Abstract
Circulating angiogenic factors (AF) reflect tissue healing capacity, although some AF can also contribute to inflammation and are indicative of endothelial dysfunction. The AF milieu in acute graft-versus-host disease (aGVHD) has not been broadly characterized. We hypothesized that patients with abundant AF involved in repair/regeneration vs. those mediating damage/inflammation would have improved outcomes. Circulating AF known predominantly for repair/regeneration (epidermal growth factor [EGF], fibroblast growth factor-1 and -2, heparin binding-EGF-like growth factor, vascular endothelial growth factor-A, -C, and -D) and for damage/inflammation (angiopoietin-2, endothelin-1, soluble endoglin [sEng], follistatin [FS], leptin, placental growth factor [PlGF]) were measured in a discovery set of HCT recipients with grade III/IV aGVHD versus controls, then validated in two aGVHD cohorts enrolled in Blood and Marrow Transplant Clinical Trials Network (BMT CTN) trials 0302 (N=105, serum) and 0802 (N=158, plasma) versus controls without aGVHD (N=53, serum). Levels of EGF and VEGF-A were lower than controls at the onset of aGVHD in both trials and higher with complete response to first-line aGVHD therapy in CTN 0802. FS and PlGF were elevated in aGVHD measured in either serum or plasma. At day 28 after initial aGVHD therapy, elevated FS was an independent negative prognostic factor for survival in both cohorts (hazard ratio 9.3 in CTN 0302, 2.8 in CTN 0802). These data suggest that circulating AF are associated with clinical outcomes after aGVHD and thus may contribute to both pathogenesis and recovery.
Keywords: Allogeneic hematopoietic cell transplantation, acute GVHD, angiogenic factor, epidermal growth factor, vascular endothelial growth factor, placental growth factor, follistatin
Background
In the United States, nearly 7,000 patients undergo allogeneic HCT annually in an effort to cure hematologic malignancies and other bone marrow disorders. Approximately 50% of these patients will experience acute graft-versus-host disease (aGVHD), a complication in which cells from the immunocompetent donor graft attack the recipient’s organs and tissues(1). Only one half of aGVHD patients achieve a durable response to first-line therapy with corticosteroids(2), and many others develop severe infections resulting from the immunocompromised state and impairment of skin and mucosal barrier integrity. As a result, many patients with severe aGVHD are at significant risk of death(3). Identification of novel treatment approaches that can alleviate inflammation, spare infectious immunity, and promote healing is critical for improving patient outcomes.
A host vascular proliferative response accompanying aGVHD was first described by Brent and Medawar in the 1960s(4), although it was not clear whether the angiogenic response in aGVHD could improve outcomes by facilitating healing, or was detrimental to outcomes by contributing to inflammation. Recently, mechanisms underlying this critical observation have been suggested using rodent models. Specifically, vasculogenesis accompanies aGVHD with a concomitant increase alpha-v integrin expression on endothelial cells in tissues targeted by aGVHD, a pathologic reaction that can be inhibited by a negative regulator of neovascularization, micro RNA-100, and by the alpha-v integrin inhibitor cilengitide (5, 6). Despite these advances, tools to therapeutically target neovascularization in human aGVHD are lacking. Recent literature suggests that angiogenic factors (AF), soluble mediators that support the development of new blood vessels, may contribute to favorable outcomes by providing trophic factors for wound healing after injury. This principle has been demonstrated in the autoimmune inflammatory bowel disease (IBD) setting with epidermal growth factor (EGF), an epithelial and endothelial mitogen that enhances angiogenic responses in tissues (7, 8). In IBD, circulating EGF levels have been shown to be low(9), and supplementation has induced remissions in a randomized trial(10).
Studies of additional such AF capable of repairing host tissues and their associations with aGVHD outcomes are emerging. Patients with single nucleotide polymorphisms (SNPs) associated with increased production of vascular endothelial growth factor (VEGF) have a reduced incidence of grade II-IV aGVHD, including gastrointestinal aGVHD(11). In addition, SNPs within the gene encoding thrombomodulin, a constitutively expressed endothelial factor that enhances angiogenesis(12), are associated with survival after onset of aGVHD(13). These genetic studies suggest that variations in the patient’s capacity to repair tissues after both the damage from the conditioning regimen and at the onset of aGVHD are clinically relevant. However, not all reports have consistently shown a beneficial role for angiogenic factors in aGVHD. High circulating levels of VEGF have been shown to be protective in some aGVHD studies(14, 15) but not others(16, 17). It is possible that other factors contributing to the angiogenic milieu may be responsible for the discrepant results. For example, angiopoietin-2 (Ang2) is an AF that can have either pro-angiogenic or anti-angiogenic function depending upon the context(18). In allogeneic HCT, elevated levels of Ang2 are reported to reflect endothelial cell activation/dysfunction and are associated with a poor prognosis in aGVHD(17, 19, 20).
Given these disparate associations of AF with outcomes in aGVHD, we sought to more broadly characterize the angiogenic milieu in aGVHD and determine association of individual factors with clinical outcomes. Furthermore, we compared serum versus plasma levels of AF, owing to the potential for variability contributed by factor release by activated platelets, a nontrivial matter for future studies of AF in human disease. Thirteen AF were first tested in a pilot study, followed by validation of 6 AF using samples from two multicenter aGVHD treatment trials, Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0302(21), a randomized four-arm phase II clinical trial for newly diagnosed aGVHD where serum was collected, and BMT CTN 0802(22), a randomized phase III study of the addition of mycophenolate mofetil versus placebo to corticosteroids in newly diagnosed aGVHD, where plasma was collected. Overall, we hypothesized that patients with abundant circulating angiogenic factors (AF) involved in repair/regeneration would have improved outcomes compared to those with higher levels of AF involved in damage/inflammation.
