Summary
The importance of vitamin A for host defense is undeniable and the study of its mechanisms is paramount. Of the estimated 250 million preschool children who are vitamin A deficient (VAD), 10% will die from their increased susceptibility to infectious disease. Vitamin A supplementation was established in the 1980s as one of the most successful interventions in the developing world. Understanding how Vitamin A controls immunity will curb the mortality and morbidity associated with VAD and exploit the immune enhancing capacity of vitamin A to heighten host resistance to infectious disease. The discoveries that retinoic acid (RA) imprints the homing of leukocytes to the gut and enhanced the induction of regulatory T-cells highlighted a potential role for RA in mucosal tolerance. However, emerging data tells of a more profound systemic impact of RA on leukocyte function and commitment. In animal models using genetic manipulation of RA signaling, we learn when and how RA controls T-cell fate. Here we review the role for RA as a critical checkpoint regulator in the differentiation of CD4+ T-cells within the immune system.
Keywords: retinoic acid, CD4 T-cells, innate lymphoid cells, T cell polarisation, plasticity, gut immune system, dendritic cells, vitamin A metabolism
Introduction
Vitamin A through its active derivative retinoic acid (RA), plays a critical role in embryogenesis, determining cell lineage and fate commitment [1]. In areas where malnutrition is endemic, vitamin A deficient (VAD) children have a severe impairment in vision due to the critical role of RA in the retina, to which it owes its name. In addition, VAD is associated with an increased burden of infectious disease [2], highlighting the importance of vitamin A for immunity. The pivotal discovery that RA was constitutively synthesised by gut dendritic cells was closely followed by several studies showing that RA was able to enhance induction of inducible regulatory T cells (iTreg). These findings led to the view that RA might act to promote oral tolerance and shifted the attention away from the critical nature of RA in peripheral, effector immune responses. In recent years a broader role for RA in systemic immunity has re-emerged. Several studies have demonstrated regional induction of RA synthesis and signaling upon inflammation [3],[4], and RA has been shown to play an essential role in Th1 responses in allograft rejection, vaccination and gut infection [4],[5]. In this review we focus on these recent advances that have shed light on a broader role for RA in directing T-helper cell fate, outside of the mucosal immune system. Given the clinical availability of retinoids, their mechanisms of action and emerging roles as immunotherapeutic agents will be discussed.
Retinoic acid: Beyond mucosal immunity
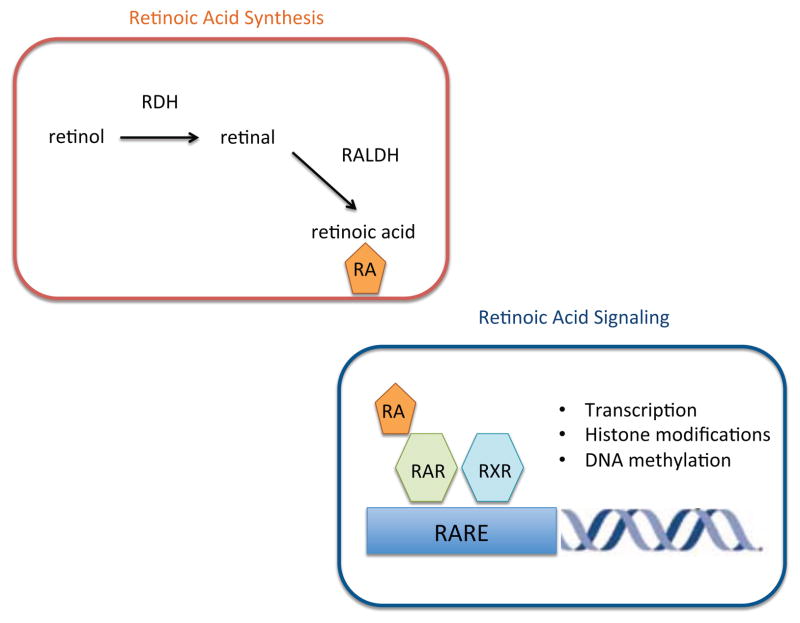
Multiple isoforms of RA exist. Of these all-trans retinoic acid (ATRA) is the predominant biological form and is the subject of this review. RA is generated from retinol, which circulates in the plasma bound to retinol binding protein. RA synthesis is restricted to cells that express the enzymes required for conversion of retinol to RA. First, retinol is converted to retinal by retinol dehydrogenase (RDH). Studies in rdh10−/− mice suggest that RDH10 is the critical isoform for retinal synthesis [6]. Retinal is then irreversibly converted to RA by one of three retinaldehyde dehydrogenase isoforms: RALDH1, RALDH2 or RALDH3 encoded by aldh1a1, aldh1a2, and aldh1a3, respectively [7],[8].
A role for RA in mucosal immunity was established by the discovery that dendritic cells within the mesenteric lymph node (MLN) and Peyer’s patches (PP) constitutively express aldh1a2, the gene that encodes RALDH2, and that RA could imprint gut tropism on T-cells through the induction of gut-homing receptors, CCR9 and α4β7 [9]. Within both human and murine MLNs, DCs that express the highest levels of aldh1a2 are the CD103+ subset [10],[11]. Recently it was shown that expression of 4-1BB, a member of the TNF receptor superfamily, correlates with CD103 positivity in dendritic cells and 4-1BB is therefore also able to identify MLN DCs with the highest levels of aldh1a2 [12]. Triggering of 4-1BB induced RALDH activity in vitro and 4-1BB deficient MLN DCs have weak RALDH activity pointing to a functional role for 4-1BB in the induction of RA synthesis.
