Abstract
BACKGROUND
Parabens are used as antimicrobial excipients in some pharmaceuticals. Parabens may adversely affect reproduction.
OBJECTIVES
Determine whether paraben-containing medication contributes to high urinary paraben concentrations.
METHODS
Individuals at a fertility clinic provided multiple urine samples during evaluation/treatment and reported 24-hour use of medications and personal care products (PCP). Repeated measures models compared specific gravity-adjusted urinary methyl, propyl, and butyl paraben concentrations between samples “exposed” and “unexposed” to paraben-containing medication.
RESULTS
Eleven participants contributed 12 exposed and 45 unexposed samples, among which paraben concentrations did not differ. Use within seven hours was associated with 8.7-fold and 7.5-fold increases in mean methyl (P=0.11) and propyl (P=0.10) paraben concentrations, respectively, after adjusting for PCP use. However, these associations decreased to 1.3-fold (P=0.76) and 2.6-fold (P=0.34), respectively, after removal of one influential individual.
CONCLUSION
Paraben-containing medications contributed to higher urinary paraben concentrations within hours of use.
INTRODUCTION
Parabens are esters of p-hydroxybenzoic acid that are used as antimicrobial preservatives in cosmetics, food packaging, and pharmaceuticals.1, 2, 3 Parabens are often used in combination, and regulations in the European Union mandate that mixtures of parabens may be used in concentrations up to 0.8% in cosmetic products, while a single paraben may be used in concentrations of up to 0.4%.4 The Cosmetic Ingredient Review, which is overseen by the U.S. Food and Drug Administration (FDA), recommends that manufacturers adhere to these levels, though they are not requirements.2
The U.S. population is widely exposed to parabens. The National Report on Human Exposure to Environmental Chemicals is a report issued by the Centers for Disease Control and Prevention (CDC) that uses samples from the National Health and Nutrition Examination Survey (NHANES) to quantify exposure among a nationally-representative segment of the population. Using data from NHANES 2005–2006, methyl and propyl paraben were detected in 99.1% and 92.7% of participants, respectively, while butyl paraben was detected in 40% of participants.5
Humans may be exposed to environmental chemicals through ingestion, inhalation, and dermal absorption, and while dermal absorption may be the most important route of paraben exposure due to their widespread use in personal care products,2 ingestion may be another important route. It has been established that pharmaceuticals can be important sources of environmental chemical exposure. This was first reported in 2004 for an individual from our fertility clinic population who was taking a pharmaceutical containing mesalamine (Asacol; Warner Chilcott Pharmaceuticals Inc, Mason, OH) and had urinary monobutyl phthalate concentrations that were approximately 1,000-fold greater than the median of the U.S. population sampled by NHANES.6 This finding was further investigated in our fertility clinic cohort for individuals using mesalamine-containing as well as other phthalate-containing medications. We reported monobutyl phthalate concentrations that were 200 times higher among participants who had used phthalate-containing medications compared to individuals who had not.7 Additionally, high urinary phthalate concentrations have been found among patients using pancreatic enzyme products for cystic fibrosis.8
In pharmaceuticals, parabens are excipients (inactive ingredients), which may be used as antimicrobial preservatives in various pharmaceutical formulations including oral solid dosage forms, as well as parenteral and topical preparations.9 While drug products with clinically-studied levels of active ingredients are approved by the FDA, individual excipients themselves are not technically ‘approved.’ Parabens are included within a class of compounds referred to as ‘generally recognized as safe’ (GRAS), which are exempt from the usual tolerance requirements in the Federal Food, Drug, and Cosmetic Act.10 A list of several hundred substances that were recognized as safe by appropriately qualified experts was developed in 1958, and substances that were already in use and that had no recognized adverse effects were allowed to remain in use and were not required to undergo any form of scientific testing for safety.11, 12
Despite the lack of required testing for GRAS products, the FDA maintains a database of inactive ingredients that have been used in approved drug products that includes the maximum approved potencies for various formulations. Butyl paraben has been approved for use at a maximum potency of 0.04 mg in a sustained action tablet formulation and 0.016% in an oral solution.13 Methyl paraben has been approved at a maximum potency of 1.8 mg in a tablet formulation and 0.15% in an oral solution, while propyl paraben has been approved for use at a maximum potency of 0.22 mg in a sustained action tablet formulation and 10% in an oral solution.13 Over-the-counter (OTC) medications are generally not approved by the FDA, and thus determining their inactive ingredients is more difficult, though they may contain the same excipients found in prescription medications. While most OTC medications list all inactive ingredients on their label, the level and type of excipient can change without notice or approval. Despite parabens being used as excipients in pharmaceuticals, to our knowledge, urinary paraben concentrations following recent ingestion of paraben-containing medications have not been examined. The aim of this study was to quantify urinary paraben concentrations in reproductive age men and women within 24 hours of self-reported use of a paraben-containing medication, and to further stratify by timing of medication use. These concentrations were compared to urine concentrations from periods when these same subjects did not report taking a paraben-containing medication.