Patients and Methods
Discovery Cohort
We measured circulating levels of EGF, fibroblast growth factor (FGF)-1, FGF-2, heparin binding-EGF-like growth factor, VEGF-A, -C, and –D, Ang2, endothelin-1, endoglin (sEng), follistatin (FS), leptin, placental growth factor (PlGF) in HCT recipients at onset of grade III-IV acute GVHD (patient discovery set, N=17) compared to recipients without aGVHD at 3 months post-HCT (N=17) and healthy stem cell donors (N=16). Therefore, samples from both HCT patients without aGVHD and healthy donors were tested as controls. Samples were analyzed by MILLIPLEX® (Millipore, Billerica, MA, USA) magnetic bead array and performed in duplicate. For the pilot study, plasma samples were collected in sodium heparin tubes, except for 13 of the acute GVHD specimens, which were serum. The mean coefficient of variation for the analytes ranged from 1.9% – 7.5%.
Validation Cohorts
AF demonstrating statistically significant <0.5-fold or >1.5-fold differences from controls in the pilot study were selected for validation in samples from two multicenter treatment trials for initial therapy of aGVHD: BMT CTN 0302 (N=105) and BMT CTN 0802 (N=158). Serum (0302) or plasma (0802) samples obtained at aGVHD onset and at day 28 post-GVHD treatment were selected for study. All patients with available aGVHD onset and day 28 post-aGVHD therapy samples were included in this study. Samples from aGVHD onset in these two trials were further compared to serum samples from a control cohort of HCT patients without acute or chronic GVHD (N=53). These control samples were collected at 3-months post-HCT in University of Minnesota allogeneic HCT recipients, a time point consistent with the pilot study. Samples were acquired after obtaining informed consent and approval from the University of Minnesota Institutional Review Board and in accordance with the Declaration of Helsinki.
Statistical analysis
Statistical comparisons across categorical variables were completed using chi-square tests. Differences in continuous variables across categories were completed using Kruskal-Wallis tests for non-parametric data(23). Differences between AF levels in onset and day 28 samples were determined using Wilcoxon signed rank tests(24). Kaplan-Meier estimates were used to determine the probability of 2-year survival, with differences between curves determined using log-rank tests(25). Cox regression was used to determine the independent effects of clinical factors and angiogenic biomarkers on 6-month and overall survival(26). Correlations between continuous variables were determined by Spearman’s rank correlation(27). Receiver operating characteristic (ROC) curves were generated to determine the value of the tested AF in discriminating aGVHD from no aGVHD. Statistical analyses were performed using JMP 10.0.0 (SAS institute, Cary, NC).
Results
Discovery Set
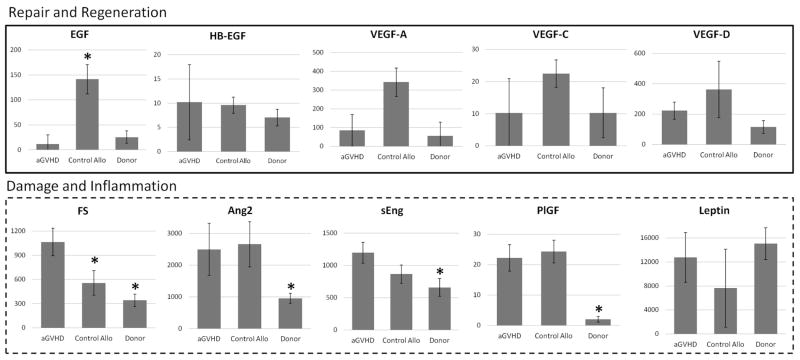
In the discovery cohort, levels of multiple angiogenic factors differed at aGVHD onset compared to controls (Figure 1). Specifically, amongst the repair and regeneration factors, the median EGF level was 10-fold lower in aGVHD compared to allogeneic HCT patients without aGVHD. Similar to EGF, the median VEGF-A level was 5-fold lower in patients with aGVHD compared to control allogeneic HCT patients and 1.5-fold higher than normal donors (overall p-value of 0.07). Based on these data and its importance in angiogenesis, VEGF-A was retained for validation studies. Four AF associated with damage/inflammation were significantly elevated in aGVHD patients compared to healthy donors: Ang2, sEng, FS, and PlGF. Too few patients had detectable levels of endothelin-1, FGF-1, and FGF-2 for statistical comparison (not shown). Using the pilot study data, 6 AF were selected for validation: FS, EGF, VEGF-A, Ang2, sEng, and PlGF.
Figure 1. Discovery set.
All results are pg/mL. *Denotes controls versus aGVHD. Abbreviations: aGVHD=acute graft-versus-host disease, allo HCT=allogeneic hematopoietic cell transplantation, Ang2=angiopoietin-2, EGF=epidermal growth factor, FS=follistatin, PlGF=placental growth factor, sEng=soluble endoglin, VEGF-A=vascular endothelial growth factor-A.
Validation cohorts
Characteristics of the patients in the validation cohorts are detailed in Table 1. The median age was 6–8 years higher in the control cohort (median 58 years vs. 50 and 52 years, p=0.01), and consequently more patients in the control cohort underwent RIC as opposed to myeloablative HCT. Patients in the control cohort had a higher proportion with umbilical cord blood as the stem cell source (p<0.001), reflecting institutional practices in unrelated donor HCT. The majority of patients in the BMT CTN trials had mild (grade I-II) aGVHD. There was no difference in the proportion of clinical responses (complete plus partial responses) to first line therapy at day 28 between the two BMT CTN studies (70.5% and 74.7%, p=0.19).