Outside of the gut, examination of peripheral DC subsets have identified DCs expressing aldh1a2 residing in the lung and skin, pointing to a role for RA in steady state immune responses at barrier sites [13],[14]. Although the majority of peripheral DCs express negligible or low levels of RALDH, the identification of cytokines and pathogen-associated molecular patterns that can induce RALDH expression indicates that RA synthesis and signaling may be a widespread occurrence during the course of a peripheral immune response. Treatment of splenic DCs with zymosan, a TLR-2 agonist, results in the induction of aldh1a2 in vitro, and stimulation of WT but not TLR2−/− splenic DCs with Candida albicans has a similar effect [15]. In vitro, GM-CSF can induce both bone marrow-derived DCs and splenic DCs to express aldh1a2 [16].
Several in vivo studies have now demonstrated local induction of RALDH activity amongst DCs in response to a diverse array of inflammatory stimuli including viral infection, alloantigen and tumour burden [3],[17],[4],[18],[19]. In addition to RA synthesis by DCs, upregulation of aldh1a2 expression has been observed in alternatively activated macrophages following infection with the helminth Shistosoma mansoni [20]. These studies suggest that RA synthesis and signaling may be a universal feature of immune responses both in the gut and the periphery. Intriguingly, peripheral induction of T and B-cell responses in the presence of RA still leads to induction of gut homing receptors [17],[21]. Induction of CCR9 expression on lung derived CD4+ T-cells following intranasal influenza infection resulted in trafficking of these cells to the small intestine. Local production of IFN-γ resulted in alterations to the gut microbiota which in turn led to increased numbers of intestinal Th17 cells. These findings explain the incidence of intestinal side effects observed in influenza patients but the functional relevance of gut homing to the primary immune response remains to be determined. Recent discoveries have shown that systemic immune responses at sites distal to the gut are modulated through the gut microbiota [22],[23] and it is possible that lymphocyte trafficking through the gut is a necessary rite of passage for effector T-cells. Regional RA production at peripheral sites of inflammation with subsequent induction of gut homing properties on lymphocytes may play a key role in shaping the course of the immune response.
RA regulation of T-helper cell fate and plasticity
RA enhances Foxp3 expression and stability
Following the initial study that identified RA synthesis by gut DCs, several groups went on to show that RA could dramatically enhance the TCR-TGF-β-mediated conversion of naïve CD4+ T cells to induced regulatory T-cells (iTreg) in vitro [10],[24],[25]. TGF-β mediated Foxp3 induction is dependent on Smad3 [26],[27]. In addition to directly regulating the expression of Smad3 [28], RA regulation of Foxp3 expression is in part mediated by binding of RAR/RXR heterodimers to a RA response element (RARE) in the enhancer 1 (CNS1) region of the Foxp3 gene, which facilitates binding of phosphorylated Smad3 to the enhancer region [29]. The first in vivo evidence supporting a role for the RA/TGF-β-Smad3 pathway in the generation of peripheral Tregs (pTreg) in the GALT comes from a recent study utilising mice lacking the Smad3 binding site within the CNS1 region. Aged mice develop deficiencies in Foxp3+ cells in the LP and PP; however, the functional significance of this is unclear since no negative impact of the pTreg deficiency was observed in T-independent or T-cell dependent models of colitis [30]. Further studies are required to understand the in vivo contribution of RA to immune tolerance, both in the gut as well as at peripheral sites of immune responses.
In addition to enhancing TCR-TGF-β mediated Foxp3 expression, RA has also been shown to confer increased stability of Foxp3 expression in the face of inflammatory cytokines and co-stimulation amongst both iTreg and thymic Treg (tTreg) [24],[28] [31]. iTregs generated in the presence of RA express reduced levels of the receptor for IL-6, a Th17 instructing cytokine [32]. Recently, RA and TGF-β were shown to induce expression of the microRNA miR-10a, which in turn inhibited expression of the follicular helper T-cell (TFH) master transcription factor, Bcl-6 [33]. Conversion of Tregs to TFH has been described in PP [34], and miR10-a overexpression in iTregs was able to reduce this conversion in vivo [33]. RA may therefore reinforce lineage stability by regulating opposing pathways that instruct alternative T-helper cell fates.
A newly described role for RA in the conversion of CD4+ T-cells to CD4+CD8αα+ T-cells within the intestinal epithelium provides a further mechanism by which RA promotes anti-inflammatory responses within the gut [35]. Cd4creRosa26dnRara/dnRara mice conditionally expressing a dominant negative form of the retinoic acid receptor RARα (dnRara) in CD4+ T-cells (henceforth referred to as dnRara mice) have severely impaired numbers of CD4+CD8α+ IELs, which appear to play a critical role in preventing intestinal inflammation. dnRara expressing CD4+ T-cells fail to upregulate T-bet, which in turn directs the expression of the CD8 lineage transcription factor, Runx3, and inhibits ThPok allowing reprogramming of CD4+ cells towards CD8α+ IELs.
