (Figures 4A and 4B). Likewise, an inhibitor of cystathionine γ-lyase, PAG (1 mM) blocked relaxations induced by AP67 and AP72 as illustrated by significant rightward shifts in the concentration-response curves to these compounds (p < 0.05) (Figures 5A and 5B). Furthermore, the maximal degree of relaxation induced by AP72 was reduced significantly (p < 0.05) from 80% ± 5.6% to 50% ± 3.6% (n = 8) at a concentration of 100 nM (Figure 5B). We next studied the effect of an activator of cystathionine p-synthase, S-adenosylmethionine (SAM, 100 μM), on AP67 and AP72- induced relaxation. On its own, SAM (100 μM) had no significant action on tone induced by phenylephrine. In concentrations up to 100 μM, SAM had no significant effect on relaxations induced by AP67 and AP72. [For AP67: IC50 in the absence of SAM: 0.08 ± 0.04 μM (n = 8) and IC50 in the presence of SAM: 0.1± 0.05 μM (n= 6); For AP72: IC50 in the absence of SAM: 4.3 ± 0.9nM (n= 8) and IC50 in the presence of SAM: 3 ± 1.5nM (n= 6)].
Figure 4.
Effect of aminooxyacetic acid (AOAA) on (A) AP67 and (B) AP72-induced relaxation of phenylephrine tone in isolated bovine posterior ciliary artery: control, and in the presence of AOAA (30 μM). AOAA blocked relaxations induced by both AP67 and AP72. Vertical bars represent means ± S.E.M.; n=5-36. *p<0.05, significantly different from control.
Figure 5.
Effect of propargylglycine (PAG) on (A) AP67 and (B) AP72-induced relaxation of phenylephrine tone in isolated bovine posterior ciliary artery: control, and in the presence of PAG (1 mM). PAG blocked relaxations induced by both AP67 and AP72. Vertical bars represent means ± S.E.M.; n=8-36. *p<0.05, significantly different from control.
Role of ATP-sensitive K+(KATP) channel and nitric oxide
There is evidence that KATP channel may be involved in vasorelaxant effect of H2S in several mammalian tissues (Zhao et al., 2001), and that H2S may induce vasorelaxation in synergy with nitric oxide (NO) (Zhao & Wang, 2002). We investigated the effect of L-NAME, a nitric oxide synthase inhibitor and glibenclamide, a KATP channel blocker on relaxations induced by AP67 and AP72 in isolated bovine PCAs. Both L-NAME (100 μM) and glibenclamide (300 μM) had no significant effect on the tone induced by phenylephrine.
L-NAME (100 uM) significantly attenuated (p < 0.05) the relaxations induced by AP67 and AP72 on bovine PCAs and increased IC50 values from of 0.08 ± 0.04 μM (n = 8) to 5.4 ± 2.0 μM (n=5) for AP67 and from 4.3 ± 0.91 nM (n=8) to 90 ± 40 nM (n=8) for AP72 (Figures 6A and 6B). Furthermore, the maximal degree of relaxation induced by AP67 was reduced significantly (p < 0.05) from 76 ± 5% to 45 ± 5% (n = 8) at a concentration of 1 μM (Figure 6A).
Figure 6.
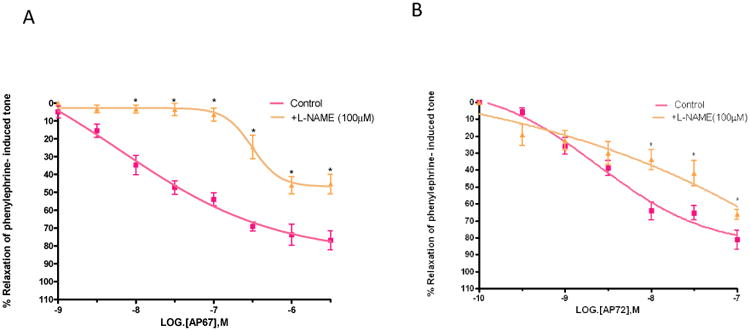
Effect of L-NAME on (A) AP67 and (B) AP72-induced relaxation of phenylephrine tone in isolated bovine posterior ciliary artery: control, and in the presence of L-NAME (100 μM). L-NAME blocked relaxations induced by both AP67 and AP72. Vertical bars represent means ± S.E.M.; n=5-36. *p<0.05, significantly different from control.