Table 1.
Patient characteristics.
| Variable | BMT CTN 0302 | BMT CTN 0802 | Control Allo HCT | p | ||||
|---|---|---|---|---|---|---|---|---|
| (N=105) | % | (N=158) | % | (N=53) | % | |||
| Age, median (range) | 50.3 (7.5 – 69.9) | 52.4 (9.1 – 76.3) | 58 (19–72) | 0.01 | ||||
| Donor type | <0.001 | |||||||
| MSD | 42 | 40.0% | 50 | 31.6% | 32 | 60.4% | ||
| Matched URD | 37 | 35.2% | 84 | 53.2% | 1 | 1.9% | ||
| Mismatched URD | 2 | 1.9% | 2 | 1.3% | 0 | 0.0% | ||
| Other related | 7 | 6.7% | 11 | 7.0% | 0 | 0.0% | ||
| UCB | 16 | 15.2% | 2 | 1.3% | 20 | 37.7% | ||
| Missing | 1 | 1.0% | 9 | 5.7% | 0 | 0.0% | ||
| Conditioning | <0.001 | |||||||
| MA | 66 | 62.9% | 97 | 61.4% | 13 | 24.5% | ||
| RIC | 38 | 36.2% | 52 | 32.9% | 40 | 75.5% | ||
| Missing | 1 | 1.0% | 9 | 5.7% | 0 | 0.0% | ||
| Days to aGVHD, median (range) | 33 (13–146) | 34 (11–165) | N/A | 0.9 | ||||
| Initial GVHD Grade | N/A | 0.48 | ||||||
| I | 12 | 11.4% | 20 | 12.7% | ||||
| II | 64 | 61.0% | 90 | 57.0% | ||||
| III | 26 | 24.8% | 29 | 18.4% | ||||
| IV | 2 | 1.9% | 5 | 3.2% | ||||
| Missing | 1 | 1.0% | 14 | 8.9% | ||||
| Day 28 response | N/A | 0.19 | ||||||
| CR | 54 | 51.4% | 76 | 48.1% | ||||
| PR | 20 | 19.0% | 42 | 26.6% | ||||
| NR | 29 | 27.6% | 31 | 19.6% | ||||
| Missing | 2 | 1.9% | 9 | 5.7% | ||||
Abbreviations: aGVHD=acute graft-versus-host disease, allo HCT=allogeneic hematopoietic cell transplant, BMT CTN=Blood and Marrow Transplant Clinical Trials Network, CR=complete response, MA-myeloablative, MSD=matched sibling donors, N/A=not applicable, NR=no response, PR=partial response, RIC=reduced intensity conditioning, UCB=umbilical cord blood, URD-unrelated donor.
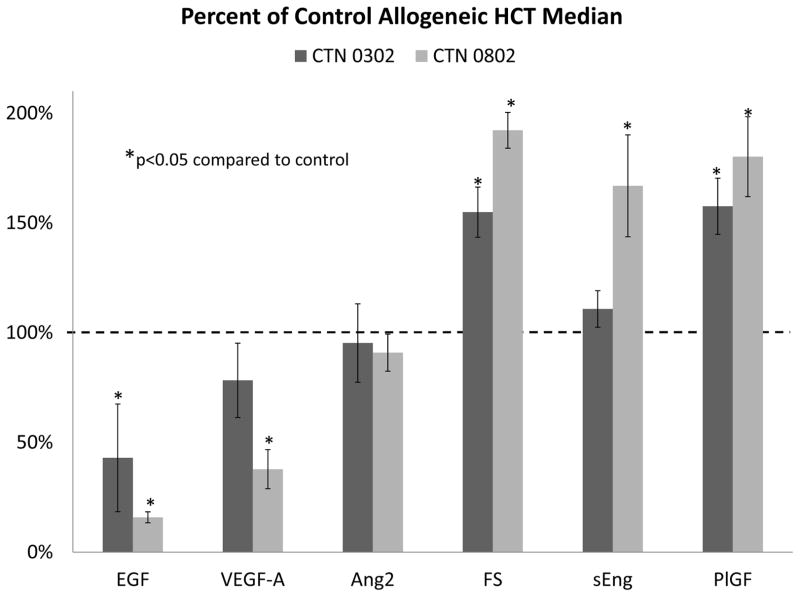
Levels of 5 of the 6 angiogenic factors studied showed significant differences at onset of aGVHD compared to control recipients without aGVHD (Figure 2, Supplemental Table S1). Initially, we compared serum levels (from CTN 0302) and plasma (from CTN 0802) angiogenic factors to serum controls. VEGF-A and EGF were significantly lower in patients with aGVHD compared to controls, similar to findings in the pilot study. Additionally, plasma levels of these factors were even lower than the serum levels. The serum levels of VEGF-A in CTN 0302 were not statistically different from controls, while EGF levels were significantly lower in both serum and plasma from aGVHD patients. Ang2 serum or plasma levels were similar to controls. Levels of both serum and plasma FS and PlGF were significantly elevated in patients with onset aGVHD compared to control allogeneic HCT patients, but the increase was greater in plasma. Plasma endoglin levels (from CTN 0802) were significantly elevated over control serum. This was not seen in aGVHD serum (from CTN 0302).
Figure 2. Circulating angiogenic factors at onset of aGVHD in two aGVHD treatment trials versus allogeneic HCT controls.
Abbreviations: Ang2=angiopoietin-2, EGF=epidermal growth factor, FS=follistatin, PlGF=placental growth factor, sEng=soluble endoglin, VEGF-A=vascular endothelial growth factor-A.