Retinoic acid is critical for CD4+ effector T-cell differentiation
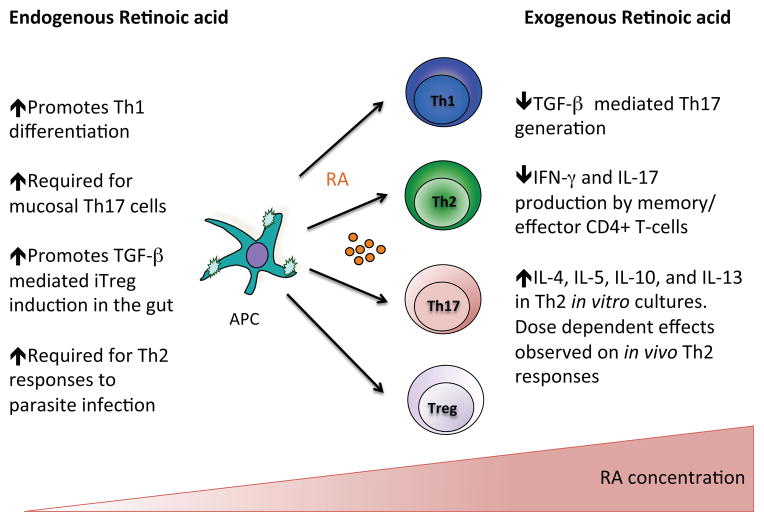
A role for RA in the generation of Tregs seemed counterintuitive given the wealth of epidemiological data supporting a role for RA in immunity to infectious disease. Emerging data examining the effects of RA on effector lymphocytes in vivo have gone someway to resolving this paradox by demonstrating a broader role for RA in Th-cell responses (Fig. 2). Our own studies using dnRara mice demonstrate a critical role for RA in the development of Th1 mediated immunity in a model of allograft rejection [4],[5]. In keeping with the ability of RA to regulate Th1 differentiation, VAD mice infected with Toxoplasma gondii were found to have significantly reduced Th1 cells in their GALT and spleen [5]. In the same study, VAD mice were also shown to have deficient Th1 cell responses in response to vaccination with ovalbumin (OVA) and E. coli toxin [5].
Fig. 2. Retinoic acid gradients regulate naïve CD4+ T-cells and cytokine responses in effector CD4+ T-cells.
Retinoic acid (RA) plays a physiological role in the differentiation of naive CD4+ T-cells. Administration of exogenous RA often has opposing effects on T-cell polarisation and inhibits production of inflammatory cytokines by effector CD4+ T-cells.
The molecular mechanism through which RA controls Th1 cell fate remains unclear. In vitro experiments conducted with CD4+ T cells isolated from RARα−/− mice suggest that disruption of RA signaling leads to impaired activation induced proliferation[5]. However, dnRara expressing CD4+ T-cells mice have normal proliferative capacity [4]. One possible explanation for this discrepancy is that unliganded RARs inhibit transcription through recruitment of co-repressors [36], thus deletion of RARα may lead to a loss of inhibition of RAR targets with a paradoxical up-regulation of RA target genes in the absence of RA. In zebrafish, deletion of RAR isoforms resulted in a paradoxical increase in RA signaling with compensatory increases in expression of alternative RARs [37]. RARα does appear to be the critical receptor in mediating the effects of RA on CD4+ T-cells as deletion of RARγ in haematopoietic cells had no impact on the development of Th1 responses following infection with Listeria monocytogenes [38]. Analysis of RARα target genes in Th1 cells will shed light on the transcriptional regulation of Th1 program by RA. In contrast to a physiological role for RA in supporting Th1 differentiation, RA treatment of naive CD4+ T-cells polarised under Th1 conditions in vitro inhibits IFN-γ production [39].
In addition to shaping Th1 responses, RA has also been shown to regulate Th17 differentiation. The earliest studies linking RA to TGF-β mediated Foxp3+ expression identified a reciprocal role for RA in the inhibition of Th17 cells differentiated from naïve T-cells with TGF-β and IL-6 in vitro. This appears to be in part due to reduced expression of IL-6R and IL-23R in responder T-cells [28]. Interestingly, the inhibitory effects of RA on Th17 differentiation are most prominent in the presence of IL-2 [33]. IL-2 activation of Stat5 antagonises Stat3 and RA inhibition of Th17 differentiation is dependent on Stat5 [28],[33]. Consistent with these in vitro findings, RA administration in vivo inhibits the development of Th17 responses and disease severity in experimental autoimmune encephalitis (EAE), a Th17 mediated autoimmune disease [28]. While pharmacological doses of RA inhibit Th17 generation, physiological levels of RA (<10nm), have been shown to enhance Th17 generation in vitro [33]. In support of an in vivo role for RA in the generation or maintenance of Th17 cells, studies in VAD mice describe a near absence of Th17 cells both during homeostasis and in response to immune-mediated inflammation [5],[40]. However, two recent studies have identified a role for RA in the generation of a type 3 innate lymphoid cells (ILC3s),, which mirror Th17 cells in their cytokine profile. VAD mice and mice treated with an RA antagonist had impaired ILC3 responses both in steady state and in response to intestinal infection with Citrobacter rodentium [41]. ILC3s produce Th17 associated cytokines and their absence in VAD mice may contribute to the impaired development of Th17 cells at the mucosal surface. Further studies are required in CD4creDNRAR mice to establish the role of RA in Th17 generation in vivo.