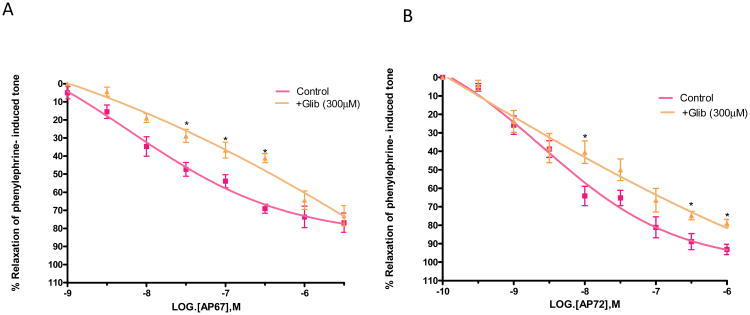
As shown in Figures 7A and 7B, glibenclamide (300 uM) significantly (p < 0.05) blocked relaxations of phenylephrine-induced tone elicited by AP67 and AP72 and increased IC50 values from 0.08 ± 0.04 μM (n = 8) to 0.9± 0.07 μM (n=8) for AP67 and from 4.3 ± 0.9 nM (n = 8) to 0.6 ± 0.2 μM (n = 12) for AP72.
Figure 7.
Effect of glibenclamide (GLIB) on (A) AP67 and (B) AP72-induced relaxation of phenylephrine tone in isolated bovine posterior ciliary artery: control, and in the presence of GLIB (300 μM). GLIB blocked relaxations induced by both AP67 and AP72. Vertical bars represent means ± S.E.M.; n=6-36. *p<0.05, significantly different from control.
Discussion
For several decades, hydrogen sulfide (H2S) gas was considered as a toxic substance until recent reports from several laboratories provided evidence for its beneficial biological role in several mammalian tissues and organs. Indeed, H2S is now recognized as a potential gaseous transmitter (with gases such as nitric oxide and carbon monoxide) which plays an important role in normal physiological processes at low concentrations (Qu et al., 2008). In its physiological role, H2S is produced in astrocytes, neurons and microglia, and it can act as an endogenous anti-inflammatory and neuroprotective agent (Li et al., 2009). In the eye, H2S is endogenously produced in ocular tissues and there is evidence supporting its pharmacological actions on neurotransmitter release from anterior uveal tissues and the retina (Kulkarni et al., 2009; Monjok et al., 2008; Njie-Mbye et al., 2010; Njie-Mbye et al., 2012; Ohia et al., 2010; Opere et al., 2009) and in protecting the retinal against insults (neuroprotection) (Osborne et al., 2010; Perrino et al., 2009). Perrino et al. (2009) showed that ACS 67, a H2S-releasing derivative of latanoprost caused a higher decrease in IOP than the parent compound in two glaucomatous rabbit models suggesting a role for H2S in the regulation of IOP. We have evidence that GYY4137, a novel slow-releasing H2S donor can relax pre-contracted isolated bovine PCAs (Chitnis et al., 2013). The PCA is a very important part of ocular blood flow and changes in vascular reactivity in these vessels can result in a variety of ocular disorders leading to vision loss.
Most studies in biomedical literature have used NaHS or Na2S (sulfide salts) as “tools” to model the biological effects of endogenous H2S gas. For instance, NaHS releases large amounts of H2S in a short duration of time. However, the release of endogenous H2S from cells may occur in lesser amounts and at a much slower rate than that from sulfide salts. Consequently, the release of H2S from sulfide salts may not mimic the biological effects of endogenously produced H2S. Recently, the availability of compounds that can release H2S slowly (e.g. AP67 and AP72) in biological systems have led to further studies of the pharmacology of these new class of gas donors (Li et al., 2009; Li et al., 2008). In the present study, we investigated the pharmacological actions of novel slow-releasing H2S donors, AP67 and AP72 on pre-contracted isolated bovine PCAs. We also compared the relaxations induced by AP67 and AP72 with that of the fast-releasing H2S donor, NaHS.
