Abstract
Rationale
Vascular smooth muscle cell (SMC) phenotypic modulation and vascular remodeling contributes to the development of a number of vascular disorders such as restenosis after angioplasty, transplant vasculopathy, and atherosclerosis. The mechanisms underlying these processes, however, remain largely unknown.
Objective
The objective of this study is to determine the role of dedicator of cytokinesis 2 (DOCK2) in SMC phenotypic modulation and vascular remodeling.
Methods and Results
Platelet-derived growth factor-BB (PDGF-BB) induced DOCK2 expression while modulating SMC phenotype. DOCK2 deficiency diminishes PDGF-BB or serum-induced down regulation of SMC markers. Conversely, DOCK2 overexpression inhibits SMC marker expression in primary cultured SMC. Mechanistically, DOCK2 inhibits myocardin expression, blocks SRF nuclear location, attenuates myocardin binding to SRF, and thus attenuates myocardin-induced smooth muscle marker promoter activity. Moreover, DOCK2 and Kruppel-like factor 4 cooperatively inhibit myocardin-SRF interaction. In a rat carotid artery balloon-injury model, DOCK2 is induced in media layer SMC initially and neointima SMC subsequently following vascular injury. Knockdown of DOCK2 dramatically inhibits the neointima formation by 60%. Most importantly, knockout of DOCK2 in mice markedly blocks ligation-induced intimal hyperplasia while restoring SMC contractile protein expression.
Conclusions
Our studies identified DOCK2 as a novel regulator for SMC phenotypic modulation and vascular lesion formation following vascular injury. Therefore, targeting DOCK2 may be a potential therapeutic strategy for the prevention of vascular remodeling in proliferative vascular diseases.
Keywords: Dedicator of cytokinesis 2, vascular smooth muscle cells, migration, proliferation, vascular remodeling
INTRODUCTION
Vascular remodeling contributes to the development of a number of vascular disorders including restenosis after angioplasty, vein graft stenosis, transplant vasculopathy, and atherosclerosis, etc 1, 2. Although neointimal formation may be controlled by different mechanisms, medial smooth muscle (SMC) phenotypic modulation from a contractile to a synthetic phenotype triggered by damaging to blood vessel walls followed by SMC migration and proliferation plays a major role in injury-induced vascular remodeling 3–6. Elucidating mechanisms underlying SMC phenotypic modulation, therefore, is critical for understanding the etiology of above-mentioned vascular proliferative disorders and for developing effective therapeutics to block the narrowing of vessel lumen due to vascular remodeling.
Dedicator of cytokinesis 2 (DOCK2) is an atypical guanine nucleotide exchange factor for the Rho-small guanine triphosphatase 7. Under physiological conditions, DOCK2 is mainly expressed in hematopoietic cells and is involved in lymphocyte activation and migration via regulating actin cytoskeleton through Rac activation 7, 8. Deletion of DOCK2 enables long-term cardiac allograft survival via suppressing graft tissue infiltration of alloreactive T cells 9. DOCK2 also controls various immunological functions including helper T cell differentiation, neutrophil chemotaxis and type I interferon induction 10–12. It is unknown, however, if DOCK2 is involved in regulating vascular function.
In this study, we found that platelet-derived growth factor-BB (PDGF-BB) induced DOCK2 expression in SMC while modulating SMC phenotype. Knockout of DOCK2 (DOCK2−/−) enhanced SMC marker gene expression in primary cultured SMCs isolated from DOCK2−/− mice. Ectopic expression of DOCK2 inhibited the marker protein expression. DOCK2 appeared to modulate SMC phenotype by suppressing myocardin/serum response factor (SRF)-mediated transcription of SMC marker genes. In vivo study using rat carotid artery balloon-injury model showed that DOCK2 is essential for injury-induced neointima formation because knockdown of DOCK2 dramatically inhibited the neointima formation by 60%. Most importantly, knockout of DOCK2 markedly blocked ligation-induced intimal hyperplasia and SMC phenotypic modulation in mouse arteries. Our study demonstrates that DOCK2 is a novel regulator of SMC phenotypic modulation and an essential factor contributing to vascular remodeling.
METHODS
Animals
Male Sprague-Dawley rats weighing 450–500 g were purchased from Harlan. DOCK2−/− mice were previously described 8. All animals were housed under conventional conditions in the animal care facilities and received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals. Animal surgical procedures were approved by the Institutional Animal Care and Use Committee of The University of Georgia.
Cell culture
SMCs were cultured by enzyme digestion method from rat or mouse thoracic aorta as described previously 13, 14. The primary cultured SMCs were confirmed by the expression of smooth muscle α-actin (α-SMA) and SM22α. SMCs less than 6 passages with 70% of confluence were used in the experiments.
Construction of Adenovirus
Adenoviral vectors expressing scramble (shCtrl) or DOCK2 short hairpin RNA (shRNA) (shDOCK2) were constructed, and the viruses were purified as described previously 14. The top and bottom strand sequences for shCtrl and shDOCK2 were included in Supplemental Materials.
Western blot analysis
Western blot (WB) was performed as described previously 15. Antibodies against DOCK2 (Millipore), α-SMA (Sigma), calponin (Abcam), SM22α (Abcam), SRF (Santa Cruz Biotechnologies), Myocardin (Myocd, Abcam), smooth muscle myosin heavy chain (SMMHC, Biomedical Technologies Inc.), Kruppel-like factor 4 (KLF4, Abcam), Flag (Sigma), GAPDH (Sigma), and α-Tubulin (Sigma) were used for immunoblotting.
Real-time quantitative PCR (qPCR)
Quantitative PCR was performed as described previously 16. The primer sequences for the involved genes were listed in the Online Table I.
Transfection and luciferase assay
Transfection of α-SMA promoter construct with myocardin and/or DOCK2 plasmids into SMCs was described previously 17. Luciferase assay was performed as described previously 16. Experiments were repeated for three times. Results shown were from a representative experiment with standard deviations.
Immunofluorescent (IF) staining
SMCs cultured form WT and DK2−/− mice or Rat were grown on glass coverslips. IF staining was performed using SRF or DOCK2 antibody as described previously 18. Stained cells were imaged using a Nikon fluorescent microscope.
Rat carotid artery injury model and adenoviral gene transfer
Rat carotid artery balloon injury model and the method that introduces adenovirus into the injured artery have been previously described 14. The balloon-injured and adenovirus-dwelled segments were collected at 3, 7, and 14 days later. Subsequent morphometric analyses were performed in a double-blinded manner.
Mouse carotid artery ligation-injury model
The mouse carotid artery ligation model was described previously 19. Mouse left common carotid arteries were exposed and ligated. The ligation-injured segments were collected at 4 weeks later. Contra-lateral non-injured carotid arteries were used as controls. Subsequent morphometric analyses were performed in a double-blinded manner.
Histomorphometric analysis and immunohistochemistry (IHC) staining
Vessel segments were removed for analysis. The dissected arteries were stained with modified hematoxylin and eosin (HE) or Elastica van Gieson staining and captured using a Nikon microscope. The circumference of lumen, internal elastic lamina, and external elastic lamina were measured by Image-pro Plus Software. IHC staining was performed using DOCK2, α-SMA, or SMMHC antibody as described previously 20.