METHODS
Study Participants
This sub-analysis included participants enrolled in a larger ongoing prospective study of women and men undergoing treatment at a fertility clinic. This cohort has been previously described in detail.14, 15 Briefly, women age 18–45 years and men age 18–51 years were enrolled either individually or as couples from the Massachusetts General Hospital (MGH) Fertility Center in the Environment and Reproductive Health Study (EARtH Study). Participants were followed from enrollment until they had a live birth or discontinued treatment at the MGH Fertility Center. All participants, regardless of treatment modality (i.e., in vitro fertilization (IVF), intrauterine insemination (IUI), or timed intercourse), were eligible for inclusion in this analysis. This study was approved by the institutional review boards at the MGH Fertility Center, the Harvard School of Public Health, and the Centers for Disease Control and Prevention (CDC).
Exposure and Outcome Measurements
Women provided two urine samples during each of their treatment cycles; one sample was provided during the monitoring phase of the cycle, and one sample was provided at the time of oocyte retrieval for those undergoing IVF or insemination for those undergoing IUI. Men provided one urine sample at the time of oocyte retrieval or insemination at each treatment cycle. Urine was collected in a clean polypropylene specimen cup. Specific gravity (SG) was measured at room temperature using a handheld refractometer (National Instrument Co. Inc., Baltimore, MD) calibrated with deionized water before each measurement. Urine samples were divided into aliquots before being frozen and stored at −80°C. Samples were then shipped overnight on dry ice to the CDC, where they were stored at ≤ −40° C until blinded analysis. The urinary concentration of free plus conjugated paraben species (i.e., total concentration) was measured using online solid phase extraction coupled to isotope dilation–high performance liquid chromatography–tandem mass spectrometry as described previously.16 Each analytical run included calibration standards, reagent blanks, and quality control materials of high and low concentration. Metabolite concentrations below the limit of detection (LOD) were assigned a value of the LOD divided by the square root of two.17 The LODs for methyl, propyl, and butyl paraben were 1.0 ng/ml, 0.2 ng/ml, and 0.2 ng/ml, respectively. Ethyl paraben was not measured due to its relatively low frequency of detection in the NHANES population.5
Starting in October 2010, participants completed a questionnaire at the time of each urine sample that included a detailed assessment of recent medication use. Participants were asked to answer the question ‘in the past 24 hours, have you taken any medications, vitamins, or supplements?’ Participants who answered ‘yes’ to this question were then asked to provide information about the medication, vitamin, or supplements they used, as well as the day (i.e., yesterday or today) and time of use. A list of medications that potentially contained parabens was developed a priori (Supplemental Table 1) using several sources,18, 19, 20, 21, 22 and all samples from any participant who reported using one or more of these medications was eligible for inclusion in the analysis. Urine samples from periods in which a participant answered yes to the question ‘in the past 24 hours, have you taken any medications, vitamins, or supplements?’ and who reported using a paraben-containing medication were considered ‘exposed urine samples’; urine samples in which participants answered ‘no’ to this question or who reported using medications that did not contain parabens were considered ‘unexposed urine samples’. Participants were included if they had at least one exposed and one unexposed urine sample. Participants also reported their use of personal care products over the previous 24 hours, and we created two variables to quantify personal care product use and its contribution to paraben exposure. The first variable was simply a count of the number of products used. The second variable was an indicator variable for whether the participant had used lotion, cosmetics, cologne/perfume, or hair gel, as users of these products were previously found to have urinary paraben concentrations 41–221% higher than non-users.23 Brand names of the personal care products were not collected on our questionnaires, and therefore we were unable to assign paraben concentrations to specific product classes.
Statistical Analysis
SG-adjusted urinary paraben concentrations were examined for all exposed samples as well as restricted to samples in which the paraben-containing medication was used on the day the urine sample was provided; these urinary concentrations were compared to concentrations from unexposed samples. Generalized estimating equations (GEE) were used to evaluate the associations between exposure status and natural log-transformed urinary methyl, propyl, and butyl paraben concentrations accounting for multiple samples per participant. Because 33% of all butyl paraben measurements were below the LOD, the proportions of detectable butyl paraben concentrations were also compared between exposed and unexposed samples using GEE models accounting for multiple samples per participant. Model-based geometric means were obtained by back-transforming GEE estimates, both unadjusted and adjusted for number of personal care products used. All analyses were conducted with SAS 9.3 (SAS Institute, Cary, North Carolina), and two-sided P-values <0.05 were considered statistically significant.