We found that H2S donors, NaHS, AP67 and AP72 produced a concentration-dependent relaxation of phenylephrine-induced tone in PCA with the following rank order of potency: AP72 > AP67 > NaHS. The slow-releasing H2S donors, AP67 and AP72 were more potent than NaHS in relaxing the pre-contracted ciliary arteries, presumably because the ciliary arteries were exposed to H2S over a longer period of time due to the sustained-release nature of these compounds. Other investigators have reported a similar high potency of a slow-releasing H2S donor, GYY4137 in comparison to NaHS, on relaxing pre-contracted rat aortic rings and in evaluating their antitumor activity in human cancer cell lines (Li et al., 2009; Li et al., 2008). It is pertinent to note that high concentrations of GYY4137 (1 mM) has been reported by Salomone et al., 2014) to potentiate phenylephrine-induced tone in rabbit ophthalmic arteries. However, it remains to be determined whether effects elicited by such higher concentrations of GYY4137 could be related to its pharmacological actions as a H2S donor. Be that is it may, the higher potency of slow-releasing H2S donors in relaxing pre-contracted blood vessels affirm their important roles as tools in studying the biology of this gas in experimental animals and humans.
To elucidate the mechanism of the relaxant action of H2S donors, we considered the possible involvement of prostanoids in this response. In blood vessels, it has been postulated that the vasodilatory effects of H2S may involve other second messengers such as nitric oxide, prostanoids, bradykinin (Bhatia, 2005; Kiss et al., 2008; Wang et al., 2008). It is well established that prostanoids play an important role in the relaxation of vascular smooth muscles to H2S donors (Koenitzer et al., 2007). Indeed, prostanoids have been shown to be involved in the relaxation of ciliary arteries (Abe et al., 2013). In the present study, we examined the possibility that intramurally generated prostanoids may contribute to our observed relaxant effects of AP67, AP72 and NaHS on precontracted PCA. Flurbiprofen shifted the inhibitory concentration-response curves of AP67 to the right suggesting that endogenous production of excitatory prostanoids may be involved in its responses. It is pertinent to note that only responses to low concentrations of AP67 were blocked by flurbiprofen. Conversely, we found that flurbiprofen had no effect on relaxations induced by NaHS or AP72 in PCA. While there is evidence that NaHS can stimulate prostanoids release in mouse hind paws (di Villa et al., 2010), it is unclear why flurbiprofen had no effect on its response in the PCA. Furthermore, the differential actions of flurbiprofen on the inhibitory response induced by AP67 and AP72 were unexpected. In a previous study, we showed evidence that prostanoids are involved in the relaxations caused by H2S donors and its substrate (L-cysteine) in the isolated iris-ciliary body (Chitnis et al., 2013; Monjok et al., 2008; Ohia et al., 2010). It may well be that the observed differences in actions of AP67 and AP72 to prostanoids could be due to additional non-specific action of AP67 on PCA and/or to an enhanced ability of AP67 to generate H2S in physiological buffers when compared to AP72 (Whiteman et al., unpublished observations). Taken together, it appears that tissue differences may exist in the ability of H2S donors to interact with the pathways leading to the production of prostanoids.
The availability of inhibitors of enzymes of the biosynthetic pathways for H2S has enabled studies to be performed on the involvement of this gas in responses produced by its donors (Kimura et al., 2005; Lowicka & Beltowski, 2007). In the current study, both the inhibitors of CBS and CSE blocked relaxations caused by AP67 and AP72 thus indicating that the production of endogenous H2S which may contribute, at least in part, to the responses elicited by these compounds in bovine PCA. Other investigators have reported that inhibitors of CBS and CSE can attenuate responses produced by NaHS on vascular and non-vascular smooth muscles (Dawe et al., 2008; Srilatha et al., 2007; Teague et al., 2002; Webb et al., 2008). For instance, at concentrations of 1 mM, the CSE inhibitor, PAG, inhibited not only the vasodilatory effects of NaHS but it also blocked the in situ production of H2S (Srilatha et al., 2007; Teague et al., 2002; Webb et al., 2008). Taken together, our results demonstrate that the inhibitory actions of AP67 and AP72 in PCA are dependent upon the endogenous biosynthesis of H2S.
Various pharmacological studies have demonstrated that activators of CBS such as SAM can lead to an increase in H2S production in several mammalian tissues (Abe & Kimura, 1996; Jensen et al., 2011; Kulkarni et al., 2011). In addition, evidence from our laboratory demonstrates that SAM can enhance relaxations caused by NaHS in isolated porcine irides (Monjok et al., 2008). In the present study, we examined the effect of SAM on AP67- and AP72-induced relaxations of the phenylephrine tone. We observed that SAM had no effect on relaxations induced by both AP67 and AP72. In a recent study, we also found that SAM did not alter the relaxation elicited by another H2S donor, GYY4137 in bovine PCA. The lack of an effect of SAM on relaxations induced by these H2S donors suggests that species and tissue differences may exist in the response of PCAs to AP67 and AP72.