Statistical analysis
All data were evaluated with a 2-tailed, unpaired Student t test or compared by one-way ANOVA followed by Fisher t test and are expressed as mean±SD. A value of P<0.05 was considered statistically significant.
RESULTS
DOCK2 was essential for SMC phenotypic modulation
PDGF-BB is a potent stimulator of SMC phenotypic modulation 21. Although DOCK2 is expressed in a very low level in normal SMC, its expression was significantly induced in SMCs when the cells were treated with PDGF-BB. Dose-dependent study showed that 5 ng/ml of PDGF-BB significantly elevated DOCK2 expression, while 10 ng/ml of PDGF-BB induced a high level of DOCK2 expression (Online Figure 1A–1B). Therefore, we used 10 ng/ml of PDGF-BB for all subsequent experiments. Since SMC phenotypic modulation is characterized by the reduction of SMC contractile proteins, we detected if DOCK2 induction by PDGF-BB correlates with the attenuation of SMC marker proteins. As shown in Fig. 1A–1B, PDGF-BB induced DOCK2 expression in a time-dependent manner while inhibiting the expression of SMC contractile proteins including SMMHC, calponin, and SM22α. These data suggest that DOCK2 may be involved in PDGF-BB-mediated SMC phenotype modulation.
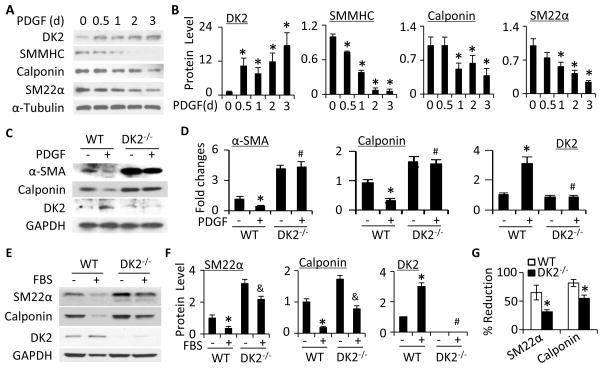
Figure 1. DOCK2 mediated SMC phenotypic modulation.
A, Time-dependent induction of DOCK2 (DK2) and SMC markers by PDGF-BB (10 ng/ml). B, Quantitative analysis of protein expression shown in A by normalizing to α-Tubulin. *, P<0.05 vs vehicle-treated group (0 h). n=3. C, Knockout of DOCK2 blocked PDGF-BB-induced downregulation of SMC marker proteins. SMCs isolated from wild type (WT) and DOCK2 knockout (DK2−/−) mice were treated with vehicle (−) or PDGF-BB (+) for 36 h. DK2, α-SMA, and calponin protein expression was detected by western blot. D, Protein levels shown in C were quantified by normalizing to GAPDH. Fold changes are shown. *, P<0.05 vs vehicle-treated WT group (−); #, P>0.05 vs vehicle-treated DK2−/− group, n=3. E, Serum (FBS)-induced SMC phenotypic modulation in WT and DK2−/− SMCs. Cells were treated with vehicle (−) or 10% FBS (+) for 48 h. SM22α, calponin, and DK2 protein expression was detected by western blot. F, Protein levels shown in E were quantified by normalizing to GAPDH. *, P<0.05 vs vehicle-treated WT group (−); &, P<0.05 vs vehicle-treated DK2−/− group; #, P>0.05 vs vehicle-treated DK2−/− group. n=3. G, DOCK2−/− attenuated FBS-induced SMC phenotypic modulation. The percentage downregulation of SMC markers by FBS was calculated by the following formula: (untreated value − FBS-treated)/untreated value)×100. *, P<0.05 vs WT group for each corresponding marker, n=3.
To determine if DOCK2 is important for SMC phenotypic modulation, we tested if DOCK2 regulates SMC marker expression. PDGF-BB or fetal bovine serum (FBS) induced DOCK2 while blocking SMC contractile protein expression in wild-type (WT) mouse primary cultured SMC (Fig. 1C–1F). However, DOCK2 knockout dramatically increased the marker protein expression even in basal condition (Fig. 1C–1F), suggesting that a low level of DOCK2 is essential for maintaining contractile protein homeostasis. PDGF-BB failed to block the contractile protein expression in DOCK2−/− SMC (Fig. 1C–1D), indicating that DOCK2 plays an essential role in PDGF-BB-induced SMC phenotypic modulation. FBS inhibited the marker expression in both WT and DOCK2−/− SMCs (Fig. 1E–1F). Although the effect of FBS in DOCK2−/− cells was also significant, the percentage down regulation of SMC proteins in DOCK2−/− SMCs was much lower comparing to the WT SMCs (Fig. 1G), indicating that DOCK2−/− also attenuated FBS-induced SMC phenotypic modulation. The discrepancy between PDGF-BB and FBS was likely due to the presence of multiple growth factors in FBS. The function of some of the factors may not be affected by DOCK2, and other intracellular factors appear to be also involved in mediating FBS-induced SMC phenotypic modulation.
DOCK2 inhibited Myocd while modulating SMC phenotype
SRF and its co-activator Myocd are well-known factors regulating SMC contractile protein expression 22–24. PDGF-BB has been shown to induce SMC phenotypic modulation by inhibiting Myocd and SRF expression or blocking Myocd-SRF interaction. In order to determine the mechanisms underlying DOCK2 function in SMC phenotypic modulation, we first tested if DOCK2 regulates Myocd or SRF expression. We found that PDGF-BB inhibited Myocd and SRF expression (Fig. 2A). Knockout of DOCK2, however, dramatically increased Myocd but not SRF expression. PDGF-BB treatment failed to reduce Myocd expression in DOCK2−/− SMCs, suggesting that DOCK2 can block Myocd expression (Fig. 2A). SRF expression was inhibited by PDGF-BB in DOCK2−/− SMCs similarly as in WT SMCs (Fig. 2A), suggesting that DOCK2 is not involved in SRF expression. Conversely, overexpression of DOCK2 blocked Myocd, but not SRF, expression (Fig. 2B) while blocking α-SMA and calponin expression (Online Figure II). Importantly, DOCK2 overexpression attenuated Myocd expression while inhibiting SMMHC expression (Fig. 2C–2D), suggesting that DOCK2 modulating SMC phenotype, at least partially, through blocking Myocd expression. Indeed, knockdown of DOCK2 enhanced Myocd expression while increased SMMHC expression (Fig. 2E–2F). These data demonstrated that down regulation of Myocd is one of the mechanisms whereby DOCK2 mediates PDGF-BB-induced SMC phenotypic modulation.
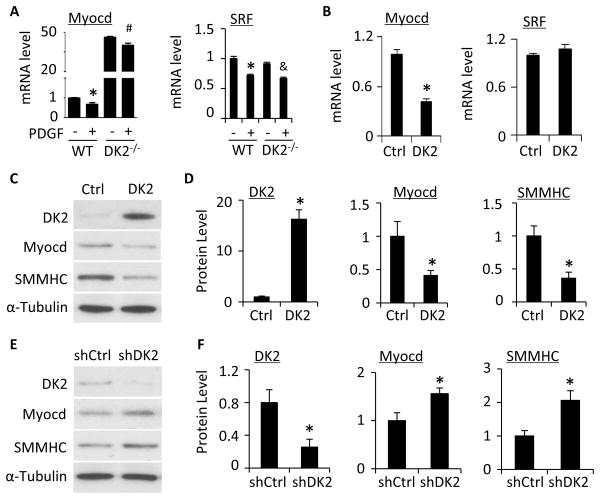
Figure 2. DOCK2 modulated SMC phenotype through downregulating myocardin.
