Abstract
In Vibrio cholerae, the genes required for biofilm development are repressed by quorum sensing at high cell density due to the accumulation in the medium of two signaling molecules, cholera autoinducer 1 (CAI-1) and autoinducer 2 (AI-2). A significant fraction of toxigenic V. cholerae isolates, however, exhibit dysfunctional quorum sensing pathways. It was reported that transition state analogs of the enzyme methylthioadenosine/S-adenosylhomocysteine nucleosidase (MtnN) required to make AI-2 inhibited biofilm formation in the prototype quorum sensing-deficient strain N16961. This finding prompted us to examine the role of both autoinducers and MtnN in biofilm development and virulence gene expression in a quorum sensing-deficient genetic background. Here we show that deletion of mtnN encoding methylthioadenosine/S-adenosylhomocysteine nucleosidase, cqsA (CAI-1), and/or luxS (AI-2) do not prevent biofilm development. However, two independent mtnN mutants exhibited diminished growth rate and motility in swarm agar plates suggesting that, under certain conditions, MtnN could influence biofilm formation indirectly. Nevertheless, MtnN is not required for the development of a mature biofilm.
1. Introduction
The formation of biofilm communities pose a challenge to the prevention and treatment of infectious diseases. It is well established that bacterial biofilms are recalcitrant to elimination with biocides, classical antibiotics and disinfectants [1-3]. The enhanced resistance of bacterial biofilms to innate host defenses and antibiotic treatment encourages chronic and/or relapsing infections [4-5] and calls for long-term antibiotic therapies that favor the acquisition of antibacterial drug resistance. Thus, novel compounds capable of inhibiting biofilm formation are commonly received with enthusiasm.
In numerous organisms, biofilm development is regulated by quorum sensing. Quorum sensing is a process by which bacteria communicate with one another by secreting and responding to extracellular signaling molecules termed autoinducers [6]. In V. cholerae, two autoinducer systems have been identified. System 1 consists of the metabolically-related molecules 3-aminotridec-2-en-4-one (Ea-CAI-1), tridecane-3,4-dione (DK-CAI-1) and (S)-3-hydroxytridecan-4-one (CAI-1) sensed by the receptor CqsS [7-9] . Ea-CAI-1 is synthesized by the activity of CqsA that catalyzes the reaction between S-adenosylmethionine (SAM) and decanoyl-coenzyme A to yield Ea-CAI-1 and methylthioadenosine [9]. Ea-CAI-1 is spontaneously converted to DK-CAI-1, which can be enzymatically reduced to CAI-1 [9]. System 2 consists of autoinducer 2 (AI-2), a set of interconverting molecules derived from the shared precursor (4S)-4,5-dihydroxypentane-2,3-dione (DPD). DPD is synthesized from S-ribosylhomocysteine (SRH) by the activity of LuxS [10]. CqsS and the AI-2 receptor LuxPQ feed cell density sensory information through a phosphorelay system to the regulator LuxO [7]. At low cell density, the autokinase domains of CqsS and LuxPQ become phosphorylated and phosphorus is transferred to LuxO [7]. Phospho-LuxO acts to destabilize the hapR mRNA encoding the master quorum sensing regulator HapR [11-12]. When the concentration of autoinducers produced by growing bacteria reaches a threshold, CqsS and LuxPQ switch from kinase to phosphatase and the flow of phosphorus is reversed. Inactivation of LuxO allows the expression of the HapR which functions to represses biofilm formation [13-17]. In V. cholerae C7258, a strain that expresses a functional HapR-dependent quorum sensing pathway, deletion of luxS enhanced biofilm formation while exogenous DPD diminished biofilm formation in a dose dependent manner [18]. Not all choleragenic Vibrios, however, express HapR. A study involving 16 geographically diverse O1, O139, nonO1/nonO139 serogroup V. cholerae strains revealed a high rate (62 %) of isolates with dysfunctional quorum sensing systems [19]. One example is the prototype strain N16961 that contains a natural frame shift mutation in hapR [20]. It has been shown that V. cholerae strains containing a natural frame shift mutation in hapR respond to autoinducers through a separate pathway involving the diguanylate cyclase VCA0939 [21].