The absence of ILC3s in VAD mice has widespread implications on adaptive immune responses since a subset of ILC3s, lymphoid tissue inducer (LTi) cells play a critical role in the development of secondary lymphoid organs and Peyer’s patches. Maternal vitamin A and fetal RA signaling were found to directly regulate the expression of RORγt, instructing the development of LTis [42]. Mice exposed to an RA-deficient environment in utero had smaller secondary lymphoid organs which persisted in adult life with impaired adaptive immune responses. Since many earlier studies have used VAD as a model for studying RA regulated dynamic T-cell responses, these studies must be reevaluated in light of the widespread homeostatic defects observed in these mice.
In addition to Th1 and Th17 mediated immune responses, RA synthesis has been reported in parasite infections suggesting a possible role for RA in shaping Th2 responses. Dietary VA levels have been shown to affect Th2 responses with enhanced production of Th2-associated cytokines and concomitant reduction in IFN-γ observed in CD4+ T cells isolated from Trichinella spiralis infected VAD mice [43]. However, in a further twist, the earlier study examining ILC dysregulation, VAD induced expansion of ILC2s with enhanced ILC2 derived Th2 type cytokines [41]. The presence of ILC2s appears to be critical for Th2 allergen responses in vivo [44], thus previously reported effects of dietary RA on Th2-cell responses in VAD mice may not be due to direct effects of RA on T-cells. Studies in which RA levels are manipulated through administration of RA are conflicting with both enhanced and reduced Th2 responses reported in murine models of asthma following RA treatment [45],[46] Genetic approaches that allow one to dissect the impact of RA signaling on defined lineages of hematopoietic cells and the ensuing immune responses will help to clarify the physiological role of RA in the regulation of Th2 mediated immunity.
Retinoic acid: an immune morphogen?
Given that the RA-RARα axis is a highly conserved signaling pathway, which plays a critical role in regulating cell fate specification during embryogenesis and cell differentiation, it is perhaps not surprising that RA has been implicated in all aspects of T-helper cell differentiation. Pleiotropic roles for RA in the generation of Treg, Th1, Th17, and Th2 cells are not mutually exclusive but rather suggest context dependent actions. The key to therapeutic targeting of the RA signaling pathway is an understanding of the dominant action of RA on T-cell specification in different in vivo settings. RA appears in part to alter the sensitivity of cells to alternative cell fates through regulation of cytokine receptors. Thus the dominant effects of RA may well be dependent on the instructive cytokine milieu. In this model, rather than specifying T-cell fate, RA enhances or stabilizes the actions of cytokines and transcription factors that guide T-helper cell programming. In this way, synthesis of RA by APCs provides an additional checkpoint for stabilisation of T-cell fate commitment.
An alternative model is that, similar to the morphogenic properties of RA during embryogenesis, the RA concentration sensed by naïve T-cells undergoing differentiation determines the dominant action of RA on T-cell fate. Over the past few years, RA has achieved recognition as a morphogen, as it has become clear that synthesis and metabolism of RA is tightly controlled resulting in concentration dependent effects on target tissues [47]. The preponderance of data from in vitro experiments in which dose titration comparisons were performed on Th1, Th2 and Th17 polarisation suggest similar dose dependent effects of RA on haematopoietic cell fate [33],[39],[48]. The paradoxical effects of RA on opposing T-cell fates may therefore be explained by concentration dependent effects of RA, allowing T-cells to act as an environmental sensor through the strength of its RA signal. Further studies of RA gradients within lymphoid tissue are required to test this hypothesis. However, the overriding message is that administration of RA either in vitro or to vitamin A replete hosts in vivo may not provide insight into the physiological actions of RA on T-cell responses.
A further layer of complexity surrounds RA regulation of Th-cell fate with bi-phasic effects of RA on Th1 and Th2 differentiation reported in vitro [39]. In Th1 differentiation, induction of T-bet is dependent on IFN-γ-Stat1 signaling whereas maintenance of T-bet expression is dependent on IL12-Stat4 activation [49], suggesting distinct roles for RA in the regulation of these signaling pathways. Similarly, a bi-phasic model for Th17 differentiation is emerging with early commitment dependent on TGF-β and IL-6 signaling but maintenance and stability dependent on IL-21 and IL-23 signaling [50]. Animal models which allow temporal control of RA signaling will be invaluable for dissecting out the role of RA in early vs. late phase differentiation.
Retinoic acid signaling: novel mechanisms of transcriptional regulation
All-trans RA, the biologically active form of VA, signals through heterodimers of the RA receptors (RARs) and retinoid X receptors (RXRs) [51]. These receptors belong to the nuclear hormone receptor family. There are three isotypes for both RAR and RXR: α, β and γ. RAR/RXR heterodimers bind to RA response elements RAREs in target genes and act as ligand dependent transcription factors. RAR/RXRs mediate transcriptional regulation through the binding of co-repressor or co-activator complexes dependent on the presence of ligand (Fig. 1). The widely accepted view is that unliganded RAR/RXR heterodimers inhibit transcription of their target gene, through recruitment of co-repressors such as NCoR or SMRT. Binding of ligand to RAR leads to release of co-repressors and recruitment of co-activators such as p300 and CBP with subsequent transcriptional activation of the target gene [52]. In addition, RA can mediate gene repression through recruitment of ligand-dependent co-repressors, such as RIP140 and PRAME, which recruit histone deacetylases to RAR/RXR complexes to repress their activities [53],[54]. RARs can also modulate transcription indirectly, through inhibition of transcription factor complexes, such as activating protein-1 (AP-1) although the underlying mechanisms remains uncertain [55].