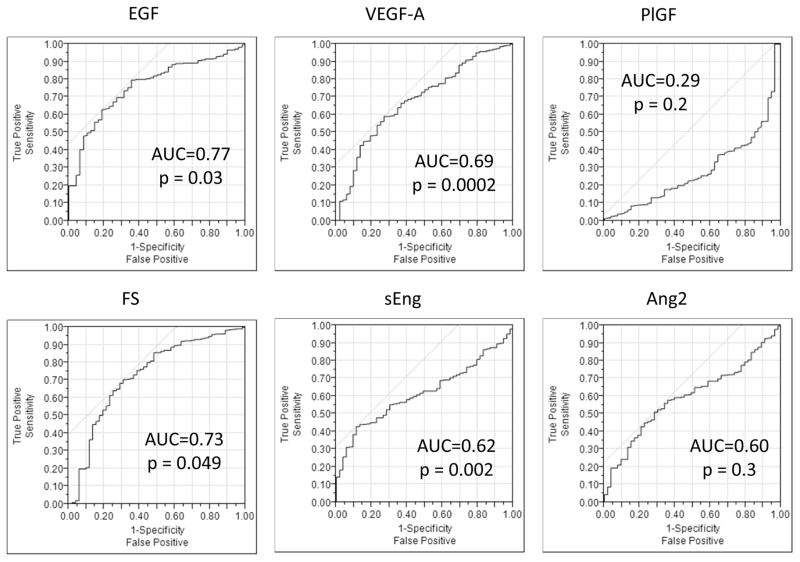
Receiver operating characteristic analyses of data pooled from BMT CTN 0302 and 0802 revealed that 4 of the 6 AF levels could discriminate aGVHD presence versus its absence: EGF, VEGF-A, FS, and sEng (Figure 3). The highest sensitivity was observed with EGF at a level of 70.9 pg/ml, demonstrating a sensitivity of 80%, albeit with a lower specificity at 35%, and an area under the curve (AUC) of 0.76 (p=0.03). sEng at a level of 1,485.7 pg/ml had the highest discrimination with, a sensitivity of 42%, a high specificity of 89%, with an AUC of 0.63 (p=0.002). ROC curves generated using only serum samples from CTN 0302 versus serum controls, only VEGF-A, with an AUC of 0.6 (p=0.046) could discriminate between aGVHD and controls without GVHD. In non-GVHD controls, donor type did influence AF levels (not shown). Reduced intensity conditioning was associated with lower Ang2 levels than myeloablative conditioning (median 3407 pg/ml vs. 5235 pg/ml, p=0.038). No other clinical variables associated with AF levels in controls and thus did not influence the ROC analysis.
Figure 3. Receiver operating characteristic (ROC) curves for 6 angiogenic factors discriminating between the presence or absence of acute graft-versus-host disease.
Abbreviations: Ang2=angiopoietin-2, AUC=area under the curve, EGF=epidermal growth factor, FS=follistatin, PlGF=placental growth factor, sEng=soluble endoglin, VEGF-A=vascular endothelial growth factor-A.
Association of AF with patient and donor factors
AF levels were not associated with time to aGVHD onset, severity, or organ tropism consistently in either aGVHD trial (Tables S2–4). EGF levels were significantly lower in recipients of myeloablative compared to reduced intensity conditioning in CTN 0302 (38 versus 89 pg/ml, p=0.005), but not CTN 0802 (p=0.4). In CTN 0802, recipient age was associated with a trend toward higher onset FS levels (p=0.07) as well as a trend toward lower day 28 EGF levels (p=0.06). PlGF levels were 1.5-fold higher at the onset of aGVHD in URD compared to HLA-matched siblings HCT in both trials (CTN 0302, p=0.03; CTN 0802, p=0.007). No other clinical factors were associated with levels of these 6 AF at onset or day 28 after initial therapy for aGVHD.
Association of AF with day 28 response to initial aGVHD therapy
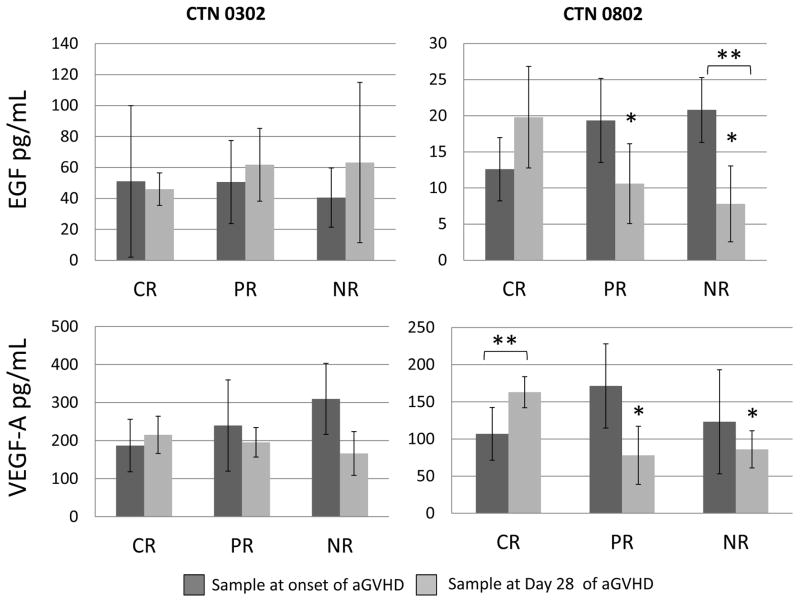
In CTN 0802 plasma samples, response to aGVHD therapy was associated with significant differences in circulating levels of EGF and VEGF-A at day 28 post-initial aGVHD therapy. EGF levels were 2-fold higher in patients with a complete response (CR, 20 pg/ml, vs. 11 pg/ml for partial response [PR] and 8 pg/ml for no response [NR] respectively, p=0.03, Figure 4). In addition, EGF levels significantly decreased from the onset of acute GVHD to day 28 of therapy in patients who had NR (p=0.01). Similarly for VEGF-A, patients with a CR had 2-fold higher levels at day 28 (CR 163 pg/ml, vs 79 pg/ml for PR and 86 pg/ml for NR, p=0.008). In patients with a CR to therapy, VEGF-A levels significantly increased from the onset of aGVHD to day 28 of therapy (p=0.006). In contrast, in CTN 0302 serum samples, we observed no differences in EGF levels with response (CR 46 pg/ml, PR 62 pg/ml vs NR 63 pg/ml, p=0.2) nor VEGF-A levels (CR 215 pg/ml, PR 195 pg/ml vs NR 166 pg/ml, p=0.8). Importantly, the 0302 samples were serum (not plasma) which generally is associated with higher levels of these markers due to release from platelets (28, 29). Additionally, levels of Ang2 and sEng significantly decreased in patients experiencing a CR or PR at day 28 (p<0.001 for each) across both cohorts. However, levels of Ang2 and sEng did not decrease (p=0.2 and p=0.1, respectively) in patients with NR at day 28 (Supplemental Figure 1).