RESULTS
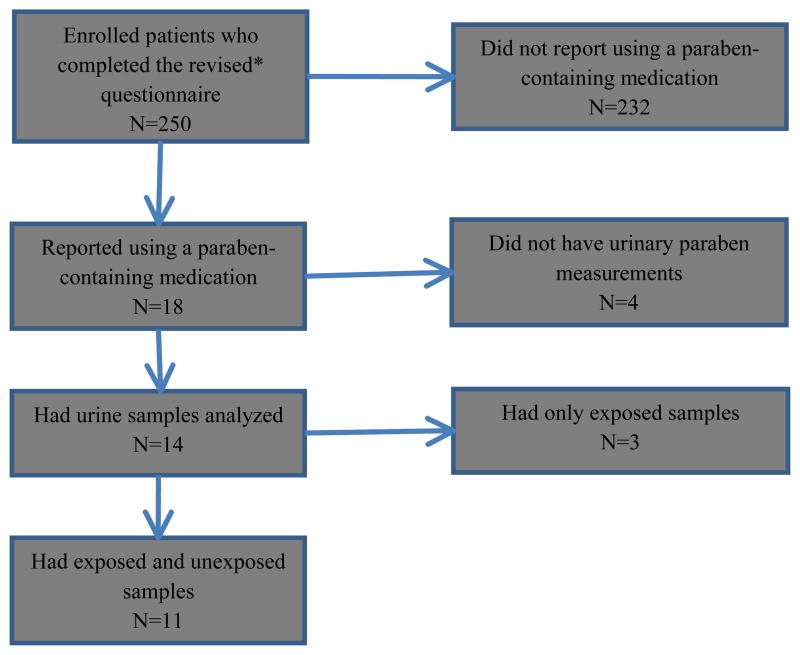
From October 15, 2010 to March 15, 2013, a total of 250 enrolled participants completed a modified medication questionnaire at the time of their urine sample, and these subjects contributed 827 urine samples. Of these 250 subjects, 18 participants (7.2%) reported using a paraben-containing medication in the prior 24 hours. Of these 18, four participants (22%) did not have urinary paraben concentration measures because they either declined to provide a urine sample (n=3) or because their urine sample had not yet been analyzed (n=1). Of the remaining 14 participants, three (21%) were excluded for having no unexposed samples. A flow-chart depicting the study population and study exclusions is shown in Figure 1. Ultimately, this analysis consisted of 11 participants, 10 female and one male, who contributed a total of 12 exposed samples and 45 unexposed samples. Individual subjects contributed a median of 3 IVF and/or IUI cycles (IQR: 2–5; range: 1–9).
Figure 1.
Flow-chart of study population
*The revised questionnaire was implemented in 2010 and included detailed questions regarding medication use
The 18 participants with at least one exposed sample were similar in terms of age to the 232 participants with no exposed samples, and while no characteristics differed significantly, exposed participants were more often female, white, and never smokers, and exposed participants had slightly higher body mass index (BMI) than unexposed participants (Table 1). Among the 11 participants included in the analysis, the mean age was 36.1 ± 3.5 years, and all participants were white. When compared to the seven participants who were excluded from the analysis, the 11 included participants were less likely to be ever smokers, and they were also more often female and had a lower BMI compared to excluded participants (Table 2).
Table 1.
Characteristics of participants who did and did not report using paraben-containing medications within 24 hours of providing at least one urine sample
| Subject characteristic | Ever-exposed N=18 |
Unexposed N=232 |
P |
|---|---|---|---|
| Gender | 0.42 | ||
| Female | 15 (83.3) | 169 (72.8) | |
| Male | 3 (16.7) | 63 (27.2) | |
| Age—mean ± SD, years | 36.1 ± 3.5 | 35.8 ± 4.1 | 0.71 |
| BMI—mean ± SD, kg/m2 | 26.5 ± 4.8 | 25.2 ± 4.6 | 0.26 |
| <25 | 7 (38.9) | 123 (53.0) | 0.49 |
| 25 – <30 | 8 (44.4) | 75 (32.3) | |
| ≥30 | 3 (16.7) | 34 (14.7) | |
| Race | 0.63 | ||
| White | 18 (100.0) | 191 (82.3) | |
| Black/African American | 0 (0.0) | 2 (0.9) | |
| Asian | 0 (0.0) | 21 (9.1) | |
| Native American/Alaska Native | 0 (0.0) | 6 (2.6) | |
| Other | 0 (0.0) | 12 (5.2) | |
| Smoking status | 0.40 | ||
| Never | 14 (77.8) | 156 (67.2) | |
| Ever | 4 (22.2) | 76 (32.8) | |
| Former | 3 (16.7) | 70 (30.2) | |
| Current | 1 (5.6) | 6 (2.6) |
SD, standard deviation; BMI, body mass index
Data are presented as n (%), or as mean (±SD) where indicated
Table 2.
Characteristics of included and excluded participants who reported using paraben-containing medications within 24 hours of at least one urine sample
| Subject characteristic | Included N=11 |
Excluded1 N=7 |
P2 |
|---|---|---|---|
| Gender | 0.53 | ||
| Female | 10 (90.9) | 5 (71.4) | |
| Male | 1 (9.1) | 2 (28.6) | |
| Age—mean ± SD | 36.1 ± 3.4 | 36.2 ± 3.8 | 0.95 |
| BMI—mean ± SD | 25.0 ± 3.6 | 28.8 ± 5.7 | 0.11 |
| <25 | 6 (54.5) | 1 (14.3) | 0.24 |
| 25 – <30 | 4 (36.4) | 4 (57.1) | |
| ≥30 | 1 (9.1) | 2 (28.6) | |
| Race | -- | ||
| White | 11 (100.0) | 7 (100.0) | |
| Smoking status | 0.01 | ||
| Never | 11 (100.0) | 3 (42.9) | |
| Ever | 0 (0.0) | 4 (57.1) | |
| Former | -- | 3 (42.9) | |
| Current | -- | 1 (14.3) |
SD, standard deviation; BMI, body mass index
Data are presented as n (%) unless otherwise specified
Participants were excluded due to lack of available urinary paraben concentration measurements (n=4) or due to a lack of unexposed samples (n=3)
P-values were obtained using Student’s t-test for continuous variables and Fisher’s exact test for categorical variables
All included participants answered ‘yes’ to the question ‘in the past 24 hours, have you taken any medications, vitamins, or supplements?’ However, after calculating time elapsed between the time they reported taking the medication and the time they provided their urine sample, for four participants we could not verify that medication use was within 24 hours of the urine sample. Among these four participants, two had times of use that were greater than 24 hours (24.7 and 25.5 hours), but they were retained in the analysis. One of the four participants did not report the day of medication use, and because her elapsed time was unknown (either 2.1 hours or 26.1 hours), she was included in the analysis of all 24-hour periods but was excluded from analyses restricted to same-day medication use. The final of these four participants did not report day or time of use; similarly, this participant was included in the analysis of all samples but excluded from further analyses. Because both the use of medication within the past 24 hours and the day and time of use were self-reported and we were unsure which measure was more likely to be correct, these four participants were retained in the overall analysis, though we did conduct a sensitivity analysis excluding these four participants.