Fig. 1. Retinoic acid synthesis and signaling.
Retinol is taken up from the blood and oxidized first to retinal by retinol dehydrogenases (RDHs) and then to all-trans-retinoic acid (RA) by retinal dehydrogenases (RALDHs). Within the immune system, RALDH2 is the predominant isoform expressed by dendritic cells. Retinoic acid receptors (RARs) are nuclear hormone receptors that function as ligand-dependent transcription factors. RARs form heterodimers with retinoid X receptors (RXRs). RA binds to RAR and triggers a conformational change that promotes recruitment of coactivator complexes to initiate transcription and modify surrounding chromatin.
In addition to its classical role as a transcriptional regulator, recent studies in embryonic stem cells have identified RA-RARα as an epigenetic regulator. Retinoids have been shown to regulate epigenetic changes including histone modifications and DNA methylation through the recruitment of co-activators with chromatin modifying properties [56]. p300, for example, possesses histone acetyltransferase activity and mediates acetylation of H3k27, a marker of active cis-regulatory regions. Epigenetic modifications have been suggested to be the determinants of lineage stability. Given the emerging data implicating RARα in lineage plasticity,, it will be important to evaluate a role for RA-RARα in the regulation of the epigenome. Recent studies have shed light on non-genomic actions of RAR [57]. In neuronal dendrites it has been shown that RARα can bind directly to mRNA, where, unliganded, it inhibits translation [58]. Binding of RA to its receptor leads to dissociation of RARα from the mRNA and relief of translational repression. In addition, RA has also been shown to activate mitogen activated protein kinases (MAPK) in a number of cell types [57]. This occurs within minutes of administration suggesting direct activation rather than transcriptional regulation [59]. Activation of p38MAPK leads to phosphorylation of RARα present in the membrane and cytosol, which in turn promotes recruitment of RAR to promoter regions of target genes. In this way, RA is able to link events at the cell membrane to its transcriptional regulation. Intriguingly, TCR-ligation and subsequent T-cell activation is required to observe RA mediated effects on lymphocytes and this non-classical pathway of RA signaling may play a key role in linking T-cell activation to subsequent transcriptional regulation by RA-RAR. However, at this stage, little is known about the importance of the non-genomic actions of RA relative to its classical regulation of transcription in haematopoietic cells. In other cell types studied, only a small fraction of RAR is available for non-genomic activities [57], suggesting that non-transcriptional activities of RA play a minor role in its overall activities.
In addition to signaling through RARs, RA is also a ligand for the nuclear receptor PPARβ (formerly PPARδ). Targeting of RA to RAR or PPARβ/δ is mediated by the cytosolic lipid binding proteins CRABP-II and FABP5 respectively. RA has a far greater affinity for CRABP-II and as such RA signaling through RAR is likely to be the dominant signaling pathway. However in cells that express high ratio of FABP5/CRABP-II, activation of PPARβ/δ is observed. Two recent studies, in adipocytes and neuronal stem cells, respectively, have shown that differentiation is accompanied by a fall in CRABP-II levels and concurrent up-regulation of FABP5 with a shift towards the RA-PPARβ signaling pathway [60],[61]. Thus both the PPARβ/δ and RAR signaling pathways can play important roles in a single cell lineage and the relative contributions of the two pathways must be explored in a temporal fashion. Lymphocytes express PPARβ/δ although until recently little was known about its function [62]. CD4+ T-cells deficient in PPARβ/δ have enhanced proliferative capacity and a greater propensity for IFN-γ and IL-17 production in vitro suggesting that PPARβ/δ acts to restrain Th1 and Th17 differentiation [63]. In keeping with this, PPARβ/δ−/− mice develop a more severe form of experimental autoimmune encephalomyelitis (EAE) which is associated with enhanced Th1 and Th17 CNS infiltration [63].
IMMUNOTHERAPY WITH RETINOIDS
Retinoids as immunosuppressive agents
Topical and oral retinoids have long been used in the treatment of psoriasis with presumed benefit related to their effects on keratinocyte differentiation and proliferation. Accumulating evidence has implicated the Th17 pathway in the pathogenesis of psoriasis [64],[65], and it may be that the therapeutic effect of retinoids in psoriasis are related to their immunoregulatory effects. The ability of RA to induce Tregs in vitro, coupled with the suppression of Th1 and Th17 responses observed with high dose retinoids, points to a potential role for retinoids as immunosuppressive agents. In support of this, retinoids have been shown to ameliorate disease in two mouse models of SLE [66],[67]. Reduction in disease was associated with inhibition of Th1 responses. RA treatment has also been shown to significantly reduce the incidence of diabetes in a spontaneous mouse model of type 1 diabetes (NOD) with established insulitis [68]. Adoptive transfer of NOD splenocytes into NOD/scid recipients leads to onset of diabetes following infiltration of the pancreas by Th1, Th17 and IFN-γ+ CD8+ T-cells. Treatment of recipient mice with RA inhibits the development of Th1 responses with a reciprocal increase in islet infiltrating Tregs [68]. Retinoids have also shown beneficial effect in mouse models of rheumatoid arthritis [69],[70]. A reduction in disease severity was associated with reduced IL-17 and IL-21 levels within the synovial fluid as well as reduced antibody production [70]. A limitation to current retinoid therapy is the adverse toxicity profile and off target effects of pan RAR agonists. Agonists selective for RARα, the isotype, implicated in effector T-cell responses, are currently in clinical development.