Figure 4. Association of circulating EGF and VEGF-A at onset of aGVHD and at day 28 after initial aGVHD therapy and clinical response.
*Denotes p<0.05 compared to complete response. **Denotes p<0.05 day 28 sample compared to the sample from the onset of aGVHD. Abbreviations: CR=complete response, EGF=epidermal growth factor, PR=partial response, NR=no response, VEGF-A=vascular endothelial growth factor-A.
Association of AF with 6-month survival
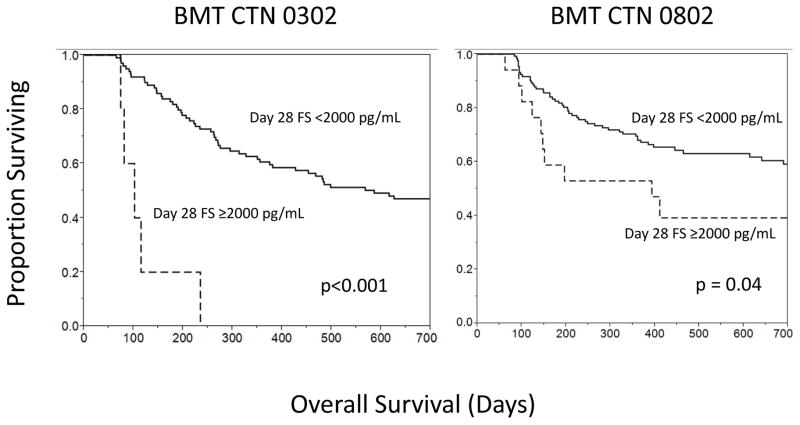
In CTN 0302 univariate survival analyses, elevated levels of sEng (p=0.04) and Ang2 at aGVHD onset (p=0.02) and at day 28 after initial aGVHD therapy (p=0.003) were associated with lower 6-month survival. In CTN 0802, elevated FS (p=0.006) and PlGF (p=0.006) levels at day 28 after aGVHD therapy were associated with lower 6-month survival. These factors – Ang2, sEng, FS, and PlGF – were tested, along with established important clinical variables for outcomes after allogeneic HCT, including patient age, aGVHD grade, and day 28 response to initial therapy (30), in a multivariate analysis of 6-month survival. In both trials, day 28 FS was an independent factor for 6-month survival (Table 2). The day 28 FS level of >2,000 pg/ml showed the strongest association with poor survival (Figure 5). In CTN 0302, 80% of patients with a FS level below the cutoff were alive at 6 months vs. 20% of patients with FS above the cutoff (p=0.001). In CTN 0802, 83% of patients with a FS below the cutoff were alive at 6 months vs. 58% with a FS above the cutoff (p=0.02). In the controls without aGVHD, PlGF at day +100 was the only AF variable associated with survival, with poorer survival when the day 100 PlGF level was > 40 pg/mL (RR 49.5, 95% CI 2.0 – 1,250, p=0.02).
Table 2.
Multivariate analysis of 6-month survival.
| BMT CTN 0302 | BMT CTN 0802 | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p | HR | 95% CI | p |
| Age > 40 years | 3.9 | 1.1 – 17.7 | 0.03 | 3.3 | 1.1 – 14.6 | 0.03 |
| Grade | 0.17 | 0.07 | ||||
| 1 | 1.0 | - | 1.0 | - | ||
| 2 | 2.0 | 0.34 – 39.0 | 0.7 | 0.2 – 2.7 | ||
| 3 | 5.1 | 0.85 – 100.8 | 2.0 | 0.5 – 8.7 | ||
| 4 | 2.8 × 108 | 0 – 132.6 | 1.8 × 109 | 0 – 2.2 | ||
| Day 28 Response | 0.01 | 0.001 | ||||
| CR | 1.0 | - | 1.0 | - | ||
| PR | 0.7 | 0.2 – 2.6 | 1.7 | 0.4 – 6.1 | ||
| NR | 3.7 | 1.2 – 11.4 | 5.0 | 2.0 – 13.7 | ||
| Day 28 FS ≥ 2000 pg/ml | 9.3 | 1.3 – 59.1 | 0.002 | 2.8 | 1.04 – 6.9 | 0.04 |
| Onset Ang2 ≥ 8000 pg/ml | 3.0 | 0.6 – 11.7 | 0.05 | 0.3 | 0.03 – 1.2 | 0.09 |
| Day 28 Ang2 ≥ 8000 pg/ml | 0.6 | 0.05 – 5.1 | 0.14 | 5.2 | 1.7 – 13.9 | 0.006 |
| Day 28 sEng ≥ 3000 pg/ml | 0.9 | 0.04 – 8.5 | 0.6 | 1.1 | 0.5 – 2.6 | 0.7 |
| Day 28 PlGF ≥ 20 pg/ml | 4.7 | 1.4 – 21.7 | 0.005 | 1.8 | 0.6 – 6.7 | 0.3 |
Abbreviations: Ang2=angiopoetin-2, CI=confidence interval, CR=complete response, FS=follistatin, HR=hazard ratio, NR=no response, PlGF=placental growth factor, PR=partial response.