In terms of medications used, three participants reported taking ibuprofen (Advil; Pfizer, Inc., New York, NY) in tablet form, three reported taking diphenhydramine (Benadryl; McNeil Consumer Healthcare McNeil-PPC, Inc., Fort Washington, PA) (two in tablet form and one in gelcap form), two reported taking dextromethorphan and guaifenesin (Robitussin DM; Pfizer Inc., New York, NY) in elixir form, two reported taking fluoxetine in capsule form, and two reported taking Tylenol (McNeil Consumer Healthcare McNeil-PPC, Inc., Fort Washington, PA) (one in gelcap form and one in tablet form). These medications are shown in Table 3. With regards to personal care product use, the mean number of products that participants reported using in the 24-hour period prior to the collection of their urine sample did not significantly differ between exposed (mean=10.3) and unexposed (mean=8.8) samples (P=0.22). No difference was observed in terms of the proportion of participants who reported using lotion, cosmetics, perfume/cologne, or hair gel (83% among exposed samples vs. 71% among unexposed samples; P=0.30).
Table 3.
Paraben concentrations in exposed and unexposed urine samples from 57 urine samples provided within 24 hours of taking or not taking a paraben-containing medication
| Subject ID | Medication* | Number of samples exposed / unexposed | Hours between exposure and urine sample | Methyl paraben (ng/ml) | Propyl paraben (ng/ml) | Butyl paraben (ng/ml) | |||
|---|---|---|---|---|---|---|---|---|---|
| Exposed | Unexposed | Exposed | Unexposed | Exposed | Unexposed | ||||
| 1 | Fluoxetine† | 1 / 1 | 6.33 | 104 | 147 | 54.0 | 138 | 9.3 | 12.9 |
| 2 | Dextromethorphan and guaifenesin† | 1 / 7 | 0.75 | 5,850 | 16.4 (5.8 – 101) | 974 | 6.0 (1.6 – 41.1) | 22.7 | 0.7 (0.3 – 12.3) |
| 3 | Dextromethorphan and guaifenesin† | 1 / 3 | 6.50 | 172 | 199 (182 – 433) | 52.4 | 56.4 (42.5 – 179) | 1.7 | 3.1 (2.3 – 3.3) |
| 4 | Ibuprofen | 1 / 8 | 24.7 | 213 | 275 (194 – 401) | 27.2 | 26.3 (21.8 – 36.0) | 0.7 | 1.3 (0.7 – 3.6) |
| 5 | Diphenhydramine | 1 / 4 | 25.5 | 159 | 86.2 (67.5 – 97.9) | 23.3 | 22.6 (9.9 – 35.0) | 9.9 | 3.8 (2.5 – 5.8) |
| 6 | Diphenhydramine, Fluoxetine† | 2 / 3 | 7.00, 23.00 | 643 (121 – 1 166) | 41.4 (25.8 – 2 499) | 124 (9.2 – 239) | 2.5 (1.5 – 445) | 0.2 (0.1 – 0.3) | 0.1 (0.1 – 5.4) |
| 7 | Ibuprofen | 1 / 7 | Unknown | 116 | 55.8 (35.0 – 111) | 12.2 | 10.7 (2.5 – 19.0) | 0.5 | 0.3 (0.2 – 0.6) |
| 8 | Acetaminophen | 1 / 2 | 22.17 | 19.8 | 32.1 (8.5 – 55.7) | 6.6 | 10.4 (0.5 – 20.2) | 1.5 | 1.5 (0.2 – 2.8) |
| 9 | Diphenhydramine | 1 / 3 | 21.33 | 78.9 | 101 (53.0 – 161) | 7.3 | 19.5 (12.9 – 60.8) | 1.6 | 19.9 (10.7 – 30.4) |
| 10 | Acetaminophen | 1 / 3 | 26.08 | 21.5 | 46.7 (40.9 – 1,619) | 5.1 | 18.6 (12.6 – 214) | 0.1 | 0.1 (0.1 – 0.2) |
| 11 | Ibuprofen | 1 / 4 | 21.58 | 8.0 | 12.9 (6.7 – 44.1) | 0.6 | 1.5 (0.4 – 8.3) | 0.2 | 0.2 (0.2 – 0.3) |
LOD, limit of detection
Data are presented as the median and interquartile range when more than one sample was available
Fluoxetine, ibuprofen, and diphenhydramine contain methyl, propyl, and butyl paraben; dextromethorphan and guaifenesin contain methyl and propyl paraben; acetaminophen contains butyl paraben
Participant reported using this medication on the day they provided their urine sample
Among all included urine samples (n=57), one-third of urinary butyl paraben concentrations were below the LOD (0.2 ng/ml); no urinary methyl or propyl paraben concentrations were below the LOD (1.0 ng/ml and 0.2 ng/ml, respectively). Of the 12 exposed samples, four were collected on the same day a participant reported taking a paraben-containing medication; the median hours elapsed between medication use and the collection of the urine sample was 6.4 hours, with a range of 0.75–7.0 hours. Of the remaining eight exposed samples, seven were collected on the day before the participant reported taking a paraben-containing medication; the median time elapsed between medication use and the collection of the urine sample was 22.6, with a range of 21.3–25.5 hours. One participant did not report the day of medication use, and thus the time elapsed between medication use and this final sample cannot be calculated.