The V. cholerae mtnN gene (VC2379) encodes an N-glycosidase with dual substrate specificity for methylthioadenosine and S-adenosylhomocysteine. Recently, transition state analogs of methylthioadenosine/S-adenosylhomocysteine nucleosidase (MtnN) were suggested to diminish biofilm formation in V. cholerae strain N16961 [22-23]. The luxS and mtnN gene products belong to a pathway that functions to recycle S-adenosylhomocysteine (SAH) [24]. MtnN converts SAH to adenine and SRH. Then, the product of luxS (S-ribosylhomocysteine lyase) converts SRH into L-homocysteine and DPD. Consequently, inhibition of MtnN activity blocks the production of the AI-2 quorum sensing signal. Since accumulation of autoinducer molecules at high cell density represses biofilm formation, the claim that blocking the biosynthesis of AI-2 inhibits biofilm formation [22-23] challenges this regulatory model.
Little knowledge exists on the role of quorum sensing and AI-2 in biofilm development in strain N16961. Thus, we undertook a genetic approach to determine the role of each autoinducer and MtnN in biofilm development in this genetic background. Our data shows that lack of both autoinducers or MtnN do not prevent nor enhance biofilm formation in this prototype cholera strain.
2. Materials and methods
2.1. Strains and media
The strains used in study were derived from V. cholerae N16961 (O1, El Tor biotype). Escherichia coli strains TOP10 (Life technologies) and S17-1λpir [25] were used for plasmid propagation and mutant construction. Bacterial strains were grown in LB medium at 37°C with agitation (250 rpm). V. cholerae MM920 [7] and V. harveyi BB170 [26] were used to measure CAI-1 and AI-2 activities, respectively. For the AI-2 assay, strain BB170 was grown in AB medium [26]. To detect the expression of the toxin co-regulated pilus (TCP), V. cholerae was grown under ToxR-permissive condition in AKI medium [27]. Ampicillin (Amp, 100 µ g/mL), polymyxin B (PolB, 100 units/mL) or kanamycin (Km, 50 µ g/mL) were added to the medium as required. The MtnN transition state analog MT-DADMe-Immucillin-A was kindly provided by Verrn Schramm (Albert Einstein College of Medicine) and added to the culture medium at a final concentration of 10 µ g/mL.
2.2. Construction of mutants
All mutants were constructed in strain N16961 by allelic exchange using derivatives of the suicide vector pCVD442 [28] as described previously [29]. The suicide vectors pCVDΔCqsA [29], pCVDluxS-Km [29], and pCVDΔluxO [30] harbor chromosome fragments containing deletions of cqsA, luxS and luxO, respectively. To construct cqsA, luxS and luxO mutants the corresponding suicide vector was transferred by conjugation from E. coli S17-1λpir to N16961. Exconjugants were selected in LB medium containing Amp and PolB. Finally, allelic exchange products containing the corresponding deletion in the N16961 background were obtained by sucrose selection as previously described [29]. To construct N16961luxOD47E, the wild type luxO gene was amplified by PCR from strain N16961 genomic DNA using the Advantage 2 PCR kit (Biosciences Clontech) and the primer combination 5'-ATGGTAGAAGACACGGC/5'-TTACCGTTCCTTCTCTT. The PCR product was cloned in pUC19 and the D47E amino acid substitution generated using the QuikChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies) following the manufacturer's instructions. The mutant allele was transferred to pCVD442 and N16961luxOD47E isolated by allelic exchange as described above. To construct N16961 derivatives lacking MtnN activity, chromosome fragments flanking the mtnN open reading frame were amplified by PCR using the primer combinations 5'-GCCGAGCTCCAGTCAGGGAGTCGATTC/5'-AAAGGATCCGATGCCGATTTTCATAGGG and 5'-GAAGGATCCGCTCTTCCGCTATGGTGCTG/5'-TAAGCATGCAGGTTTCCACTCCCGCCTTG and sequentially cloned in pUC19. A Km resistance cassette from plasmid pUC4K (GenBank accession number X06404) was inserted in place of the mtnN gene. Finally, the mtnN flanking DNA fragments and Km cassette was transferred to pCVD442 to yield pCVDΔmtnN. The above suicide vector harboring the mtnN deletion and Km insertion was used to construct the N16961ΔmtnN strains by conjugation and sucrose selection as described above. All mutations and deletions were confirmed by PCR and DNA sequencing.