One possible future use for retinoids is the ex vivo generation of stable Tregs for cellular immunotherapy in the context of autoimmune disease and transplantation. The stability of ex vivo expanded Tregs has been a significant concern. RA treatment of Tregs has been shown to constrain their plasticity within an inflammatory setting and RA treatment could be incorporated into protocols for Treg expansion. RA mediated induction of CCR9 and α4β7 may also generate Tregs with enhanced capacity for gut homing which could be advantageous in the setting of inflammatory bowel disease.
Concluding remarks
Recent advances have led to an increased understanding of why VA is so critical for immunological health and has extended the importance of this vitamin beyond the malnourished patient. Given the pleiotropic effects of RA, the challenge for successful immunotherapy will rely on a detailed understanding of the molecular mechanisms through which RA receptors mediate their effects, opening the door for new immunomodulatory drugs.
Acknowledgments
This work was supported the Wellcome Trust (Research Training Fellowship to CB and Program Grant to RJN) and the NIH (R01AT005382 to RJN).
References
- 1.Niederreither K, Dollé P. Retinoic acid in development: towards an integrated view. Nature Reviews Genetics. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- 2.Sommer A, Djunaedi E, Loeden AA, Tarwotjo I, West K, JR, Tilden R, Mele L, et al. Impact Of Vitamin A Supplementation on Childhood Mortality. The Lancet. 1986;327:1169–1173. doi: 10.1016/s0140-6736(86)91157-8. [DOI] [PubMed] [Google Scholar]
- 3.Guo Y, Pino-Lagos K, Ahonen CA, Bennett KA, Wang J, Napoli JL, Blomhoff R, et al. A Retinoic Acid--Rich Tumor Microenvironment Provides Clonal Survival Cues for Tumor-Specific CD8+ T Cells. Cancer Res. 2012;72:5230–5239. doi: 10.1158/0008-5472.CAN-12-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pino-Lagos K, Guo Y, Brown C, Alexander MP, Elgueta R, Bennett KA, De Vries V, et al. A retinoic acid-dependent checkpoint in the development of CD4+ T cell-mediated immunity. Journal of Experimental Medicine. 2011;208:1767–1775. doi: 10.1084/jem.20102358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall JA, Cannons JL, Grainger JR, Santos Dos LM, Hand TW, Naik S, Wohlfert EA, et al. Essential Role for Retinoic Acid in the Promotion of CD4+ T Cell Effector Responses via Retinoic Acid Receptor Alpha. Immunity. 2011;34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Napoli JL. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim Biophys Acta. 2012;1821:152–167. doi: 10.1016/j.bbalip.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–8. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 8.Lin M, Zhang M, Abraham M, Smith SM. Mouse retinal dehydrogenase 4 (RALDH4), molecular cloning, cellular expression, and activity in 9-cis-retinoic acid biosynthesis in intact cells. J Biol Chem. 2003;278:9856–61. doi: 10.1074/jbc.M211417200. [DOI] [PubMed] [Google Scholar]
- 9.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song S-Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Coombes J, Siddiqui K, Arancibia-C C. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. The Journal of Experimental Medicine. 2007;204:1757. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. Journal of Experimental Medicine. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S-W, Park Y, Eun S-Y, Madireddi S, Cheroutre H, Croft M. Cutting Edge: 4-1BB Controls Regulatory Activity in Dendritic Cells through Promoting Optimal Expression of Retinal Dehydrogenase. The Journal of Immuunology. 2012;189:2697–701. doi: 10.4049/jimmunol.1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, de Bovis B, et al. Skin-draining lymph nodes contain dermis-derived CD103- dendritic cells that constitutively produce retinoic acid and induce Foxp3+ regulatory T cells. Blood. 2010;115:1958–1968. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- 14.Gyöngyösi A, Szatmari I, Pap A, Dezso B, Pos Z, Széles L, Varga T, et al. RDH10, RALDH2, and CRABP2 are required components of PPARγ-directed ATRA synthesis and signaling in human dendritic cells. J Lipid Res. 2013;54:2458–2474. doi: 10.1194/jlr.M038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manicassamy S, Pulendran B. Retinoic acid-dependent regulation of immune responses by dendritic cells and macrophages. Seminars in Immunology. 2009;21:22–27. doi: 10.1016/j.smim.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokota A, Takeuchi H, Maeda N, Ohoka Y, Kato C, Song S-Y, Iwata M. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. International Immunology. 2009;21:361–377. doi: 10.1093/intimm/dxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Li F, Wei H, Lian Z-X, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. Journal of Experimental Medicine. 2014;211:2397–2410. doi: 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allie SR, Zhang W, Tsai C-Y, Noelle RJ, Usherwood EJ. Critical role for all-trans retinoic acid for optimal effector and effector memory CD8 T cell differentiation. The Journal of Immunology. 2013;190:2178–2187. doi: 10.4049/jimmunol.1201945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mielke LA, Jones SA, Raverdeau M, Higgs R, Stefanska A, Groom JR, Misiak A, et al. Retinoic acid expression associates with enhanced IL-22 production by γδ T cells and innate lymphoid cells and attenuation of intestinal inflammation. Journal of Experimental Medicine. 