A, Knockout of DOCK2 (DK2−/−) increased myocardin (Myocd) but not SRF mRNA expression. SMCs isolated from WT or DK2−/− mice were treated with vehicle (−) or PDGF-BB (+) for 24 h. Myocd and SRF mRNA expression was detected by qPCR. *, P<0.05 vs vehicle-treated (−) WT group, #, P>0.05 vs vehicle-treated DK2−/− group. &, P<0.05 vs vehicle-treated DK2−/− group. n=3. B, Ectopic expression of DK2 inhibited Myocd but not SRF mRNA expression. Myocd and SRF mRNA expression was detected by qPCR. *, P<0.05 compared with control plasmid group (Ctrl), n=3. C, Ectopic expression of DK2 blocked Myocd and SMC marker protein expression. The protein expression of DK2, Myocardin, and SMMHC was detected by western blot. D, Quantification of protein levels shown in C by normalized to α-Tubulin. *, P<0.05 compared with Ctrl group, n=3. E, Knockdown of DK2 by shRNA enhanced Myocd and SMMHC protein expression in SMC. Primary cultured SMCs were transfected with control (shCtrl) or DOCK2 shRNA (shDK2) followed by PDGF-BB-treatment. The protein expression of DK2, Myocd, and SMMHC was detected by western blot. F, Quantification of protein levels shown in E by normalized to α-Tubulin. *, P<0.05 compared with shCtrl group, n=3.
DOCK2 mediated PDGF-BB function in blocking SRF nuclear location and attenuated Myocd-induced SMC marker promoter activity
Although DOCK2 did not affect SRF expression, SRF plays a very important role in SMC marker gene expression and SMC phenotype 22. Since SRF has to be translocated into nuclei to serve as a transcription factor, we sought to determine if DOCK2 is involved in SRF nuclear location. PDGF-BB inhibited SRF nuclear location (Fig. 3A–3B), consistent with a previous report 25. However, DOCK2−/− blunted the effect of PDGF-BB and restored SRF nuclear location (Fig. 3A–3B), suggesting that DOCK2 mediated PDGF-BB function in inhibiting SRF nuclear location.
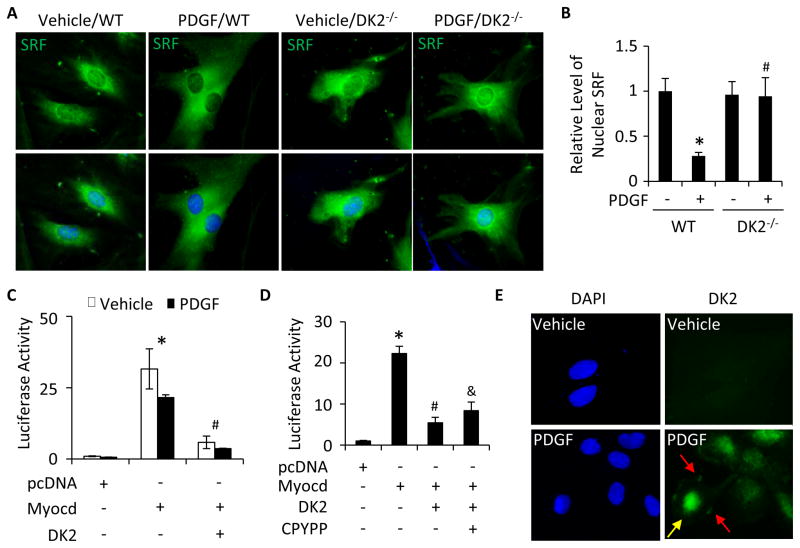
Figure 3. DOCK2 blocked SRF nuclear translocation and myocardin-mediated SMC marker gene transcription.
A, DOCK2 deficiency (DK2−/−) restored PDGF-BB-blocked SRF nuclear location. WT and DK2−/− SMCs were treated with vehicle or PDGF-BB for 24 h. SRF cellular location was detected by immunostaining. DAPI stained the nuclei. B, Quantification of nuclear SRF level shown in A by normalizing to the signal intensity in vehicle-treated WT SMCs (set as 1). *, P<0.05 vs vehicle-treated WT SMCs (−); #, P>0.05 vs vehicle-treated DK2−/− SMCs (−). n=3. C, DK2 blocked Myocd-activated α-SMA promoter activity. pcDNA, Myocd, or DK2 plasmid were co-transfected with α-SMA promoter as indicated into SMCs followed by vehicle or PDGF-BB treatment for 24 h. Luciferase assay was performed. *, P<0.05 vs pcDNA-transfected groups; #, P<0.05 vs Myocd alone-transfected groups. n=3. D, DK2 blocked α-SMA promoter activity independent of Rac. pcDNA, Myocd, or DK2 plasmid were co-transfected with α-SMA promoter into SMCs followed by vehicle (−) or CPYPP (25 μM) treatment as indicated for 24 h. Luciferase assay was performed. *, P<0.05 vs pcDNA-transfected group; #, P<0.05 vs Myocd alone-transfected group. &, P>0.05 vs Myocd/DK2 co-transfected group (n=3). E, DOCK2 nuclear location. DOCK2 cellular location was detected by immunostaining. DK2 was located on both cytoplasm membrane (red arrows) and nuclei (yellow arrow) of SMCs upon PDGF-BB treatment in addition to the cytoplasm.
Because Myocd is a coactivator of SRF in regulating SMC marker gene transcription 24, 26, we sought to determine if DOCK2 alters Myocd/SRF-activated SMC marker gene transcription. As shown in Figure 3C, Myocd strongly induced the transcription of α-SMA promoter. PDGF-BB inhibited the promoter activity, likely due to PDGF-BB-induced DOCK2 expression. Myocd-induced promoter activity was not completely blocked by PDGF-BB probably because of the limited DOCK2 induced by PDGF-BB. However, overexpression of DOCK2 almost completely blocked Myocd-activated α-SMA promoter activity (Fig. 3C). These data demonstrate that DOCK2 regulates SMC phenotype through inhibiting Myocd-activated transcription of SMC contractile genes. Previous studies have shown that DOCK2 function in lymphocyte migration is mainly mediated by Rac1 8. We thus tested if DOCK2 function in SMC promoter activity is also associated with Rac1. As shown in Fig. 3D, a specific inhibitor CPYPP 27 that blocks DOCK2 activity in activating Rac1 (Online Figure III) did not affect DOCK2 function in inhibiting Myocd-induced α-SMA promoter activity, suggesting that DOCK2 modulates SMC phenotype independent of its role in activating Rac1.
The role of DOCK2 in SMC marker gene transcription motivated us to hypothesize that DOCK2 may be a nuclear factor in SMCs. Indeed, immunostaining of DOCK2 showed that PDGF-BB-induced DOCK2 were located in the nuclei of SMCs in addition to the cytoplasm membrane and the cytoplasm (Fig. 3E), consistent with its function in inhibiting Myocd/SRF-activated α-SMA promoter activity and SRF nuclear location.