2.3. Autoinducer bioassays
To measure the production of CAI-1 activity, V. cholerae strains were grown in LB medium to late log phase and the cultures were centrifuged at 12,000 rpm for 10 min. The supernatants were filtered through a 0.22-µm syringe filter and tested for the presence of CAI-1 activity by inducing light production in the V. cholerae reporter strain MM920 [7]. The reporter strain was grown overnight with shaking at 30°C, diluted 1:10 in fresh medium and 70 µ L aliquots transferred to an opaque-wall 96-well microtiter plate. Cell-free culture fluids were added to a final concentration of 30% (v/v). The plates were incubated at 30°C with agitation and light production was measured at 30 min intervals in a Synergy 2 BioTek plate reader. Results are expressed as light fold induction relative to a sterile medium control. For AI-2 activity, cell-free culture supernatants were tested for light production in the AI-2 reporter strain V. harveyi BB170 as described in [26].
2.4. Measurement of MtnN activity
To determine MtnN activity cell pellets were resuspended in 10 mM HEPES (pH 7.4) - 1 mM dithiothreitol (DTT) and disrupted by sonication. The cleared cell extracts were dialyzed against buffer containing 100 mM HEPES, 1 mM DTT, 20% glycerol. Reactions mixtures (1 mL) consisted of 100 mM HEPES, 50 mM potassium phosphate, 100 µM methylthioadenosine and crude extract so that linear increase in product formation could be followed over time. Reactions were incubated for 0, 0.25, 0.5, 1, and 2 hours at 37 °. At each time point 150 µl of reaction mixture was removed, mixed with 150 µl of water, and the reaction was stopped by boiling. The cell debris was removed by filtration and the sample was injected onto a 5 µm BDS Hypersil C-18 column (150 × 4.6 mm) (Keystone Scientific Inc., State College, PA) equilibrated with 50 mM ammonium dihydrogen phosphate buffer (pH 4.5) containing 2.5% acetonitrile (flow rate of 1 ml/minute). Adenine and methylthioadenosine were eluted from the column by isocratic elution for 10 min with 50 mM ammonium dihydrogen phosphate buffer (pH 4.5) containing 2.5% acetonitrile followed by 20 min linear gradient to 50 mM ammonium dihydrogen phosphate buffer (pH 4.5) containing 50% acetonitrile. Adenine and methylthioadenosine were detected as they eluted from the column by their absorbance at 254 nm.
2.5. Biofilm and motility assays
Biofilm formation was measured by the crystal violet staining method and results were normalized for growth and expressed as the OD570 nm/OD600 nm ratio [13]. For motility detection, swarm agar plates consisting of LB medium containing 0.3% agar were inoculated by stabbing with overnight cultures grown from single colonies in LB medium. The plates were incubated for 24 h at 30°C, imaged and the swarm halo (mm) was measured.
2.6. Western blot detection of the toxin co-regulated pilus production
TcpA, the major TCP subunit, was detected in Western blots using a rabbit anti-TcpA serum kindly provided by Biao Kan (CDC Beijing). Cell pellets corresponding to the same number of cells based on OD600 readings were boiled in 100 µ L of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer, and proteins were separated in a 12% polyacrylamide gel. Gels were transferred to polyvinylidene difluoride (PVDF) membranes, and TCP protein was detected with the BM chemiluminescence kit (Roche Applied Sciences, Indianapolis, IN) following the manufacturer's recommended procedure.