2013;210:1117–1124. doi: 10.1084/jem.20121588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broadhurst MJ, Leung JM, Lim KC, Girgis NM. Upregulation of Retinal Dehydrogenase 2 in Alternatively Activated Macrophages during Retinoid-dependent Type-2 Immunity to Helminth Infection in Mice. PLoS Pathog. 2012;8:e1002883. doi: 10.1371/journal.ppat.1002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammerschmidt S, Friedrichsen M, Boelter J. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J Clin Invest. 2011;121:3051–3061. doi: 10.1172/JCI44262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H-J, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2011;108:4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benson M, Pino-Lagos K, Rosemblatt M, Noelle R. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. Journal of Experimental Medicine. 2007;204:1765. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mucida D, Park Y, Kim G, Turovskaya O, Scott I. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 26.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nature Immunology. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 27.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. The Journal of Immunology. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L, Kitani A, Stuelten C, McGrady G, Fuss I. Positive and negative transcriptional regulation of the Foxp3 gene is mediated by access and binding of the Smad3 protein to enhancer I. Immunity. 2010;33:313–25. doi: 10.1016/j.immuni.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlenner SM, Weigmann B, Ruan Q, Chen Y, Boehmer von H. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. Journal of Experimental Medicine. 2012;209:1529–1535. doi: 10.1084/jem.20112646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou X, Kong N, Wang J, Fan H, Zou H. Cutting Edge: All-Trans Retinoic Acid Sustains the Stability and Function of Natural Regulatory T Cells in an Inflammatory Milieu. The Journal of Immunology. 2010;185:2675–9. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill JA, Hall JA, Sun C-M, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, et al. Retinoic Acid Enhances Foxp3 Induction Indirectly by Relieving Inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi H, Kanno T, Nakayamada S, Hirahara K, egrave GS, Muljo SA, Kuchen S, et al. TGF-β and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nature Immunology. 2012:1–11. doi: 10.1038/ni.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 35.Reis BS, Rogoz A, Costa-Pinto FA, Taniuchi I, Mucida D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4+ T cell immunity. Nature Immunology. 2013;14:271–280. doi: 10.1038/ni.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurokawa R, Söderström M, Hörlein A, Halachmi S, Brown M, Rosenfeld MG, Glass CK. Polarity-specific activities of retinoic acid receptors determined by a co-repressor. Nature. 1995;377:451–454. doi: 10.1038/377451a0. [DOI] [PubMed] [Google Scholar]
- 37.D’Aniello E, Rydeen AB, Anderson JL, Mandal A, Waxman JS. Depletion of retinoic acid receptors initiates a novel positive feedback mechanism that promotes teratogenic increases in retinoic acid. PLoS Genet. 2013;9:e1003689. doi: 10.1371/journal.pgen.1003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dzhagalov I, Chambon P. Regulation of CD8+ T Lymphocyte Effector Function and Macrophage Inflammatory Cytokine Production by Retinoic Acid Receptor γ. The Journal of Immunology. 2007;178:2113–21. doi: 10.4049/jimmunol.178.4.2113. [DOI] [PubMed] [Google Scholar]
- 39.Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. International Immunology. 2003;15:1017–1025. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- 40.Wang C, Kang SG, HogenEsch H, Love PE, Kim CH. Retinoic acid determines the precise tissue tropism of inflammatory Th17 cells in the intestine. The Journal of Immunology. 2010;184:5519–5526. doi: 10.4049/jimmunol.0903942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, et al. Adaptation of Innate Lymphoid Cells to a Micronutrient Deficiency Promotes Type 2 Barrier Immunity. Science. 2014;343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cantorna MT, Nashold FE, Hayes CE. In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J Immunol. 1994;152:1515–1522. [PubMed] [Google Scholar]
- 44.Halim TYF, Steer CA, Mathä L, Gold MJ, Martinez-Gonzalez I, Mcnagny KM, McKenzie ANJ, et al. Group 2 Innate Lymphoid Cells Are Critical for the Initiation of Adaptive T Helper 2 Cell-Mediated Allergic Lung Inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuster GU, Kenyon NJ, Stephensen CB. Vitamin A deficiency decreases and high dietary vitamin A increases disease severity in the mouse model of asthma. J Immunol. 2008;180:1834–1842. doi: 10.4049/jimmunol.180.3.1834. [DOI] [PubMed] [Google Scholar]