There is evidence that H2S promotes vasorelaxation by directly opening ATP-sensitive K+ (KATP) channels or voltage dependent K+ channels (Cheng et al., 2004; Zhao et al., 2001). In addition, studies from our laboratory have shown that glibenclamide, a specific KATP channel antagonist, attenuated relaxations induced by H2S donors (NAHS and GYY4137) and the H2S substrate (L-cysteine) on ocular smooth muscles (Chitnis et al., 2013; Ohia et al., 2010). In the present study, we investigated the effect of glibenclamide on relaxations induced by AP67 and AP72. We found that glibenclamide blocked relaxations of phenylephrine-induced tone elicited by AP67 and AP72. A similar glibenclamide-induced antagonism of relaxations induced by H2S donors (GYY4137 and NaHS) has been reported in other vascular beds (Chen et al., 2005; Ghasemi et al., 2012; Li et al., 2008; Tang et al., 2005; Webb et al., 2008). Since data from the present study demonstrates a role for NO in the relaxations induced by AP67 and AP72, it is important to highlight the observation by Murphy & Brayden (1995) in which they reported that NO caused hyperpolarization of rabbit mesenteric arteries by activating KATP channels. It is, therefore, tempting to speculate that the effect of AP67 and AP72 on KATP channels may be due to a direct action on these channels and/or an indirect effect on the channels through the release of NO. In contrast, other investigators have found that the relaxations caused by H2S in the guinea-pig ileum and trout urinary bladder were not mediated by KATP channels (Dombkowski et al., 2006;,Teague et al., 2002). The observed sensitivity of relaxations induced by AP67 and AP72 to glibenclamide indicates that KATP channels partially mediate these responses in the isolated bovine PCA. Taken together, it appears that species and tissue differences may exist in the ability of H2S to interact with KATP channels.
It is well known that H2S and NO are vasorelaxant factors that possess the ability to ‘cross talk’ through formation of a nitrosothiol moiety (Whiteman et al., 2006). However, there are conflicting reports in biomedical literature pertaining to the pharmacological outcomes of this interaction. For instance, some studies on the thoracic aorta of rats report that H2S can enhance the vasorelaxant effect of NO (Hosoki et al., 1997), whilst others report that H2S reduced NO induced vasorelaxation (Ali et al., 2006; Zhao & Wang, 2002). In the present study, we examined the effect of L-NAME, a NO synthase inhibitor, on relaxations induced by AP67 and AP72. L-NAME shifted the concentration response curves of AP67 and AP72 to the right suggesting that endogenous NO may be involved in its responses. In support of this observation, other investigators have shown that H2S-induced vascular response was reduced by NO inhibitors in rat aorta, ileum, liver and vascular tissues (Ali et al., 2006; Chen et al., 2005; Hosoki et al., 1997; Whiteman et al., 2006; Zhao et al., 2003; Zhao et al., 2001). Interestingly, previous studies from our laboratory have reported that, L-NAME did not alter the relaxation effects of NaHS and L-cysteine in isolated porcine irides (Ohia et al.,2010; Monjok et al., 2008; Chitnis et al., 2013). In the salamander retina, Pong & Eldred (2009) found that NaHS reduced NO-induced cGMP-like immunoreactivity, a response that was not significantly affected by the addition of CuCl2 indicating that the decrease caused by the H2S donor was not due to the sequestering of H2S and NO to form a novel nitrosothiol. In the rabbit ophthalmic artery,Salomone et al. (2014) found that the ability of a high concentration of GYY4137 to potentiate phenylephrine-induced contractions was unaffected by the presence of NG-nitro-L-arginine (LNNA). Furthermore, relaxations of residual tone in the rabbit ophthalmic artery by acetylcholine and sodium nitroprusside (in the presence of LNNA) was not blocked by GYY4137 (1 mM). Based on these observations,Salomone et al. (2014) suggested that GYY4137 interfered with basal release of NO, an action that occurred upstream of guanylyl cyclase. It is important to note that at a lower concentration of GYY4137 (100 μM) had no direct effect on cGMP accumulation in the rat isolated aorta (Li et al., 2008). Be that as it may, observations made in the present study about an interaction of AP67 and AP72 with the NO pathway indicates that NO accounts at least, in part, for the pharmacological actions of these donors in the isolated bovine PCA.