3. Results and discussion
In this study we first examined if quorum sensing could activate rather than repress biofilm formation in V. cholerae strains exhibiting a dysfunctional quorum sensing system. To this end, we constructed a set of isogenic quorum sensing deletion mutants starting from strain N16961 (Fig. 1). Since SAM serves as a precursor for the biosynthesis of both autoinducers, we first examined the possibility that a blockage in the biosynthesis of one autoinducer could indirectly affect the production of the other. As expected, ΔcqsA and ΔluxS mutants did not produce detectable CAI-1 and AI-2 activity, respectively (Fig. 1). Further, the ΔcqsA deletion mutant produced wild type levels of AI-2 while the ΔluxS mutant produced wild type levels of CAI-1 indicating that blocking the biosynthesis of one autoinducer does not impact the production of the second (Fig. 1). We also constructed a ΔluxO mutant in which quorum sensing is permanently active and a luxOD47E mutant. The D47E mutation in LuxO renders this protein active irrespective of its phosphorylation stage to permanently turn off quorum sensing [7]. We next examined the effect of these mutations on biofilm formation using the crystal violet staining assay. As shown in Fig. 2, none of these mutations prevented biofilm formation and the modest differences observed between strains did not reach statistical significance. In V. cholerae, biofilm development and the expression of virulence factors are both negatively regulated by quorum sensing [13]. Thus, we asked if the N16961 quorum sensing mutants expressed elevated virulence factors. To this end, we chose to measure the expression of TCP. Expression of TCP is co-regulated to that of cholera toxin and the pilus is required for surface adherence to the intestinal mucosa, microcolony formation and attachment to chitinous surfaces [31-32]. As shown in Fig. 3, alteration of quorum sensing by mutation did not significantly impact TCP expression. From the above results, we conclude that quorum sensing does not regulate biofilm development and virulence gene expression in strain N16961 that lacks the HapR regulator.
Fig.1. Production of CAI-1 and AI-2 in quorum sensing mutants.
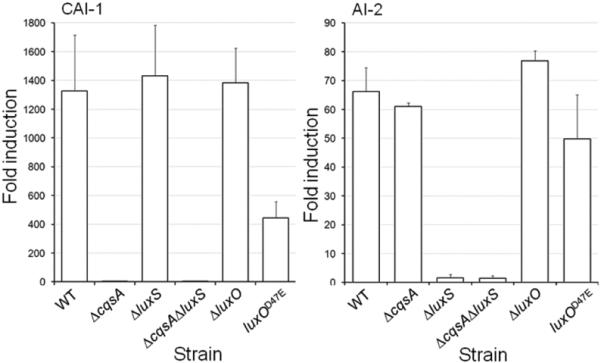
Strain N16961 (WT) and its isogenic mutants were grown in LB medium at 37°C to late log phase and the corresponding autoinducer activity measured as described in materials and methods. Each value represents the mean of three independent experiments. Error bars denote the standard deviation (STDEV).
Fig.2. Biofilm formation in quorum sensing mutants.
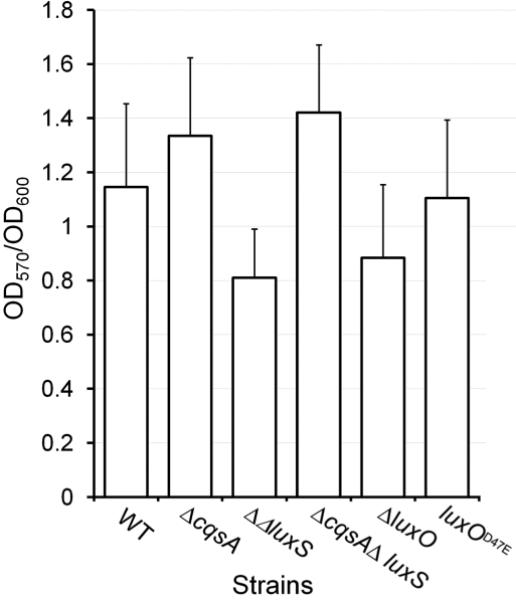
Strain N16961 (WT) and its isogenic mutants were grown in LB medium at 37°C to stationary phase. The cultures were diluted 100-fold in fresh medium and biofilms allowed to develop under static conditions for 24 h in 96-well microtiter plates. Biofilm formation was measured using the crystal violet straining method (OD570) and the data normalized for growth (OD600). Each value represents the mean of three independent experiments. Error bars denote the standard deviation (STDEV).
Fig.3. Expression of the toxin co-regulated pilus in quorum sensing mutants.