DOCK2 inhibited Myocd-SRF interaction
Since PDGF-BB also inhibits Myocd binding to SRF, which blocks SMC marker transcription 28, we explored if DOCK2 modulates SMC phenotype by influencing Myocd-SRF interaction. Thus, Flag-tagged Myocd plasmids were transfected into SMC, and the interaction of Myocd with endogenous SRF was detected. As shown in Figure 4A and 4B, Myocd indeed interacted with SRF in SMCs, and PDGF-BB inhibited their interaction. However, when DOCK2 was knocked down by shRNA, the reduced Myocd-SRF interaction by PDGF-BB was significantly increased. These data suggest that DOCK2 mediates PDGF-BB-induced reduction of mycardin binding to SRF. To confirm DOCK2 function in blocking myocarin-SRF binding, we overexpressed DOCK2 in SMCs and found that DOCK2 indeed significantly blocked the interaction between Myocd and SRF (Fig. 4C–4D). To determine how DOCK2 inhibits Myocd-SRF interaction, we tested if DOCK2 interacts with myocardin or SRF. As shown in Fig. 4E–4F, DOCK2 interacted with Myocd, but not SRF. PDGF-BB treatment significantly enhanced DOCK2-Myocd interaction. These data suggest that DOCK2 may inhibit Myocd-SRF interaction by directly interacting with Myocd, which likely diminished the availability of Myocd for SRF.
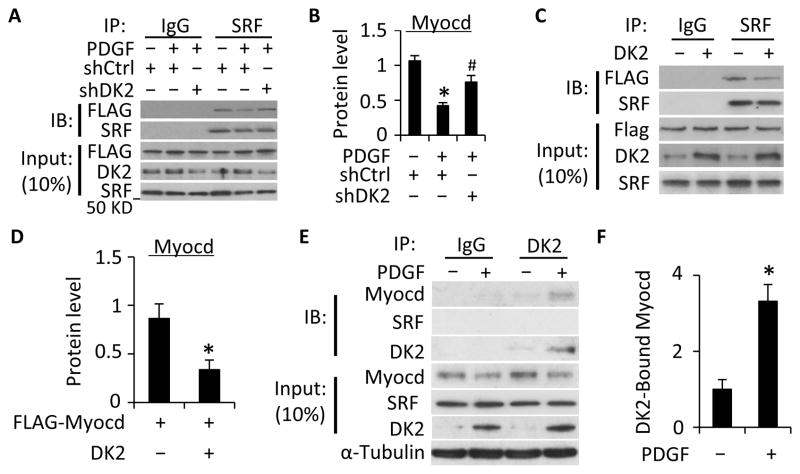
Figure 4. DOCK2 inhibited Myocd-SRF interaction.
A, Knockdown of DOCK2 (DK2) restored PDGF-BB-blocked Myocardin-SRF interaction. SMCs were transduced with scramble (shCtrl) or DK2 shRNA (shDK2) and transfected with Flag-tagged Myocd cDNA as indicated. Cells were then treated with PDGF-BB. Cell lysates were immunoprecipitated (IP) with normal IgG or SRF antibody followed by immunobloting (IB) with Flag or SRF antibody. B, Quantification of SRF-bound Myocd by normalizing to the input Myocd level in each treatment and set the vehicle-treated group as 1. *, P<0.05 vs the vehicle treated group (−); #, P<0.05 vs PDGF-BB and shCtrl-treated groups (n=3). C. DK2 overexpression suppressed Myocardin-SRF interaction. Control (−) or DK2 expression plasmids were co-transfected with Flag-tagged Myocd into SMCs followed by Co-IP with IgG or SRF antibody and IB with Flag antibody. D, SRF-bound Myocd was quantified similarly as in B. *, P<0.05 vs control plasmid-transfected group (−), n=3. E, Co-IP with endogenous proteins indicated that Myocd physically interacted with DK2. Primary cultured SMCs were treated with vehicle (−) or PDGF-BB (+) for 24 h. Cell lysates were Co-IP with normal IgG or DK2 antibody, and blotted with Myocd or SRF antibody as indicated. Myocd-DK2 interaction was enhanced by PDGF-BB. F, Quantification of DK2-bound Myocd shown in E. *, P<0.05 vs vehicle-treated group, n=3.
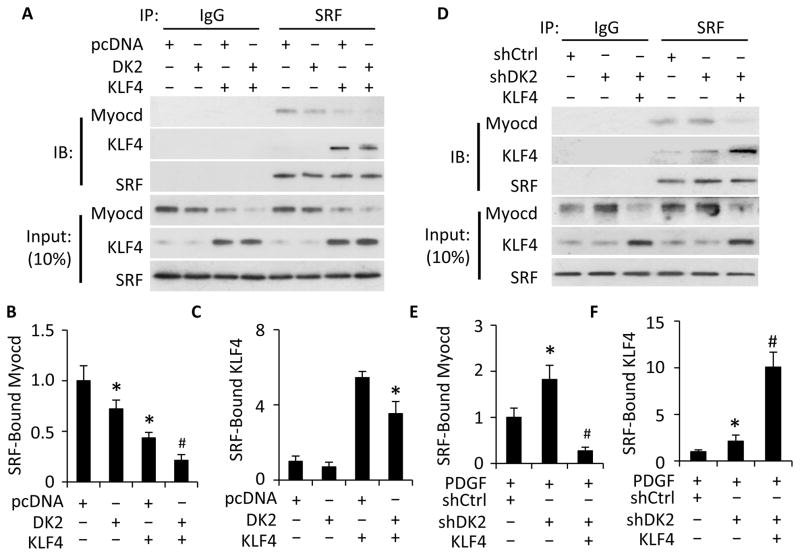
DOCK2 and KLF4 cooperatively blocked Myocd-SRF interaction
KLF4 is found to be a critical factor in modulating PDGF-BB-induced SMC phenotypic modulation 29. Since both KLF4 and DOCK2 modulates SMC phenotype by blocking Myocd-SRF interaction 29, we sought to determine if KLF4 and DOCK2 work together to regulate Myocd-SRF interaction. As shown in Fig. 5A–5B, individual expression of either DOCK2 or KLF4 significantly attenuated Myocd-SRF binding. Co-expression of DOCK2 and KLF4 further and almost completely blocked the Myocd-SRF interaction (Fig. 5A–5B), suggesting that DOCK2 and KLF4 cooperatively regulate Myocd-SRF interaction in SMC phenotypic modulation. In addition, knockdown of DOCK2 enhanced Myocd-SRF interaction. But this effect was blocked by the overexpression of KLF4 (Fig. 5D–5E), probably due to a deprivation of SRF by a strong interaction between KLF4 and SRF, as shown in Fig. 5A and previous findings 30. KLF4 appeared to compete with Myocd for binding to SRF because the KLF4-SRF binding was significantly increased while Myocd-SRF binding was diminished (Fig. 5D–5F). Interestingly, the KLF4-SRF interaction was regulated by DOCK2 because overexpression of DOCK2 attenuated KLF4 binding to SRF (Fig. 5A and 5C) while knockdown of DOCK2 increased KLF4-SRF binding (Fig. 5D and 5F). This effect of DOCK2 was likely attributed to the role of DOCK2 in SRF nuclear location (Fig. 3A–3B). Collectively, these data indicate that DOCK2 can block both the Myocd-SRF interaction and KLF4-SRF interaction in phenotype modulated SMCs.