We conclude that H2S donors NaHS, AP67 and AP72 can relax pre-contracted isolated bovine ciliary artery, an effect dependent on endogenous production of H2S. The inhibitory action of AP67 (but not that of AP72 and NaHS) on pre-contracted PCA involves the production of inhibitory endogenous prostanoids. Furthermore, the observed inhibitory action of AP67 and AP72 on ciliary arteries is dependent, at least in part, on the endogenous biosynthesis of NO and by an action of KATP channels.
Highlights.
H2S donors can relax pre-contracted isolated bovine posterior ciliary arteries
Prostanoids are involved in the inhibitory action of AP67 on pre-contracted isolated bovine ciliary arteries.
The observed vascular relaxation of H2S donors in isolated bovine posterior ciliary artery is dependent on endogenous biosynthesis of H2S by cystathionine γ-lyase and cystathionine β-synthase.
The inhibitory action of H2S donors on posterior ciliary arteries is dependent on the endogenous biosynthesis of NO and by an action of KATP channels
Acknowledgments
This work was supported in part by National Institutes of Health/National Eye Institute Grant R15EY022215-01.
Abbreviations
- H2S
Hydrogen sulfide
- CBS
Cystathionine β-synthase
- CSE
Cystathionine γ -lyase
- PAG
Propargylglycine
- AOAA
Aminooxyacetic acid
- SAM
S-adenosyl-L-methionine
- L-NAME
L-nitroarginine methyl ester
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe S, Watabe H, Takaseki S, Aihara M, Yoshitomi T. The effects of prostaglandin analogues on intracellular Ca2+ in ciliary arteries of wild-type and prostanoid receptor-deficient mice. J Ocul Pharmacol Ther. 2013;29:55–60. doi: 10.1089/jop.2011.0197. [DOI] [PubMed] [Google Scholar]
- Ali MY, Ping CY, Mok YY, Ling L, Whiteman M, Bhatia M, Moore PK. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br J Pharmacol. 2006;149:625–634. doi: 10.1038/sj.bjp.0706906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M. Hydrogen sulfide as a vasodilator. IUBMB Life. 2005;57:603–606. doi: 10.1080/15216540500217875. [DOI] [PubMed] [Google Scholar]
- Bucolo C, Drago F. Carbon monoxide and the eye: Implications for glaucoma therapy. Pharmacol Ther. 2011;130:191–201. doi: 10.1016/j.pharmthera.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Chen YH, Yao WZ, Geng B, Ding YL, Lu M, Zhao MW, Tang CS. Endogenous hydrogen sulfide in patients with chronic obstructive pulmonary disease. Zhonghua Jie He He Hu Xi Za Zhi. 2005;28:694–697. [PubMed] [Google Scholar]
- Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287:H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- Chitnis MK, Njie-Mbye YF, Opere CA, Wood ME, Whiteman M, Ohia SE. Pharmacological actions of the slow release hydrogen sulfide donor GYY4137 on phenylephrine-induced tone in isolated bovine ciliary artery. Exp Eye Res. 2013;116:350–354. doi: 10.1016/j.exer.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Dalske HF. Pharmacological reactivity of isolated ciliary arteries. Invest Ophthalmol. 1974;13:389–392. [PubMed] [Google Scholar]
- Dawe GS, Han SP, Bian JS, Moore PK. Hydrogen sulphide in the hypothalamus causes an ATP-sensitive K+ channel-dependent decrease in blood pressure in freely moving rats. Neuroscience. 2008;152:169–177. doi: 10.1016/j.neuroscience.2007.12.008. [DOI] [PubMed] [Google Scholar]
- di Villa BR, Coletta C, Mitidieri E, De DG, Rossi A, Sautebin L, Cirino G, Bucci M, Sorrentino R. Hydrogen sulphide induces mouse paw oedema through activation of phospholipase A2. Br J Pharmacol. 2010;161:1835–1842. doi: 10.1111/j.1476-5381.2010.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombkowski RA, Doellman MM, Head SK, Olson KR. Hydrogen sulfide mediates hypoxia-induced relaxation of trout urinary bladder smooth muscle. J Exp Biol. 2006;209:3234–3240. doi: 10.1242/jeb.02376. [DOI] [PubMed] [Google Scholar]