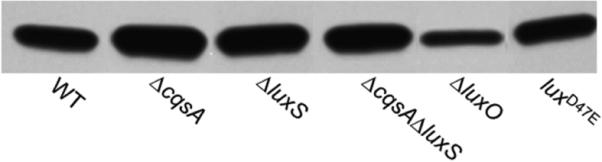
Strain N16961 (WT) and its isogenic mutants were grown in AKI cultures and production of TcpA, the TCP major subunit, was determined by Western blot as described in materials and methods.
Based on the above findings, our data is not consistent with inhibition of MtnN diminishing biofilm formation by disrupting quorum sensing. The fact that mtnN is required for the biosynthesis of AI-2 and at the same time is part of a metabolic pathway that recycles SAH can complicate the interpretation of mtnN phenotypes. Thus, we examined if MtnN could affect biofilm formation by a mechanism independent of quorum sensing. To this end, we constructed two independent mtnN deletion mutants. The MtnN activity of wild type strain N16961 was 2717 ± 535 nmoles/mg/h. In contrast, both mtnN mutants exhibited activities < 10 nmoles/mg/h under identical conditions. This result indicates that mtnN is the only gene encoding this enzyme activity in the V. cholerae genome. As expected, we could not detect AI-2 activity in the supernatants of ΔmtnN mutants (data not shown). As shown in Fig. 4A, deletion of mtnN did not prevent the formation of wild type biofilms. Loss of mtnN, however, resulted in diminished growth rate, which could be due to the accumulation of potentially toxic SAH (Fig. 4B). Further, the mtnN deletion mutants exhibited reduced motility in swarm agar plates (Fig. 4C). We suggest that the reduced swarm halo could be due to SAH inhibition of SAM-dependent methylation of methyl-accepting chemotaxis proteins required to transduce chemotactic signals to the flagellar motor. Consistent with the above genetic data, strain N16961 was capable of developing a wild type biofilm in the presence of the transition state analog MT-DADMe-Immucillin-A. Addition of the transition state analog to the medium, however, resulted in 80 % inhibition of MtnN activity. This level of inhibition suggests that a significant fraction of MT-DADMe-Immucillin-A remained bound to MtnN after cell disruption and dialysis. This result is consistent with MT-DADMe-Immucillin-A binding to MtnN with a dissociation constant in the picomolar range [23].
Fig.4. Phenotypic analysis of mtnN mutants.
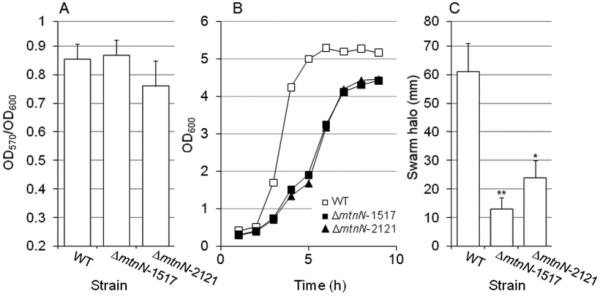
Strain N16961 (WT) and two independent isogenic ΔmtnN mutants were grown in LB medium at 37°C. A. Biofilm formation. The cultures were diluted 100-fold in fresh medium and biofilm formation measured as described for the quorum sensing mutants in 96-well microtiter plates. Each value represents the mean of three independent experiments. Error bars denote the standard deviation (STDEV). B. Growth curve. Overnight cultures were diluted 1000-fold in fresh LB medium and incubated with agitation (250 rpm) at 37°C. At different time points, growth was estimated by measuring the OD600. C. Motility. Wild type and two independent isogenic ΔmtnN mutants were grown in LB medium to stationary phase at 37°C. Swarm (soft) agar plates were stabbed with cultures of each mutant and the plates were incubated 24 h. Motility was estimated by measuring the swarm halo (mm). Each value represents the mean of three independent experiments. Error bars denote the standard deviation (STDEV). *Swarm significantly different than wild type (p < 0.05); ** swarm significantly different than wild type (p< 0.01).