Figure 5.
2 year survival by follistatin (FS) level at day 28 of aGVHD.
Discussion
Without adequate levels of important trophic angiogenic factors, host tissues may have compromised healing in the wake of severe aGVHD. Our study is the first to identify markedly low EGF levels at the onset of aGVHD. Low circulating EGF levels have been identified in inflammatory bowel disease(9), which has similarities in presentation and perhaps pathogenesis with aGVHD(31). Our results provide a rationale for supplementation of deficient factors to aid in repair as an adjunct to immunosuppression. The utility of angiogenic factor replacement has been demonstrated with the use of EGF-containing enemas in left-sided ulcerative colitis(10). The widespread tissue damage associated with aGVHD suggests that systemic administration of such factors may be needed. During steady state, the predominant luminal sources of EGF in the gastrointestinal tract are salivary and Brunner’s glands, although Paneth cells, a known target of GVHD, have also been identified as producers of EGF(32, 33). We hypothesize that the low EGF levels we observed in GVHD are related to an alloimmune attack on EGF-producing cells, although increased EGF loss through protein-losing enteropathy or other mechanism is also possible.
Our initial pilot data were validated in two separate cohorts of patients from multicenter GVHD trials, lending external validity to the results. We also analyzed both serum and plasma in the different cohorts and were able to confirm that plasma is the preferred sample type for measuring EGF and VEGF-A. We note that only a few patients had severe grade III-IV aGVHD in the BMT CTN trials, and this may have compromised our ability to observe differences in these analytes with varying aGVHD severity. These validated findings associating angiogenic factors, aGVHD response, and survival can be further hypothesis-generating for future studies into the pathophysiology and therapy of aGVHD.
Levels of EGF and VEGF-A were significantly lower and more closely associated with response in CTN 0802, where plasma, as opposed to serum, was analyzed. Serum has higher levels of EGF and VEGF-A relative to plasma due to release from activated platelets(28, 29). Some of the differences we observed regarding AF and outcomes in the two trials may reflect the different sample sources, and further studies of these factors should ideally use plasma samples. Nonetheless, EGF serum levels were still significantly lower in aGVHD in CTN 0302 compared to recipient controls which were 2–3 times the reported normal serum levels22,23. It is possible that increased EGF production beyond the normal basal state accompanies post-HCT recovery, but this requires further study.
In addition to the relative deficiency of circulating trophic growth factors for healing in aGVHD (EGF and VEGF-A), we also identified markers of endothelial damage after allogeneic HCT which were further dysregulated in aGVHD (FS and PIGF). Increased levels of circulating PlGF may reflect an underlying enhanced inflammatory state with higher levels of TNFα (rank correlation 0.58, p=0.001, unpublished), and increasing levels of FS may reflect greater endothelial damage and/or neovascularization. The results of this study contributes to the findings of endothelial-derived factors that are abnormal in aGVHD (34, 35). Interestingly, despite widespread inflammation and endothelial activation/injury, thrombosis is infrequent in severe aGVHD(36).
We observed that FS levels are significantly elevated post-allogeneic HCT and are even higher after onset of aGVHD in both serum and plasma. The tissue source of excess FS in aGVHD is unknown. FS was first identified for its follicle-stimulating hormone inhibitory activity in ovarian follicular fluid,(37) although it has more recently been shown to play a role in angiogenesis and function as a specific binding protein (and neutralizer) of activin-A, a ubiquitously expressed protein with pro-inflammatory and pro-fibrotic properties that is elevated after allogeneic HCT(15, 38, 39). However, FS is also a key regulator of tissue regeneration after injury(40). Supplemental FS was recently shown to reduce mortality in rodent models of gram-negative sepsis(41) and inflammatory bowel disease(42), and FS overexpression was associated with improved muscle healing after laceration(43). We recently identified that circulating FS is significantly higher in healthy pregnant women carrying a male fetus as opposed to a female fetus(44), suggesting that elevated FS may reflect adaptation to a greater immunologic challenge for the mother. Its association with poor outcomes in both GVHD trials suggest that marked elevations in FS may reflect endothelial damage(45, 46), an established manifestation of steroid-refractory aGVHD(19), and/or neovascuarlization(43), which is associated with worsened aGVHD in rodents(5, 6).
In summary, we observed that EGF and VEGF-A, factors involved in repair and regeneration, are low in aGVHD compared to controls. EGF and VEGF-A are also associated with response to therapy, best recognized in plasma samples. AFs indicative of damage and inflammation, FS and PlGF, were elevated at the onset of aGVHD in both serum and plasma. Elevated FS at day 28 after initial aGVHD is associated with poor 6-month survival in both CTN 0302 and 0802. Ang2 and sEng decrease over time after effective aGVHD therapy. Finally, PlGF levels are disproportionately elevated in aGVHD after URD HCT but are also associated with overall survival in patients without aGVHD. Mechanistic studies are needed to clarify the role of these factors in post-HCT recovery, yet these data support the critical impact of the host healing capacity as well as endothelial damage for clinical response and survival after aGVHD.