- Ghasemi M, Dehpour AR, Moore KP, Mani AR. Role of endogenous hydrogen sulfide in neurogenic relaxation of rat corpus cavernosum. Biochem Pharmacol. 2012;83:1261–1268. doi: 10.1016/j.bcp.2012.01.026. [DOI] [PubMed] [Google Scholar]
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- Jensen KK, Geoghagen NS, Jin L, Holt TG, Luo Q, Malkowitz L, Ni W, Quan S, Waters MG, Zhang A, Zhou HH, Cheng K, Luo MJ. Pharmacological activation and genetic manipulation of cystathionine beta-synthase alter circulating levels of homocysteine and hydrogen sulfide in mice. Eur J Pharmacol. 2011;650:86–93. doi: 10.1016/j.ejphar.2010.09.080. [DOI] [PubMed] [Google Scholar]
- Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal. 2010;12:1111–1123. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- Kimura H, Nagai Y, Umemura K, Kimura Y. Physiological roles of hydrogen sulfide: synaptic modulation, neuroprotection, and smooth muscle relaxation. Antioxid Redox Signal. 2005;7:795–803. doi: 10.1089/ars.2005.7.795. [DOI] [PubMed] [Google Scholar]
- Kiss L, Deitch EA, Szabo C. Hydrogen sulfide decreases adenosine triphosphate levels in aortic rings and leads to vasorelaxation via metabolic inhibition. Life Sci. 2008;83:589–594. doi: 10.1016/j.lfs.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenitzer JR, Isbell TS, Patel HD, Benavides GA, Dickinson DA, Patel RP, Darley-Usmar VM, Lancaster JR, Jr, Doeller JE, Kraus DW. Hydrogen sulfide mediates vasoactivity in an O2-dependent manner. Am J Physiol Heart Circ Physiol. 2007;292:H1953–H1960. doi: 10.1152/ajpheart.01193.2006. [DOI] [PubMed] [Google Scholar]
- Kulkarni KH, Monjok EM, Zeyssig R, Kouamou G, Bongmba ON, Opere CA, Njie YF, Ohia SE. Effect of hydrogen sulfide on sympathetic neurotransmission and catecholamine levels in isolated porcine iris-ciliary body. Neurochem Res. 2009;34:400–406. doi: 10.1007/s11064-008-9793-7. [DOI] [PubMed] [Google Scholar]
- Kulkarni M, Njie-Mbye YF, Okpobiri I, Zhao M, Opere CA, Ohia SE. Endogenous production of hydrogen sulfide in isolated bovine eye. Neurochem Res. 2011;36:1540–1545. doi: 10.1007/s11064-011-0482-6. [DOI] [PubMed] [Google Scholar]
- Li L, Salto-Tellez M, Tan CH, Whiteman M, Moore PK. GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free Radic Biol Med. 2009;47:103–113. doi: 10.1016/j.freeradbiomed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- Lowicka E, Beltowski J. Hydrogen sulfide (H2S) - the third gas of interest for pharmacologists. Pharmacol Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- Monjok EM, Kulkarni KH, Kouamou G, McKoy M, Opere CA, Bongmba ON, Njie YF, Ohia SE. Inhibitory action of hydrogen sulfide on muscarinic receptor-induced contraction of isolated porcine irides. Exp Eye Res. 2008;87:612–616. doi: 10.1016/j.exer.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Murphy ME, Brayden JE. Nitric oxide hyperpolarizes rabbit mesenteric arteries via ATP-sensitive potassium channels. J Physiol. 1995;486(Pt 1):47–58. doi: 10.1113/jphysiol.1995.sp020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal. 2009a;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, Gadalla MM, Snyder SH. Signaling by gasotransmitters. Sci Signal. 2009b;2:re2. doi: 10.1126/scisignal.268re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njie-Mbye YF, Bongmba OY, Onyema CC, Chitnis A, Kulkarni M, Opere CA, Leday AM, Ohia SE. Effect of hydrogen sulfide on cyclic AMP production in isolated bovine and porcine neural retinae. Neurochem Res. 2010;35:487–494. doi: 10.1007/s11064-009-0085-7. [DOI] [PubMed] [Google Scholar]