Our results don't rule out the possibility that deletion/inhibition of MtnN could affect biofilm development under certain experimental conditions. If any, the effect of MtnN on biofilm formation could result from reduced motility/chemotaxis and/or growth rate rather than disruption of quorum sensing. Indirect effects of this kind could be subject to significant variability in the crystal violet microtiter plate assay. For instance, it has been shown that flagellum and pili accelerate surface attachment and facilitates bacterial spreading along abiotic surfaces [33-34]. Nevertheless, non-motile mutants can still develop normal biofilms provided sufficient time. Another source of variability is the strength with which biofilm cells are anchored to the surface. This could vary with strain and growth medium due to the expression of matrix proteins such as Bap1 [35]. An additional complicating factor is the non homogenous distribution of adherent cells in the crystal violet microtiter plate assay. In addition to adhering to the bottom and walls of a well, many V. cholerae strains form a thick biofilm at the liquid-air interface that could be difficult to quantitate or could detach with the washings. Our study, nevertheless, shows that V. cholerae mutants lacking MtnN activity, can form a biofilm similar to wild type.
Supplementary Material
Highlights.
Quorum sensing and autoinducer 2 modulate biofilm formation in Vibrio cholerae.
S-adenosylhomocysteine nucleosidase activity is required to produce autoinducer 2.
S-adenosylhomocysteine nucleosidase was not required for biofilm development.
Lack of this enzyme resulted in reduced growth rate and flagellar motility.
Acknowledgements
This study was supported by National Institutes of Health Research Grants AI081039 to AJS, AI090397 to JAB and by a Southern Research Institute endowment fund to JAB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. The Lancet. 2001;358:135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 2.Mah TF, O’Toole GA. Mechanism of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 3.Hogan D, Kolter R. Why are bacteria refractory to antimicrobials? Curr. Opinion. Microbiol. 2002;5:472–477. doi: 10.1016/s1369-5274(02)00357-0. [DOI] [PubMed] [Google Scholar]
- 4.Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trend Microbiol. 2004;13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Del Pozo JL, Patel R. The challenge of treating biofilm-associated bacterial infections. Clin. Pharmacol. Ther. 2007;82:204–209. doi: 10.1038/sj.clpt.6100247. [DOI] [PubMed] [Google Scholar]
- 6.Ng W-L, Bassler BL. Bacterial quorum sensing network architectures. Ann. Rev. Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell. 2002;110:303–314. doi: 10.1016/s0092-8674(02)00829-2. [DOI] [PubMed] [Google Scholar]
- 8.Higgins DA, Poimianek ME, Krami CM, Taylor RK, Semmelhack MF, Bassler BL. The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature. 2007;450:883–886. doi: 10.1038/nature06284. [DOI] [PubMed] [Google Scholar]
- 9.Wei Y, Perez LJ, Ng W-L, Semmelhack MF, Bassler BL. Mechanism of Vibrio cholerae autoinducer-1 biosynthesis. ACS Chem. Biol. 2011;6:356–365. doi: 10.1021/cb1003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 11.Jobling MG, Holmes RK. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol. Microbiol. 1997;26:1023–1034. doi: 10.1046/j.1365-2958.1997.6402011.x. [DOI] [PubMed] [Google Scholar]
- 12.Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell. 2004;118:69–82. doi: 10.1016/j.cell.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA. 2002;99:3129–3134. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammer BK, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 2003;50:101–114. doi: 10.1046/j.1365-2958.2003.03688.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Mekalanos JJ. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Develop. Cell. 2003;5:647–656. doi: 10.1016/s1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
- 16.Yildiz FH, Liu XS, Heydorn A, Schoolnik GK. Molecular analysis of rugosity in a Vibrio cholerae O1 El Tor phase variant. Mol. Microbiol. 2004;53:497–515. doi: 10.1111/j.1365-2958.2004.04154.x. [DOI] [PubMed] [Google Scholar]