- 46.Wu J, Zhang Y, Liu Q, Zhong W, Xia Z. All-trans retinoic acid attenuates airway inflammation by inhibiting Th2 and Th17 response in experimental allergic asthma. BMC Immunol. 2013;14:28. doi: 10.1186/1471-2172-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White RJ, Nie Q, Lander AD, Schilling TF. Complex Regulation of cyp26a1 Creates a Robust Retinoic Acid Gradient in the Zebrafish Embryo. PLoS Biol. 2007;5:e304. doi: 10.1371/journal.pbio.0050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uematsu S, Fujimoto K, Jang MH, Yang B-G, Jung Y-J, Nishiyama M, Sato S, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nature Immunology. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 49.Schulz E, Mariani L, Radbruch A, Höfer T. Sequential Polarization and Imprinting of Type 1 T Helper Lymphocytes by Interferon-γ and Interleukin-12. Immunity. 2009;30:673–683. doi: 10.1016/j.immuni.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 50.Basu R, Hatton RD, Weaver CT. The Th17 family: flexibility follows function. Immunological reviews. 2013;252:89–103. doi: 10.1111/imr.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chambon P. A decade of molecular biology of retinoic acid receptors. The FASEB Journal. 1996;10:940–54. [PubMed] [Google Scholar]
- 52.Nagy L, Schwabe J. Mechanism of the nuclear receptor molecular switch. Trends in biochemical sciences. 2004;29:317–24. doi: 10.1016/j.tibs.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Nicholson RC, Mader S, Nagpal S, Leid M, Rochette-Egly C, Chambon P. Negative regulation of the rat stromelysin gene promoter by retinoic acid is mediated by an AP1 binding site. EMBO J. 1990;9:4443–4454. doi: 10.1002/j.1460-2075.1990.tb07895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Epping MT, Wang L, Edel MJ, Carlée L, Hernandez M, Bernards R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835–847. doi: 10.1016/j.cell.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Perissi V, Jepsen K, Glass CK, Rosenfeld MG. Deconstructing repression: evolving models of co-repressor action. Nature Reviews Genetics. 2010;11:109–123. doi: 10.1038/nrg2736. [DOI] [PubMed] [Google Scholar]
- 56.Gudas LJ. Retinoids induce stem cell differentiation via epigenetic changes. Semin Cell Dev Biol. 2013;24:701–5. doi: 10.1016/j.semcdb.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rochette-Egly C, Germain P. Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (RARs) Nuclear receptor signaling. 2009;7:e005. doi: 10.1621/nrs.07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sarti F, Schroeder J, Aoto J, Chen L. Conditional RARα knockout mice reveal acute requirement for retinoic acid and RARα in homeostatic plasticity. Frontiers in Molecular Neuroscience. 2012;5:16. doi: 10.3389/fnmol.2012.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masiá S, Alvarez S, de Lera AR, Barettino D. Rapid, nongenomic actions of retinoic acid on phosphatidylinositol-3-kinase signaling pathway mediated by the retinoic acid receptor. Mol Endocrinol. 2007;21:2391–2402. doi: 10.1210/me.2007-0062. [DOI] [PubMed] [Google Scholar]
- 60.Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Molecular and Cellular Biology. 2009;29:3286–3296. doi: 10.1128/MCB.01742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu S, Levi L, Siegel R, Noy N. Retinoic acid induces neurogenesis by activating both retinoic acid receptors (RAR) and peroxisome proliferator-activated receptor β/δ (PPARβ/δ) Journal of Biological Chemistry. 2012;287:42195–42205. doi: 10.1074/jbc.M112.410381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schote AB, Turner JD, Schiltz J, Muller CP. Nuclear receptors in human immune cells: expression and correlations. Molecular immunology. 2007;44:1436–1445. doi: 10.1016/j.molimm.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 63.Dunn SE, Bhat R, Straus DS, Sobel RA, Axtell R, Johnson A, Nguyen K, et al. Peroxisome proliferator-activated receptor delta limits the expansion of pathogenic Th cells during central nervous system autoimmunity. Journal of Experimental Medicine. 2010;207:1599–1608. doi: 10.1084/jem.20091663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, Matsunami N, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kinoshita K, Yoo B-S, Nozaki Y, Sugiyama M, Ikoma S, Ohno M, Funauchi M, et al. Retinoic acid reduces autoimmune renal injury and increases survival in NZB/W F1 mice. J Immunol. 2003;170:5793–5798. doi: 10.4049/jimmunol.170.11.5793. [DOI] [PubMed] [Google Scholar]
- 67.Pérez de Lema G, Lucio-Cazaña FJ, Molina A, Luckow B, Schmid H, de Wit C, Moreno-Manzano V, et al. Retinoic acid treatment protects MRL/lpr lupus mice from the development of glomerular disease. Kidney international. 2004;66:1018–1028. doi: 10.1111/j.1523-1755.2004.00850.x. [DOI] [PubMed] [Google Scholar]
- 68.Van Y-H, Lee W-H, Ortiz S, Lee M-H, Qin H-J, Liu C-P. All-trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)-dependent suppression of interferon-gamma-producing T-cells without affecting Th17 cells. Diabetes. 2009;58:146–155. doi: 10.2337/db08-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nozaki Y, Yamagata T, Sugiyama M, Ikoma S, Kinoshita K, Funauchi M. Anti-inflammatory effect of all-trans-retinoic acid in inflammatory arthritis. Clinical Immunology. 2006;119:272–279. doi: 10.1016/j.clim.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 70.Kwok S-K, Park M-K, Cho M-L, Oh H-J, Park E-M, Lee D-G, Lee J, et al. Retinoic acid attenuates rheumatoid inflammation in mice. The Journal of Immunology. 2012;189:1062–1071. doi: 10.4049/jimmunol.1102706. [DOI] [PubMed] [Google Scholar]