Figure 5. DOCK2 and KLF4 cooperatively inhibited Myocd-SRF binding.
A, Co-expression of DOCK2 (DK2) and KLF4 completely blocked Myocd-SRF binding. SMCs were co-transfected with pcDNA, DK2, and/or KLF4 expression plasmids as indicated followed by serum starvation for 24 h. Cell lysates were immunoprecipitated (IP) with IgG and SRF antibody. The immunoprecipitates were blotted (IB) with Myocd, KLF4 and SRF antibody as indicated. B, Quantification of SRF-bound Myocd in A. *, P<0.05 vs pcDNA-transfected group (set as 1); #, P<0.05 vs all other groups (n=3). C, Quantification of SRF-bound KLF4 in A. *, P<0.05 vs pcDNA/KLF4 plasmid-transfected group, n=3. D, KLF4 blocked DOCK2 knockdown-enhanced Myocd-SRF binding. SMCs were transfected with control (shCtrl) or DK2 shRNA (shDK2) for 48 h followed by transfection of pcDNA (−) or KLF4 expression plasmid (+) as indicated and PDGF-BB treatment for 24 h. Cell lysates were Co-IP with IgG and SRF antibody. The precipitated proteins were blotted (IB) with Myocd, KLF4 and SRF antibody as indicated. E, Quantification of SRF-bound Myocd in D. *, P< 0.05 compared with shCtrl group (set as 1); #, P<0.05 vs shDK2-treated cells without KLF4 plasmid (n=3). F. Quantification of SRF-bound KLF4 in D. *, P< 0.05 compared with shCtrl group (set as 1); #, P<0.05 compared other two groups (n=3).
DOCK2 expression was activated during injury-induced SMC phenotypic modulation and was essential for injury-induced vascular remodeling
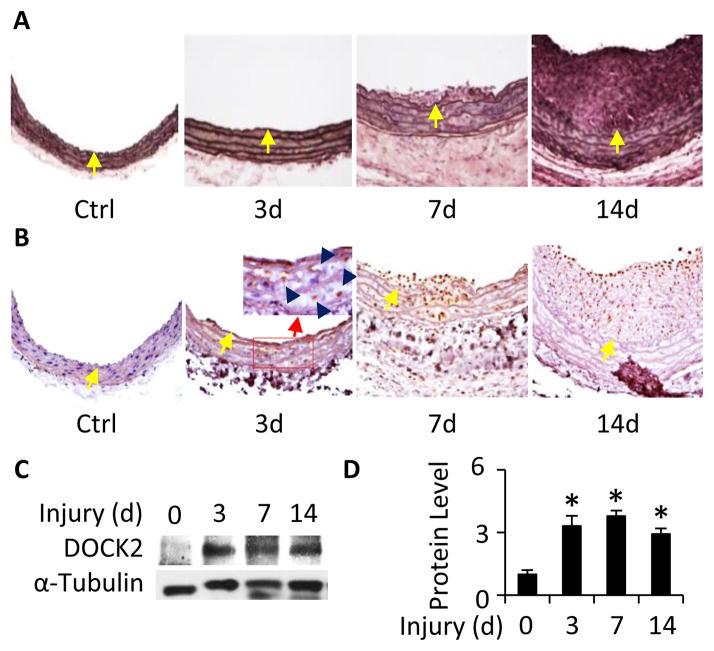
Mechanical injury to artery causes phenotypic modulation of medial layer SMC followed by neointimal formation. DOCK2 is clearly important for PDGF-BB-induced SMC phenotypic modulation in vitro. To determine if DOCK2 is involved in SMC phenotypic modulation and vascular remodeling in vivo, we used balloon catheter to mechanically injure rat carotid artery. As shown in Figure 6A, balloon injury induced a progressive neointima formation, consistent with previous reports 31. DOCK2 expression is barely detectable in SMCs of the normal artery, but was highly induced in the medial layer SMCs 3 days after the injury (Fig. 6B), a time when SMC phenotypic modulation occurs without evident neointima formation. 7 or 14 days after the injury, DOCK2 was expressed in the neointimal SMCs (Fig. 6B). Notably, strong DOCK2 expression was mainly observed in the neointimal SMCs near or on the luminal surface of arteries with 14 days of injury (Figs. 6B and 7A), consistent with it’s role in modulating SMC phenotype because luminal surface SMCs exhibit de-differentiated state while SMCs in other neointima area start to re-differentiate at this stage 32. Time course quantitative analysis of DOCK2 expression revealed that DOCK2 protein was highly induced as early as 3 days after the injury (Fig. 6C–6D), indicating that DOCK2 may be involved in vascular lesion formation.
Figure 6. Balloon injury induced DOCK2 expression in tunica media and neointima SMCs.
A, Balloon injury induced progressive neointima formation. Rat left carotid arteries were injured for 3, 7, and 14 days as indicated. The right non-injured carotid arteries were used as controls (Ctrl). Artery sections were stained with Elastica van Gieson solution. Yellow arrows indicate the internal elastin lamina. B, DOCK2 was induced in tunica media initially and neointima VSMCs subsequently following injury. Artery sections were incubated with DOCK2 antibody followed by horseradish peroxidase-conjugated secondary antibody and DAB staining. Dark blue arrows in the enlarged image of the red box in the 3d sections show representative SMCs expressing DOCK2. Yellow arrows indicate the internal elastin lamina. C, DOCK2 expression in injured arteries was detected by western blot. Data shown are a representative result of 3 independent experiments. D, Quantification of DOCK2 expression by normalizing to α-Tubulin. *, P<0.05 compared to uninjured arteries (0 d).
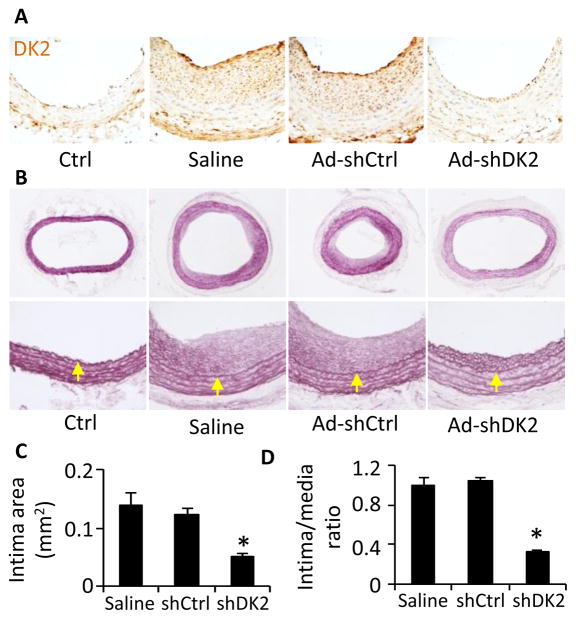
Figure 7. Knockdown of DOCK2 blocked neointima formation.
A, DOCK2 expression was efficiently blocked by adenovirus delivery of DOCK2 shRNA. Immediately after balloon injury, the injured rat carotid arteries were incubated with sterile saline solution, adenovirus expressing scramble (Ad-shCtrl), or DOCK2 shRNA (Ad-shDK2) as indicated. 14 days later, artery sections were stained with DOCK2 antibody. DOCK2 (DK2) expression was visualized by DAB staining. B, DOCK2 knockdown blocked injury-induced neointima formation. Artery sections were stained with Elastica van Gieson solution. Yellow arrows indicate internal elastic lamina. C and D, Quantification of intima area and intima/media ratio. *, P<0.05 compared with saline- or Ad-shCtrl-treated arteries (n=6).