Supplementary Material
Supplemental Figure 1. Association of Ang2 and sEng with response to acute graft-versus-host disease therapy at day 28. Asterisk denotes p<0.05 by Wilcoxon signed rank.
Abbreviations: Ang2=angiopoietn-2, CR=complete response, NR=no response, NS=not significant, PR=partial response
Table S1. Differences in 6 AF at onset of aGVHD compared to controls.
Table S2. Correlation of 6 AF with time to onset of aGVHD. Lower levels of VEGF-A and EGF were associated with the development of aGVHD earlier post-HCT in CTN 0302, while higher levels of Ang2 were associated with earlier development of aGVHD in CTN 0802.
Table S3. Association of 6 AF and aGVHD severity. PlGF was associated aGVHD severity, with higher levels observed in less severe disease at onset and at day 28 in CTN 0302.
Table S4. Association of 6 AF and severity of organ involvement. We evaluated whether levels of any of the 6 AF were associated with severity of organ involvement at onset and at day 28 after initial aGVHD therapy. Associations of severity of organ involvement were observed only in CTN 0302. At aGVHD onset, patients in CTN 0302 with stage 1 liver aGVHD had significantly elevated FS (median 1,250 pg/ml for stage 1, compared 671 pg/ml for stage 0, 438 pg/ml for stage 1, 458 pg/ml for stage 3, p=0.004). Also at onset, sEng was significantly lower in patients with upper GI aGVHD (median 827 pg/ml versus 1,162 pg/ml, p=0.02). At day 28, patients with stage 3 liver aGVHD had the highest Ang2 (median 3,807 pg/ml for stage 3, compared to 2,359 pg/ml for stage 0, 2,072 pg/ml for stage 1, and 1,746 for stage 2, p=0.02).
Circulating levels of EGF and VEGF-A are low at the onset of aGVHD.
GF and VEGF-A are associated with day 28 response to aGVHD therapy in CTN 0802.
FS and PlGF are elevated at the onset of aGVHD in both serum and plasma.
Elevated FS at day 28 after initial aGVHD is associated with poor 6-month survival
Acknowledgments
The authors gratefully acknowledge the BMT CTN 0302 and 0802 study investigators and participating centers. The studies conducted by the BMT CTN were completed with the support of grant U10HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute at the National Institutes of Health. The authors would also like to thank Mr. Michael Ehrhardt from the Cytokine Reference Laboratory of the University of Minnesota for laboratory analyses. This study was supported in part by the Office of Research in Women’s Health and the National Institute of Child Health and Human Development, Oregon BIRCWH Award Number 2K12HD043488 (SGH and LFN), the Medical Foundation of Oregon (SGH), and by NIH P30 CA77598 and P01CA111412, utilizing the Biostatistics and Bioinformatics Shared Resource of the Masonic Cancer Center.
Footnotes
Financial disclosure: The authors of this manuscript have no relevant conflicts of interest to disclose.
Authorship
SGH designed the study, provided patient samples for analysis, analyzed data, and wrote the manuscript. MRV, KRS, LFN, GM, and SAC provided patient samples and edited the manuscript. FH and TED analyzed data and edited manuscript. APM analyzed patient samples and edited the manuscript. GMV, AS, MLM, BRB, and DJW edited the manuscript. All authors approved of the final version of the manuscript.
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holtan SG, Pasquini M, Weisdorf DJ. Acute graft-versus-host disease: a bench-to-bedside update. Blood. 2014;124:363–373. doi: 10.1182/blood-2014-01-514786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 3.Cahn JY, Klein JP, Lee SJ, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint Societe Francaise de Greffe de Moelle et Therapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood. 2005;106:1495–1500. doi: 10.1182/blood-2004-11-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brent L, Medawar P. Quantitative studies on tissue transplantation immunity. VII. The normal lymphocyte transfer reaction. Proceedings of the Royal Society of London Series B, Containing papers of a Biological character. 1966;165:281–307. doi: 10.1098/rspb.1966.0069. [DOI] [PubMed] [Google Scholar]
- 5.Penack O, Henke E, Suh D, et al. Inhibition of neovascularization to simultaneously ameliorate graft-vs-host disease and decrease tumor growth. Journal of the National Cancer Institute. 2010;102:894–908. doi: 10.1093/jnci/djq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonhardt F, Grundmann S, Behe M, et al. Inflammatory neovascularization during graft-versus-host disease is regulated by alphav integrin and miR-100. Blood. 2013;121:3307–3318. doi: 10.1182/blood-2012-07-442665. [DOI] [PubMed] [Google Scholar]
- 7.Hase S, Nakazawa S, Tsukamoto Y, Segawa K. Effects of prednisolone and human epidermal growth factor on angiogenesis in granulation tissue of gastric ulcer induced by acetic acid. Digestion. 1989;42:135–142. doi: 10.1159/000199838. [DOI] [PubMed] [Google Scholar]
- 8.Ravindranath N, Wion D, Brachet P, Djakiew D. Epidermal growth factor modulates the expression of vascular endothelial growth factor in the human prostate. Journal of andrology. 2001;22:432–443. [PubMed] [Google Scholar]
- 9.Oikonomou KA, Kapsoritakis AN, Kapsoritaki AI, et al. Downregulation of serum epidermal growth factor in patients with inflammatory bowel disease. Is there a link with mucosal damage? Growth factors. 2010;28:461–466. doi: 10.3109/08977194.2010.527967. [DOI] [PubMed] [Google Scholar]