Individual urinary paraben concentrations for exposed and unexposed samples are shown in Table 3. The mean urinary paraben concentrations in exposed and unexposed urine samples controlling for multiple samples per person are shown in Table 4, and the median SG-adjusted concentrations of urinary methyl, propyl, and butyl paraben by time since medication use are shown in Figure 2. After controlling for multiple samples per individual, having taken a paraben-containing medication in the 26-hour period prior to the urine sample was associated with 1.6-fold and 1.7-fold increases in mean urinary methyl (121 ng/ml in exposed samples vs. 69.6 ng/ml in unexposed samples; P=0.33) and propyl (20.8 ng/ml in exposed samples vs. 13.0 ng/ml in unexposed samples; P=0.37) paraben concentrations, respectively. These differences decreased slightly in magnitude after adjusting for the number of personal care products used during exposed and unexposed periods, which was more strongly associated with urinary paraben levels than an indicator for whether the participant had used lotion, cosmetics, perfume/cologne, or hair gel (Table 4). The mean butyl paraben concentrations did not differ, though there was a slight (0.9-fold) decrease in exposed compared to unexposed samples after adjusting for the number of personal care products used.
Table 4.
Mean paraben concentrations in exposed and unexposed urine samples, stratified by time (hours) of exposure relative to urinary paraben concentration measurements
| Unadjusted model | Adjusted model* | |||||||
|---|---|---|---|---|---|---|---|---|
| Time** (hours) | Number of samples exposed | Number of samples unexposed | Exposed | Unexposed | P | Exposed | Unexposed | P |
| Methyl paraben (ng/ml) | ||||||||
| <26 | 12 | 45 | 121.3 (58.8) | 69.6 (21.6) | 0.33 | 101 (57.5) | 73.9 (20.3) | 0.62 |
| 0.75–7.0 | 4 | 14 | 590 (473.0) | 58.1 (34.5) | 0.08 | 527 (472) | 60.8 (34.3) | 0.11 |
| 21.3–<26 | 6 | 24 | 60.9 (29.4) | 67.7 (26.5) | 0.51 | 49.2 (23.6) | 77.4 (29.8) | 0.16 |
| Propyl paraben (ng/ml) | ||||||||
| <26 | 12 | 45 | 20.8 (10.5) | 13.0 (4.1) | 0.37 | 16.6 (10.0) | 13.9 (3.7) | 0.75 |
| 0.75–7.0 | 4 | 14 | 160 (96.8) | 11.5 (3.9) | 0.01 | 99.9 (94.1) | 13.4 (3.7) | 0.10 |
| 21.3–<26 | 6 | 24 | 7.5 (3.8) | 9.5 (4.0) | 0.28 | 5.5 (2.6) | 11.2 (3.6) | 0.02 |
| Butyl paraben (ng/ml) | ||||||||
| <26 | 12 | 45 | 1.16 (0.60) | 1.12 (0.47) | 0.94 | 1.04 (0.54) | 1.15 (0.47) | 0.82 |
| 0.75–7.0 | 4 | 14 | 2.36 (2.51) | 1.40 (0.55) | 0.55 | 1.79 (2.10) | 1.53 (0.40) | 0.88 |
| 21.3–<26 | 6 | 24 | 1.00 (0.52) | 1.33 (0.76) | 0.50 | 0.76 (0.28) | 1.51 (0.77) | 0.07 |
| N (%) of butyl paraben > LOD | ||||||||
| <26 | 12 | 45 | 9 (75.0) | 29 (64.4) | 0.16 | 9 (75.0) | 29 (64.4) | 0.16 |
| 0.75–7.0 | 4 | 14 | 3 (75.0) | 11 (78.6) | 0.72 | 3 (75.0) | 11 (78.6) | 0.85 |
| 21.3–<26 | 6 | 24 | 5 (83.3) | 18 (75.0) | 0.39 | 5 (83.3) | 18 (75.0) | 0.90 |
LOD, limit of detection
Data are presented as the mean and standard error, accounting for multiple samples per subject using GEE models and back-transforming estimates from the log scale, or as n (%)
Adjusted model includes number of personal care products reported during the 24 hours prior to sample collection; the estimates presented are the mean urinary paraben concentrations for the mean number of personal care products used by all participants (9.1), those with medication use 0.75–7.0 hours (7.4), and those with medication use 21.3–<26 hours (9.3)
Time is defined as the number of hours elapsed between the reported medication use and the collection of the urine sample; two subjects with 2 exposed and 7 unexposed samples had an unknown time of medication use and were excluded from the stratified analysis
Figure 2.