To test if DOCK2 plays a role in balloon injury-induced vascular remodeling, we used adenovirus to deliver DOCK2 shRNA to the injured arteries. As shown in Figure 7A, DOCK2 shRNA effectively blocked DOCK2 expression in neointima SMCs. Saline or control shRNA did not affect the injury-induced neointima formation. However, knockdown of DOCK2 dramatically blocked the neointima formation (Fig. 7B). Morphometric quantification of elastic-stained sections showed that DOCK2 shRNA inhibited the neointima formation by 60% (0.050±0.006 versus 0.123±0.011 mm2; P<0.01, n=6, Fig. 7C). The Intima/Media area ratios (Fig. 7D) showed similar results as in Figure 7C. These results demonstrate that DOCK2 is a novel regulator essential for injury-induced vascular remodeling.
Knockout of DOCK2 in mice blocked ligation injury-induced intimal hyperplasia and SMC phenotypic modulation
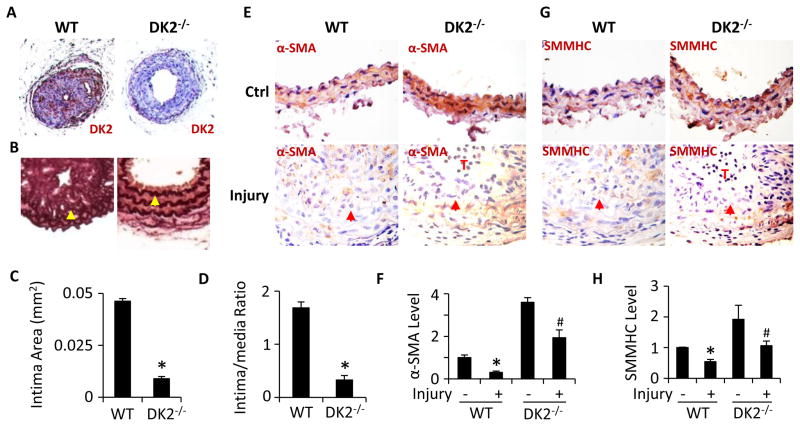
Adenovirus-mediated shRNA delivery approach may not be able to fully reveal DOCK2 function in vascular remodeling because adenoviral vector can only modify DOCK2 expression in cells with a direct contact with the virus. Therefore, we used DOCK2−/− mice and carotid artery ligation injury model to further confirm the role of DOCK2 in vascular remodeling. Mouse carotid artery ligation injury elicits a remodeling response similar to rat carotid artery balloon injury although the small size of the vessel and thrombus often limit the sample size for molecular analysis. In addition, the degree of neointimal development in various distance from the ligature may cause variations in the measurements of the response. These variations can be eliminated by comparing the neointima development in areas with the same distance from the ligature among mice with artery ligation. As shown in Figure 8, knockout of DOCK2 (Fig. 8A) dramatically inhibited ligation injury-induced neointima formation (Fig. 8B). Morphometric quantification of the elastic-stained sections showed that the neointima was reduced by 80% in carotid arteries of DOCK2−/− mice as compared with the WT mice (0.009±0.001 versus 0.048±0.001 mm2; P<0.01, n=6, Fig. 8C). The Intima/Media area ratios (Fig. 8D) showed similar results as in Figure 8C. These data further demonstrate a critical role of DOCK2 in injury-induced vascular remodeling.
Figure 8. DOCK2 deficiency attenuated injury-induced neointima formation and SMC phenotypic modulation in mouse arteries.
A, Ligation injury induced DOCK2 expression in wild type (WT), but not DOCK2 knockout (DK2−/−) mouse carotid arteries. Mouse left carotid arteries were injured by ligation for 28 days. DOCK2 (DK2) expression was detected by immunohistochemistry (IHC) staining using DK2 antibody and DAB visulization. B, DK2 knockout blocked ligation-induced neointima formation. Artery sections were stained with Elastica van Gieson solution. Arrows indicate internal elastic lamina. C-D, Quantification of intima area (C) and intima/media ratio (D) of the injured arteries. *, P<0.05 vs WT mouse carotid arteries with ligation (n=6). E-H, DK2−/− increased α-SMA (E) and SMMHC (G) expression in both control (Ctrl) and ligation-injured arteries. α-SMA and SMMHC was detected by IHC staining using α-SMA (E) and SMMHC (G) antibodies followed by DAB visualization. Arrows indicate internal elastic lamina. T indicates the thrombus in injured DK2−/− arteries. α-SMA (F) and SMMHC (H) expression was quantified by normalizing to the DAB intensity in the WT Ctrl arteries (set as 1). *, P<0.05 vs WT arteries without injury (−); #, P<0.05 vs WT arteries with injury for each individual proteins (n=6).
To determine if DOCK2−/− affects injury-induced SMC phenotypic modulation in vivo, we detected α-SMA and SMMHC expression in WT and DOCK2−/− mouse carotid arteries with or without ligation injury. As shown in Fig. 8E–8H, in control arteries without ligation, DOCK2−/− increased α-SMA and SMMHC expression. Injury caused a down regulation of both α-SMA and SMMHC in WT mouse arteries. However, DOCK2−/− increased the α-SMA and SMMHC expression as compared to the injured WT arteries (Fig. 8E–8H), suggesting that DOCK2 mediates the injury-induced SMC phenotypic modulation in vivo.
DISCUSSION
In the present study, we have identified DOCK2 as a novel regulator in SMC phenotypic modulation and vascular remodeling. DOCK2 is scarcely detectable in normal SMCs. However, it is induced during SMC phenotypic modulation induced by PDGF-BB or serum in vitro and vascular injury in vivo. DOCK2 deficiency increased the expression of SMC marker proteins down-regulated by PDGF-BB or serum in vitro and by vascular injury in vivo, demonstrating that DOCK2 promotes SMC phenotypic modulation, leading to vascular remodeling/neointima formation. Interestingly, DOCK2 knockout in normal SMCs also causes a dramatic increase of contractile proteins, suggesting that a low level of DOCK2 in quiescent SMCs may be necessary for maintaining the proper level of contractile proteins while excessive DOCK2 expression results in SMC phenotype alteration, similar to the treatment with PDGF-BB or serum. Importantly, vascular injury induces DOCK2 expression initially in medial SMCs and subsequently neointima SMCs. The initial expression of DOCK2 in medial SMCs is likely attributed to the SMC phenotypic modulation in injured arteries because at this time (1–3 days after injury), SMCs are responding to injury-triggered serum factors such as PDGF-BB to modulate its phenotype. The absence of intimal hyperplasia during the initial expression of DOCK2 also suggests that DOCK2 may be transforming SMCs from a contractile to a synthetic phenotype at this stage. Blocking the initial expression of DOCK2 immediately after injury using adenovirus-delivered shRNA effectively blocks injury-induced neointima formation, indicating that DOCK2-induced SMC phenotype modulation is essential for injury-induced vascular remodeling. The definitive role of DOCK2 in vascular remodeling and SMC phenotypic modulation in vivo is supported by the blockade of intimal hyperplasia and increased expression of SMC contractile proteins in ligation-injured DOCK2−/− mouse arteries.