- 17.Waters CM, Lu W, Rabinowitz JD, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J. Bacteriol. 2008;190:2527–2536. doi: 10.1128/JB.01756-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali SA, Benitez JA. Differential response of Vibrio cholerae planktonic and biofilm cells to autoinducer 2 deficiency. Microbiol. Immunol. 2009;53:582–586. doi: 10.1111/j.1348-0421.2009.00161.x. [DOI] [PubMed] [Google Scholar]
- 19.Joelsson A, Liu Z, Zhu J. Genetic and phenotypic diversity of quorum-sensing systems in clinical and environmental isolates of Vibrio cholerae. Infect. Immun. 2006;74:1141–1147. doi: 10.1128/IAI.74.2.1141-1147.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidelberg JF, Eisen JA, Nelson WC, et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. 2000;406:477–83. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammer BK, Bassler BL. Regulatory small RNAs circumvent the conventional quorum sensing pathway in pandemic Vibrio cholerae. Proc. Natl. Acad. Sci USA. 2007;104:11145–11149. doi: 10.1073/pnas.0703860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schramm VL, Gutierrez JA, Cordovano G, Basu I, Guha C, Belbin TJ, Evans GB, Tyler PC, Furneaux RH. Transition state analogs in quorum sensing and SAM recycling. Nucleic Acids Symp. Ser. 2008;52:75–76. doi: 10.1093/nass/nrn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutierrez JA, Crowder T, Rinaldo-Matthis A, Ho M, Almo SC, Schramm VL. Transition state analogs of 5’methylthioadenosine nucleosidase disrupt quorum sensing. Nature Chem. Biol. 2009;5:251–257. doi: 10.1038/nchembio.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winzer K, Hardie KR, Burgess N, Doherty N, Kirke D, Holden MTG, Linforth R, Cornell KA, Taylor AJ, Hill PJ, Williams P. LuxS: its role in central metabolism and in vitro synthesis of 4-hydroxi-5-methyl-3(2H)-furanone. Microbiology. 2002;148:909–922. doi: 10.1099/00221287-148-4-909. [DOI] [PubMed] [Google Scholar]
- 25.de Lorenzo V, Eltis L, Kessler B, Timmis KN. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene. 1993;123:17–24. doi: 10.1016/0378-1119(93)90533-9. [DOI] [PubMed] [Google Scholar]
- 26.Taga ME, Xavier ME,KB. Methods for analysis of bacterial autoinducer-2 production. Curr. Protoc. Microbiol. 2011 doi: 10.1002/9780471729259.mc01c01s23. Chapter 1. Unit1C.1. doi: 10.1002/9780471729259.mc01c01s23. [DOI] [PubMed] [Google Scholar]
- 27.Iwanaga M, Yamamoto K, Higa N, Ichinose Y, Nakasone N, Tanabe M. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol. Immunol. 1986;30:1075–1083. doi: 10.1111/j.1348-0421.1986.tb03037.x. [DOI] [PubMed] [Google Scholar]
- 28.Donnenberg MS, Kaper JB. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang W, Pascual-Montano A, Silva AJ, Benitez JA. The cyclic AMP receptor protein modulates quorum sensing, motility and multiple genes that affect intestinal colonization in Vibrio cholerae. Microbiology. 2007;153:2964–2975. doi: 10.1099/mic.0.2007/006668-0. [DOI] [PubMed] [Google Scholar]
- 30.Sultan SZ, Silva AJ, Benitez JA. The PhoB regulatory system modulates biofilm formation and stress response in El Tor biotype Vibrio cholerae. FEMS Microbiol. Lett. 2010;302:22–31. doi: 10.1111/j.1574-6968.2009.01837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jude BA, Taylor RK. The physical basis of type 4 pilus-mediated microcolony formation by Vibrio cholerae O1. J. Struct. Biol. 2011;175:1–9. doi: 10.1016/j.jsb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reguera G, Kolter R. Virulence and the environment: a novel role for Vibrio cholerae toxin-coregulated pili in biofilm formation on chitin. J. Bacteriol. 2005;187:3551–3555. doi: 10.1128/JB.187.10.3551-3555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watnick PI, Kolter R. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 1999;34:586–595. doi: 10.1046/j.1365-2958.1999.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Utada AS, Bennett RR, Fong JC, Gibiansky ML, Yildiz FH, Golestanian R, Wong GC. Vibrio cholerae use pili and flagella synergistically to effect motility switching and conditional surface attachment. Nature Communications. 2014;5 doi: 10.1038/ncomms5913. Article 4913. doi: 10.1038/ncomms5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berk V, Fong JC, Dempsey GT, Develioglu ON, Zhuang X, Liphardt J, Yildiz FH, Chu S. Molecular architecture and assembly principles of Vibrio cholerae biofilms. Science. 2012;337:236–239. doi: 10.1126/science.1222981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