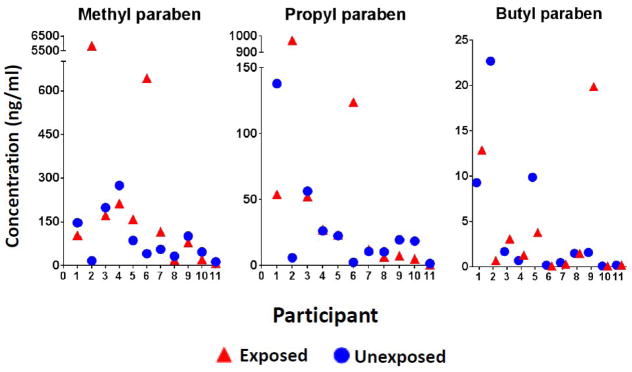
Median urinary paraben levels in exposed and unexposed samples
While there were no significant differences between exposed and unexposed samples collected in the prior 24 hours, having taken a paraben-containing medication on the same day as the urine sample (range: 0.75–7.0 hours) was associated with a nearly 14-fold increase in mean urinary methyl paraben concentration (590 ng/ml in exposed samples vs. 58.1 ng/ml in unexposed samples; P=0.08) and a greater than 10-fold difference in propyl paraben concentration (160 ng/ml in exposed samples vs. 11.5 ng/ml in unexposed samples; P=0.01). There was a 1.7-fold increase in mean urinary butyl paraben concentrations (2.36 ng/ml in exposed samples vs. 1.40 ng/ml in unexposed samples; P=0.55). These differences decreased in magnitude to 8.7-fold, 7.5-fold, and 1.2-fold, respectively, after adjustment for the number of personal care products used. Because one individual with extremely high urinary methyl and propyl paraben concentrations may have largely driven the results, a sensitivity analysis excluding this participant was performed. When this participant was excluded, the differences in urinary methyl and propyl paraben concentrations decreased to 1.3-fold (P=0.76) and 2.6-fold (P=0.34), respectively.
Taking a paraben-containing medication on the day before the urine sample (range: 21.3–25.5 hours) was associated with mean urinary methyl, propyl and butyl paraben concentrations that were approximately 0.8-fold lower in exposed compared to unexposed urine samples. After adjusting for the number of personal care products used, these differences increased in magnitude to 0.55-fold for all comparisons.
DISCUSSION
Overall, SG-adjusted urinary methyl and propyl paraben concentrations were approximately 1.65-fold greater in urine samples collected within 26 hours of use of a paraben-containing medication than they were in unexposed periods. These differences in methyl and propyl paraben concentrations were nearly 14-fold and more than 10-fold greater, respectively, when restricting to participants who reported using a paraben-containing medication on the day (within 7 hours) of their urine sample. Even after adjusting for personal care product use, the differences were approximately 8-fold greater among exposed samples. This is plausible given the short biological half-lives of parabens. A study in rats detected methyl and propyl paraben metabolites in the urine as soon as 30 minutes after oral administration, and excretion peaked at 90 minutes after dosing.24 Among these four samples in which participants reported taking a paraben-containing medication on the day of their urine sample, two had used medications containing methyl and propyl paraben, and two had used medications containing methyl, propyl, and butyl paraben. Among samples in which participants reported taking a paraben-containing medication on the day prior to their urine sample, which was a median of 22.6 hours prior to their sample, five had taken butyl paraben-containing medications and three had taken medications containing methyl, propyl, and butyl paraben. Use of paraben-containing medications was not associated with butyl paraben concentrations as either a continuous or dichotomous measure. It appears that these differences in mean methyl and propyl paraben concentrations seen between exposed and unexposed samples in which the participant reported taking a paraben-containing medication on the day of the urine collection may have been driven by one participant in particular. This participant reported using dextromethorphan and guaifenesin 45 minutes prior to one of her urine samples, which is within the time range when paraben metabolites may be detected in urine,24 and the unadjusted geometric means of her urinary methyl, propyl, and butyl paraben concentrations were 257-fold, 137-fold, and 17-fold higher, respectively, than the concentrations in her unexposed samples. When this participant was excluded in a sensitivity analysis, the differences in methyl and propyl paraben concentrations among participants who reported taking a paraben-containing medication on the day of their urine sample (within 7 hours) decreased to 1.3-fold (P=0.76) and 2.6-fold (P=0.34), respectively. While the differences without this participant were not statistically significant, it is important to note that the results for this one participant provide compelling evidence that medications may contribute to high paraben exposure. The very large increase in her urinary paraben levels likely reflects the timing of urine collection relative to her use of medication.