DOCK2 appears to modulate SMC phenotype by inhibiting Myocd/SRF-mediated SMC marker gene transcription. DOCK2 regulates Myocd/SRF function via inhibiting Myocd expression, SRF nuclear location and Myocd-SRF interaction. DOCK2 may inhibit Myocd expression by regulating Myocd transcription similar to SMC marker genes because DOCK2 is a nuclear factor. DOCK2 blocks SRF nuclear translocation probably via an indirect mechanism because DOCK2 does not directly interact with SRF. These mechanisms may include SRF nuclear transporting or SRF exporting machinery. Although the mechanisms by which DOCK2 inhibits Myocd expression and SRF nuclear location require further investigation, DOCK2 is likely to block Myocd-SRF interaction through diminishing the availability of Myocd for SRF binding because DOCK2 inhibits Myocd expression as well as directly binding to Myocd. The combined effects of DOCK2 on Myocd expression, SRF nuclear location and Myocd-SRF interaction make DOCK2 a powerful regulator mediating SMC phenotypic modulation.
DOCK2 and KLF4 appear to cooperatively mediate SMC phenotypic modulation because co-expression of DOCK2 and KLF4 has a greater effect in reducing Myocd-SRF interaction comparing to each of the individual effect. KLF4 is known to inhibit SMC marker expression and modulate SMC phenotype also by inhibiting Myocd expression and Myocd-SRF interaction. However, the mechanisms underlying DOCK2 and KLF4 are different. DOCK2 interacts with Myocd, but not SRF to attenuate Myocd-SRF interaction while KLF4 interacts with SRF, but not Myocd to block Myocd-SRF binding. DOCK2 also inhibits KLF4 binding to SRF (Fig. 5), which is likely due to the role of DOCK2 in blocking SRF nuclear translocation (Fig. 3A–3B). Since PDGF-BB induces the expression of both DOCK2 and KLF4, the cooperative action of DOCK2 and KLF4 may be required for suppressing the strong activity of Myocd in SMC marker expression, leading to a successful phenotypic modulation.
The present study has used both rat balloon carotid artery injury model and mouse carotid artery ligation model to study the role of DOCK2 in vascular remodeling. The mechanisms underlying the balloon injury-induced remodeling are not identical to that of ligation injury. Balloon catheter completely denudes endothelium, which triggers the production of inflammatory factors such as PDGF-BB 21. Ligation injury maintains intact endothelium initially although the endothelium may be partially detached by persistent vessel constriction 19. The near-stasis conditions in the ligated vessel may prompt significant inflammatory response 19. Balloon injury-induced vascular response resembles the remodeling of vascular structures in restenosis after angioplasty while ligation injury-mediated remodeling partially recapitulates the development of intimal lesions in human vascular diseases that occurs in the absence of noticeable endothelial denudation. Knockdown or knockout of DOCK2 blocks neointima formation in both the balloon- and ligation-injured arteries suggest that DOCK2 mediates a key mechanism, i.e., the SMC phenotypic modulation, which is shared by these two different vascular responses.
Taken together, our study has identified DOCK2 as a novel regulator for SMC phenotypic modulation and vascular remodeling. Blocking DOCK2 expression, therefore, may be an effective approach to treat proliferative vascular disorders. Since DOCK2 is also functionally involved in inflammation, an essential process contributing to neointima formation, targeting DOCK2 may hamper SMC phenotypic modulation and inflammation simultaneously to achieve a better vascular repair.
Supplementary Material
Novelty and Significance.
What Is Known?
Smooth muscle cell (SMC) phenotypic modulation is an essential process initiating vascular remodeling/neointima formation in proliferative vascular diseases.
Myocardin (Myocd)-serum response factor (SRF) interaction is critical for maintaining the contractile SMC phenotype
Kruppel-like factor 4 (KLF4) blocks Myocd-SRF interaction by binding to SRF, resulting in SMC phenotypic modulation.
What New Information Does This Article Contribute?
Dedicator of cytokinesis 2 (DOCK2) is a novel protein factor regulating SMC phenotypic modulation and vascular remodeling. Deletion of DOCK2 blocks injury-induced vascular remodeling while restoring SMC contractile protein expression.
DOCK2 modulates SMC phenotype by inhibiting Myocd expression, blocking SRF nuclear localization, attenuating Myocd binding to SRF, and consequently diminishing smooth muscle marker promoter activity.
DOCK2 and KLF4 cooperatively modulate SMC phenotype. Unlike KLF4 that binds to SRF, DOCK2 attenuates Myocd-SRF interaction by binding to Myocd.
Prior studies have shown that SMC phenotypic modulation plays an important role in injury-induced vascular remodeling, but the mechanisms underlying this process remain incompletely elucidated. Here, we used a DOCK2 knockout mouse model as well as molecular and cellular analyses to identify a novel mechanism underlying SMC phenotypic modulation. Our studies demonstrate for the first time that DOCK2 is a novel regulator for SMC phenotype modulation and injury-induced vascular remodeling. DOCK2 modulates SMC phenotype by decreasing Myocd expression, blocking SRF nuclear localization, and attenuating Myocd binding to SRF, thus inhibiting Myocd-induced SMC marker promoter activity. Importantly, our studies indicate that mechanisms underlying DOCK2 and KLF4 functions are different. DOCK2 interacts with Myocd, but not SRF, to attenuate Myocd-SRF interaction, while KLF4 interacts with SRF, but not Myocd, to block Myocd-SRF binding. In a rat carotid artery balloon-injury model, DOCK2 is induced in medial SMC initially and neointimal SMC subsequently following vascular injury. Knockdown of DOCK2 inhibits the neointima formation by 60%. Most importantly, knockout of DOCK2 in mice markedly blocks ligation-induced intimal hyperplasia while restoring SMC contractile protein expression, demonstrating a critical role of DOCK2 in SMC phenotypic modulation in vivo.
Acknowledgments
SOURCES OF FUNDING
This work was supported by grants from National Institutes of Health (HL123302, HL119053, and HL107526) and National Natural Science Foundation of China (No. 81328002). X. G. was a recipient of American Heart Association Pre-doctoral Fellowship.
Nonstandard Abbreviations and Acronyms
- SMC
Smooth muscle cell
- PDGF-BB
Platelet-derived growth factor
- DOCK2
dedicator of cytokinesis 2
- SRF
serum response factor
- α-SMA
smooth muscle α-actin
- shRNA
short hairpin RNA
- H&E
hematoxylin and eosin
- PFA
paraformaldehyde
- IHC
immunohistochemistry
- FBS
fetal bovine serum
Footnotes
DISCLOSURE
None.