- Njie-Mbye YF, Kulkarni M, Opere CA, Ohia SE. Mechanism of action of hydrogen sulfide on cyclic AMP formation in rat retinal pigment epithelial cells. Exp Eye Res. 2012;98:16–22. doi: 10.1016/j.exer.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Ohia SE, Opere CA, Monjok EM, Kouamou G, Leday AM, Njie-Mbye YF. Role of hydrogen sulfide production in inhibitory action of L-cysteine on isolated porcine irides. Curr Eye Res. 2010;35:402–407. doi: 10.3109/02713680903576716. [DOI] [PubMed] [Google Scholar]
- Ohia SE, Kulkarni-Chitnis M, Njie-Mbye YF, Robinson J, Mitchell L, Opere CA, Whiteman M. Effect of Novel Hydrogen Sulfide Donors (AP67 and AP72) on Isolated Bovine Ciliary Artery. ARVO Meeting Abstracts. 2014;55:4348. [Google Scholar]
- Opere CA, Monjok EM, Kulkarni KH, Njie YF, Ohia SE. Regulation of [3H] D-aspartate release from mammalian isolated retinae by hydrogen sulfide. Neurochem Res. 2009;34:1962–1968. doi: 10.1007/s11064-009-9984-x. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Ji D, Abdul Majid AS, Fawcett RJ, Sparatore A, Del SP. ACS67, a hydrogen sulfide-releasing derivative of latanoprost acid, attenuates retinal ischemia and oxidative stress to RGC-5 cells in culture. Invest Ophthalmol Vis Sci. 2010;51:284–294. doi: 10.1167/iovs.09-3999. [DOI] [PubMed] [Google Scholar]
- Perrino E, Uliva C, Lanzi C, Soldato PD, Masini E, Sparatore A. New prostaglandin derivative for glaucoma treatment. Bioorg Med Chem Lett. 2009;19:1639–1642. doi: 10.1016/j.bmcl.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Pong WW, Eldred WD. Interactions of the gaseous neuromodulators nitric oxide, carbon monoxide, and hydrogen sulfide in the salamander retina. J Neurosci Res. 2009;87:2356–2364. doi: 10.1002/jnr.22042. [DOI] [PubMed] [Google Scholar]
- Qu K, Lee SW, Bian JS, Low CM, Wong PT. Hydrogen sulfide: neurochemistry and neurobiology. Neurochem Int. 2008;52:155–165. doi: 10.1016/j.neuint.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Salomone S, Foresti R, Villari A, Giurdanella G, Drago F, Bucolo C. Regulation of vascular tone in rabbit ophthalmic artery: cross talk of endogenous and exogenous gas mediators. Biochem Pharmacol. 2014;92:661–668. doi: 10.1016/j.bcp.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Srilatha B, Adaikan PG, Li L, Moore PK. Hydrogen sulphide: a novel endogenous gasotransmitter facilitates erectile function. J Sex Med. 2007;4:1304–1311. doi: 10.1111/j.1743-6109.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- Tang G, Wu L, Liang W, Wang R. Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol Pharmacol. 2005;68:1757–1764. doi: 10.1124/mol.105.017467. [DOI] [PubMed] [Google Scholar]
- Teague B, Asiedu S, Moore PK. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br J Pharmacol. 2002;137:139–145. doi: 10.1038/sj.bjp.0704858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Mainali P, Tang CS, Shi L, Zhang CY, Yan H, Liu XQ, DU JB. Effects of nitric oxide and hydrogen sulfide on the relaxation of pulmonary arteries in rats. Chin Med J (Engl) 2008;121:420–423. [PubMed] [Google Scholar]
- Webb GD, Lim LH, Oh VM, Yeo SB, Cheong YP, Ali MY, El OR, Lee CN, Wong PS, Caleb MG, Salto-Tellez M, Bhatia M, Chan ES, Taylor EA, Moore PK. Contractile and vasorelaxant effects of hydrogen sulfide and its biosynthesis in the human internal mammary artery. J Pharmacol Exp Ther. 2008;324:876–882. doi: 10.1124/jpet.107.133538. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Li L, Kostetski I, Chu SH, Siau JL, Bhatia M, Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem Biophys Res Commun. 2006;343:303–310. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- Zhao W, Ndisang JF, Wang R. Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol. 2003;81:848–853. doi: 10.1139/y03-077. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wang R. H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol. 2002;283:H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]