Among samples in which the participant reported taking a paraben-containing medication on the day before the urine sample, the mean urinary methyl, propyl, and butyl paraben concentrations of exposed samples were unexpectedly lower than mean concentrations of unexposed samples. Given that most of the paraben should have been excreted over this time period,2, 25 this finding may reflect variation in an individual’s day-to-day exposure to parabens from sources other than medications. Overall, the differences in these means were small.
At the time of their designation in the Food Additives Amendment of 1958 as GRAS substances, the endocrine-disrupting abilities of parabens had not been recognized. Since then, parabens have been linked to adverse reproductive outcomes, and thus these findings may be of particular importance to couples wanting a child. Previous work by our group has found parabens to be associated with sperm DNA damage in men as measured by the percentage of DNA in comet tail.26 Methyl and propyl paraben have been shown to affect mitochondrial activity in isolated rat hepatocytes,27 which may be a mechanism of male infertility,28, 29 and parabens have also been shown to bind estrogen receptors in rats.30
In addition to their use in medications, other sources of paraben exposure are personal care products.23 A particular strength of our study was our comparison of exposed and unexposed samples from the same participants, as this allowed us to control for paraben exposure from these other sources; we believe that an individual’s use of personal care products is unlikely to change drastically over a relatively short time period, as season has not been shown to affect patterns of personal care product use.31 The mean number of personal care products that participants reported in the 24-hour period prior to the collection of their urine sample was 10.3 among exposed samples and 8.8 among unexposed samples. Among pregnant women in Puerto Rico, users of lotion had urinary propyl paraben concentrations that were 3.4 greater than concentrations among non-users,32 which is slightly higher than the difference of 2.2-fold found among a cohort of women from the EARtH Study.23 Based on the present data, it appears that in the 1–2 hours following their use, paraben-containing medications may contribute to urinary paraben concentrations that are up to 257-fold greater than concentrations in periods of non-use of these medications. In the 6–7 hours following their use, it appears that the use of paraben-containing medications contributes to urinary paraben concentrations on a similar magnitude to that of personal care products.
A limitation of our study is the potential for exposure misclassification. Because the paraben content of medications is not readily available and can change over time, we were unable to quantify the amount of parabens in each medication dose. Similarly, while we accounted for the number of personal care products used, which are known sources of paraben exposure, we were not able to quantify paraben exposure from other sources such as foods. Medication use was self-reported by the participants, and it is possible that some participants did not correctly report the name of their medication or the time when they used the medication. This bias is expected to be minimal as participants were asked only to report medication use in the previous 24 hours, and any misclassification is expected to be non-differential and thus bias results towards the null. It may also be possible that certain kinds of medications are not reported; there is evidence to suggest that women undergoing infertility treatment may be more likely to disclose use of anti-depressants when they do so electronically.33 In this case, the bias would be expected to be towards the null. Additionally, approximately one-third of butyl paraben concentrations were below the LOD. When modeled as a continuous measure, the lowest third of butyl paraben values were assigned a value of the LOD divided by the square root of two, though this may not be representative of the participants’ actual butyl paraben concentrations. To account for the high proportion of samples below the LOD, we also dichotomized butyl paraben into detectable and undetectable concentrations, though in this case we may be missing a finer relationship that cannot be detected with a dichotomous classification. In the dichotomous classification, all concentrations above 0.2 ng/ml were considered to be the same, whereas in reality there were some detectable concentrations that were over 99-fold higher than the LOD, though not all of these samples were associated with reporting taking a paraben-containing medication, and the dichotomous classification cannot distinguish between very small and very large differences, which is likely important. Finally, our sample was small, included 10 women and only one man, and our findings are largely driven by one participant with very high paraben concentrations following use of a paraben-containing medication.
Our results suggest that medications, independent of personal care products, may be an important route of human paraben exposure, though our results were largely driven by one participant with very high urinary paraben levels. Our sample size was small and these results should be confirmed in a larger cohort. Regardless, these findings may have important implications for future research into effects of environmental chemicals, and when assessing parabens, it may be important to assess medication use as a source of human exposure.
Supplementary Material
Parabens are used in some medications and may adversely affect reproduction
We compare urinary paraben concentrations in relation to medication use
We control for use of personal care products, an important exposure source
Paraben-containing medications were associated with increased urinary concentrations
Paraben-containing medications can be a source of very high paraben exposure
Acknowledgments
This work was supported by NIEHS ES009718, training grant T32 ES 07069, which supported LED, and R01 HD 059861, which funded SHD.
Footnotes
Competing interests: The authors have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. National Biomonitoring Program: Paraben. Sep 7, 2013. [Google Scholar]
- 2.CIR Expert Panel. Final amended report on the safety assessment of Methylparaben, Ethylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(Suppl 4):1–82. doi: 10.1080/10915810802548359. [DOI] [PubMed] [Google Scholar]
- 3.Liao C, Liu F, Kannan K. Occurrence of and dietary exposure to parabens in foodstuffs from the United States. Environ Sci Technol. 2013;47:3918–3925. doi: 10.1021/es400724s. [DOI] [PubMed] [Google Scholar]
- 4.Regulation (EC) No 1223.2009 of the European Parliament and of the Council. 30 Nov 2009.