References
- 1.Schwartz SM, deBlois D, O’Brien ER. The intima. Soil for atherosclerosis and restenosis. Circulation research. 1995;77:445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- 2.Neitzel GF, Barboriak JJ, Pintar K, Qureshi I. Atherosclerosis in aortocoronary bypass grafts. Morphologic study and risk factor analysis 6 to 12 years after surgery. Arteriosclerosis. 1986;6:594–600. doi: 10.1161/01.atv.6.6.594. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz SM. Smooth muscle migration in atherosclerosis and restenosis. The Journal of clinical investigation. 1997;100:S87–89. [PubMed] [Google Scholar]
- 4.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: New perspectives and therapeutic strategies. Nature medicine. 2002;8:1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 5.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological reviews. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 6.Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovascular research. 2012;95:156–164. doi: 10.1093/cvr/cvs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishihara H, Kobayashi S, Hashimoto Y, Ohba F, Mochizuki N, Kurata T, Nagashima K, Matsuda M. Non-adherent cell-specific expression of dock2, a member of the human cdm-family proteins. Biochimica et biophysica acta. 1999;1452:179–187. doi: 10.1016/s0167-4889(99)00133-0. [DOI] [PubMed] [Google Scholar]
- 8.Fukui Y, Hashimoto O, Sanui T, Oono T, Koga H, Abe M, Inayoshi A, Noda M, Oike M, Shirai T, Sasazuki T. Haematopoietic cell-specific cdm family protein dock2 is essential for lymphocyte migration. Nature. 2001;412:826–831. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 9.Jiang H, Pan F, Erickson LM, Jang MS, Sanui T, Kunisaki Y, Sasazuki T, Kobayashi M, Fukui Y. Deletion of dock2, a regulator of the actin cytoskeleton in lymphocytes, suppresses cardiac allograft rejection. The Journal of experimental medicine. 2005;202:1121–1130. doi: 10.1084/jem.20050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka Y, Hamano S, Gotoh K, Murata Y, Kunisaki Y, Nishikimi A, Takii R, Kawaguchi M, Inayoshi A, Masuko S, Himeno K, Sasazuki T, Fukui Y. T helper type 2 differentiation and intracellular trafficking of the interleukin 4 receptor-alpha subunit controlled by the rac activator dock2. Nature immunology. 2007;8:1067–1075. doi: 10.1038/ni1506. [DOI] [PubMed] [Google Scholar]
- 11.Kunisaki Y, Nishikimi A, Tanaka Y, Takii R, Noda M, Inayoshi A, Watanabe K, Sanematsu F, Sasazuki T, Sasaki T, Fukui Y. Dock2 is a rac activator that regulates motility and polarity during neutrophil chemotaxis. The Journal of cell biology. 2006;174:647–652. doi: 10.1083/jcb.200602142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotoh K, Tanaka Y, Nishikimi A, Nakamura R, Yamada H, Maeda N, Ishikawa T, Hoshino K, Uruno T, Cao Q, Higashi S, Kawaguchi Y, Enjoji M, Takayanagi R, Kaisho T, Yoshikai Y, Fukui Y. Selective control of type i ifn induction by the rac activator dock2 during tlr-mediated plasmacytoid dendritic cell activation. The Journal of experimental medicine. 2010;207:721–730. doi: 10.1084/jem.20091776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golovina VA, Blaustein MP. Preparation of primary cultured mesenteric artery smooth muscle cells for fluorescent imaging and physiological studies. Nature protocols. 2006;1:2681–2687. doi: 10.1038/nprot.2006.425. [DOI] [PubMed] [Google Scholar]
- 14.Wang JN, Shi N, Xie WB, Guo X, Chen SY. Response gene to complement 32 promotes vascular lesion formation through stimulation of smooth muscle cell proliferation and migration. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:e19–26. doi: 10.1161/ATVBAHA.111.230706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo X, Jose PA, Chen SY. Response gene to complement 32 interacts with smad3 to promote epithelial-mesenchymal transition of human renal tubular cells. American journal of physiology. Cell physiology. 2011;300:C1415–1421. doi: 10.1152/ajpcell.00204.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo X, Stice SL, Boyd NL, Chen SY. A novel in vitro model system for smooth muscle differentiation from human embryonic stem cell-derived mesenchymal cells. American journal of physiology. Cell physiology. 2013;304:C289–298. doi: 10.1152/ajpcell.00298.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F, Luo Z, Huang W, Lu Q, Wilcox CS, Jose PA, Chen S. Response gene to complement 32, a novel regulator for transforming growth factor-beta-induced smooth muscle differentiation of neural crest cells. The Journal of biological chemistry. 2007;282:10133–10137. doi: 10.1074/jbc.C600225200. [DOI] [PubMed] [Google Scholar]
- 18.Shi N, Guo X, Chen SY. Olfactomedin 2, a novel regulator for transforming growth factor-beta-induced smooth muscle differentiation of human embryonic stem cell-derived mesenchymal cells. Molecular biology of the cell. 2014 doi: 10.1091/mbc.E14-08-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arteriosclerosis, thrombosis, and vascular biology. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 20.Cui XB, Guo X, Chen SY. Response gene to complement 32 deficiency causes impaired placental angiogenesis in mice. Cardiovascular research. 2013;99:632–639. doi: 10.1093/cvr/cvt121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marmur JD, Poon M, Rossikhina M, Taubman MB. Induction of pdgf-responsive genes in vascular smooth muscle. Implications for the early response to vessel injury. Circulation. 1992;86:III53–60. [PubMed] [Google Scholar]
- 22.Miano JM, Long X, Fujiwara K. Serum response factor: Master regulator of the actin cytoskeleton and contractile apparatus. American journal of physiology. Cell physiology. 2007;292:C70–81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: A component of a molecular switch for smooth muscle differentiation. Journal of molecular and cellular cardiology. 2002;34:1345–1356. doi: 10.1006/jmcc.2002.2086. [DOI] [PubMed] [Google Scholar]
- 24.Du KL, Ip HS, Li J, Chen M, Dandre F, Yu W, Lu MM, Owens GK, Parmacek MS. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Molecular and cellular biology. 2003;23:2425–2437. doi: 10.1128/MCB.23.7.2425-2437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan-Albuquerque N, Garat C, Desseva C, Jones PL, Nemenoff RA. Platelet-derived growth factor-bb-mediated activation of akt suppresses smooth muscle-specific gene expression through inhibition of mitogen-activated protein kinase and redistribution of serum response factor. The Journal of biological chemistry. 2003;278:39830–39838. doi: 10.1074/jbc.M305991200. [DOI] [PubMed] [Google Scholar]
- 26.Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 27.Nishikimi A, Uruno T, Duan X, Cao Q, Okamura Y, Saitoh T, Saito N, Sakaoka S, Du Y, Suenaga A, Kukimoto-Niino M, Miyano K, Gotoh K, Okabe T, Sanematsu F, Tanaka Y, Sumimoto H, Honma T, Yokoyama S, Nagano T, Kohda D, Kanai M, Fukui Y. Blockade of inflammatory responses by a small-molecule inhibitor of the rac activator dock2. Chemistry & biology. 2012;19:488–497. doi: 10.1016/j.chembiol.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Pipes GC, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: Versatile regulators of cell growth, migration, and myogenesis. Genes & development. 2006;20:1545–1556. doi: 10.1101/gad.1428006. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circulation research. 2005;96:280–291. doi: 10.1161/01.RES.0000155951.62152.2e. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. The Journal of biological chemistry. 2005;280:9719–9727. doi: 10.1074/jbc.M412862200. [DOI] [PubMed] [Google Scholar]
- 31.Tulis DA. Rat carotid artery balloon injury model. Methods in molecular medicine. 2007;139:1–30. doi: 10.1007/978-1-59745-571-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei GL, Krasinski K, Kearney M, Isner JM, Walsh K, Andres V. Temporally and spatially coordinated expression of cell cycle regulatory factors after angioplasty. Circulation research. 1997;80:418–426. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.