- 5.Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables. Department of Health and Human Services, Centers for Disease Control and Prevention; 2013. [Google Scholar]
- 6.Hauser R, Duty S, Godfrey-Bailey L, Calafat AM. Medications as a source of human exposure to phthalates. Environ Health Perspect. 2004;112:751–753. doi: 10.1289/ehp.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez-Diaz S, Su YC, Mitchell AA, Kelley KE, Calafat AM, Hauser R. Medications as a potential source of exposure to phthalates among women of childbearing age. Reprod Toxicol. 2013;37:1–5. doi: 10.1016/j.reprotox.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller BO, Davidson AG, Innis SM. Phthalate metabolites in urine of CF patients are associated with use of enteric-coated pancreatic enzymes. Environ Toxicol Pharmacol. 2009;27:424–427. doi: 10.1016/j.etap.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Rowe RC, Sheshky PJ, Quinn ME. Handbook of Pharmaceutical Excipients. 6. Washington, DC: American Pharmaceutical Association; 2009. [Google Scholar]
- 10.Federal Drug Administration. [Accessed 28 Feb 2014];Generally Recognized as Safe (GRAS) Available at http://www.fda.gov/food/Ingredientspackaginglabeling/GRAS/ Last updated 12 Feb 2014.
- 11.Goodrich W. Safe food additives and additives generally recognized as safe --there is a difference. The Business Lawyer. 1960;16:98–106. [Google Scholar]
- 12.The Food Additives Amendment of 1958. 72 Stat. 1784. 1958.
- 13.Federal Drug Administration. [Accessed 24 Oct 2013];Inactive Ingredient Search for Approved Drug Products. Available at http://www.accessdata.fda.gov/scripts/cder/iig/index.Cfm. Last updated 23 Oct 2013.
- 14.Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ Health Perspect. 2012;120:739–745. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith KW, Braun JM, Williams PL, Ehrlich S, Correia KF, Calafat AM, et al. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ Health Perspect. 2012;120:1538–1543. doi: 10.1289/ehp.1104614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77:5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- 17.Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 18.United States National Library of Medicine, National Institutes of Health. [Accessed 31 Mar 2014];Pillbox: prototype pill identification system. Available at http://pillbox.nlm.nih.gov/
- 19.Federal Drug Administration Center for Drug Evaluation and Research. [Accessed 31 Mar 2014];FDA Approved Drugs Products database. Available at http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm/
- 20.United States National Library of Medicine. [Accessed 31 Mar 2014];DailyMed. Available at http://dailymed.nlm.nih.gov/
- 21.PDR Network. Physicians’ Desk Reference. Montvale, NJ: PDR Network; 2013. [Google Scholar]
- 22.Physicians’ Desk Reference for Nonprescription Drugs, Dietary Supplements, and Herbs. Montvale, NJ: 2013. [Google Scholar]
- 23.Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol. 2013 doi: 10.1038/jes.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derache R, Gourdon J. Metabolism of a food preservative: p-hydroxybenzoic acid and its esters. Food and Cosmetics Toxicology. 1963;1:189–195. doi: 10.1016/s0015-6264(63)80686-0. [DOI] [PubMed] [Google Scholar]
- 25.Janjua NR, Frederiksen H, Skakkebaek NE, Wulf HC, Andersson AM. Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int J Androl. 2008;31:118–130. doi: 10.1111/j.1365-2605.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- 26.Meeker JD, Ehrlich S, Toth TL, Wright DL, Calafat AM, Trisini AT, et al. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod Toxicol. 2010;30:532–539. doi: 10.1016/j.reprotox.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawa Y, Moldeus P. Mechanism of p-hydroxybenzoate ester-induced mitochondrial dysfunction and cytotoxicity in isolated rat hepatocytes. Biochem Pharmacol. 1998;55:1907–1914. doi: 10.1016/s0006-2952(98)00097-5. [DOI] [PubMed] [Google Scholar]
- 28.Soni MG, Taylor SL, Greenberg NA, Burdock GA. Evaluation of the health aspects of methyl paraben: a review of the published literature. Food Chem Toxicol. 2002;40:1335–1373. doi: 10.1016/s0278-6915(02)00107-2. [DOI] [PubMed] [Google Scholar]
- 29.Tavares RS, Martins FC, Oliveira PJ, Ramalho-Santos J, Peixoto FP. Parabens in male infertility-is there a mitochondrial connection? Reprod Toxicol. 2009;27:1–7. doi: 10.1016/j.reprotox.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Routledge EJ, Parker J, Odum J, Ashby J, Sumpter JP. Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic. Toxicol Appl Pharmacol. 1998;153:12–19. doi: 10.1006/taap.1998.8544. [DOI] [PubMed] [Google Scholar]
- 31.Wu XM, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. Usage pattern of personal care products in California households. Food Chem Toxicol. 2010;48:3109–3119. doi: 10.1016/j.fct.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, Ferguson KK, Mukherjee B, Calafat AM, et al. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environ Sci Technol. 2013;47:3439–3447. doi: 10.1021/es400510g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domar AD, Moragianni VA, Ryley DA, Urato AC. The risks of selective serotonin reuptake inhibitor use in infertile women: a review of the impact on fertility, pregnancy, neonatal health and beyond. Hum Reprod. 2013;28:160–171. doi: 10.1093/humrep/des383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.