- 10.Sinha A, Nightingale J, West KP, Berlanga-Acosta J, Playford RJ. Epidermal growth factor enemas with oral mesalamine for mild-to-moderate left-sided ulcerative colitis or proctitis. The New England journal of medicine. 2003;349:350–357. doi: 10.1056/NEJMoa013136. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Lee NY, Lee MH, Sohn SK. Vascular endothelial growth factor gene polymorphisms may predict the risk of acute graft-versus-host disease following allogeneic transplantation: preventive effect of vascular endothelial growth factor gene on acute graft-versus-host disease. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14:1408–1416. doi: 10.1016/j.bbmt.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Li JY, Su CH, Wu YJ, et al. Therapeutic angiogenesis of human early endothelial progenitor cells is enhanced by thrombomodulin. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2518–2525. doi: 10.1161/ATVBAHA.111.235143. [DOI] [PubMed] [Google Scholar]
- 13.Rachakonda SP, Penack O, Dietrich S, et al. Single-Nucleotide Polymorphisms Within the Thrombomodulin Gene (THBD) Predict Mortality in Patients With Graft-Versus-Host Disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:3421–3427. doi: 10.1200/JCO.2013.54.4056. [DOI] [PubMed] [Google Scholar]
- 14.Min CK, Kim SY, Lee MJ, et al. Vascular endothelial growth factor (VEGF) is associated with reduced severity of acute graft-versus-host disease and nonrelapse mortality after allogeneic stem cell transplantation. Bone marrow transplantation. 2006;38:149–156. doi: 10.1038/sj.bmt.1705410. [DOI] [PubMed] [Google Scholar]
- 15.Nachbaur D, Schumacher P, Auberger J, Clausen J, Kircher B. Vascular endothelial growth factor and activin-a serum levels following allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13:942–947. doi: 10.1016/j.bbmt.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Lunn RA, Sumar N, Bansal AS, Treleaven J. Cytokine profiles in stem cell transplantation: possible use as a predictor of graft-versus-host disease. Hematology. 2005;10:107–114. doi: 10.1080/10245330400001975. [DOI] [PubMed] [Google Scholar]
- 17.Porkholm M, Bono P, Saarinen-Pihkala UM, Kivivuori SM. Higher angiopoietin-2 and VEGF levels predict shorter EFS and increased non-relapse mortality after pediatric hematopoietic SCT. Bone marrow transplantation. 2013;48:50–55. doi: 10.1038/bmt.2012.101. [DOI] [PubMed] [Google Scholar]
- 18.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luft T, Dietrich S, Falk C, et al. Steroid-refractory GVHD: T-cell attack within a vulnerable endothelial system. Blood. 2011;118:1685–1692. doi: 10.1182/blood-2011-02-334821. [DOI] [PubMed] [Google Scholar]
- 20.Tichelli A, Gratwohl A. Vascular endothelium as ‘novel’ target of graft-versus-host disease. Best practice & research Clinical haematology. 2008;21:139–148. doi: 10.1016/j.beha.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Alousi AM, Weisdorf DJ, Logan BR, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114:511–517. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolanos-Meade J, Logan BR, Alousi AM, et al. Phase III clinical trial steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute graft-versus-host disease: BMT CTN 0802. Blood. 2014 [Google Scholar]
- 23.Kruskal WH, Wallis WA. Use of Ranks in One-Criterion Variance Analysis. Journal of the American Statistical Association. 1952;47:583–621. [Google Scholar]
- 24.Wilcoxon F. Individual Comparisons by Ranking Methods. Biometrics Bulletin. 1945;1:80–83. [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 26.Cox DR. Regression models and life-tables. J Roy Statist Soc Ser B. 1972;34:187–220. [Google Scholar]
- 27.Lehman A. Jmp For Basic Univariate And Multivariate Statistics: A Step-by-step Guide. SAS Press. 2005:123. [Google Scholar]
- 28.Biancotto A, Feng X, Langweiler M, Young NS, McCoy JP. Effect of anticoagulants on multiplexed measurement of cytokine/chemokines in healthy subjects. Cytokine. 2012;60:438–446. doi: 10.1016/j.cyto.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnan VV, Ravindran R, Wun T, Luciw PA, Khan IH, Janatpour K. Multiplexed measurements of immunomodulator levels in peripheral blood of healthy subjects: Effects of analytical variables based on anticoagulants, age, and gender. Cytometry Part B, Clinical cytometry. 2014;86:426–435. doi: 10.1002/cyto.b.21147. [DOI] [PubMed] [Google Scholar]
- 30.MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood. 2010;115:5412–5417. doi: 10.1182/blood-2009-12-258442. [DOI] [PubMed] [Google Scholar]
- 31.Asplund S, Gramlich TL. Chronic mucosal changes of the colon in graft-versus-host disease. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1998;11:513–515. [PubMed] [Google Scholar]
- 32.Poulsen SS, Nexo E, Olsen PS, Hess J, Kirkegaard P. Immunohistochemical localization of epidermal growth factor in rat and man. Histochemistry. 1986;85:389–394. doi: 10.1007/BF00982668. [DOI] [PubMed] [Google Scholar]
- 33.Levine JE, Huber E, Hammer ST, et al. Low Paneth cell numbers at onset of gastrointestinal graft-versus-host disease identify patients at high risk for nonrelapse mortality. Blood. 2013;122:1505–1509. doi: 10.1182/blood-2013-02-485813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leblond V, Salehian BD, Borel C, et al. Alterations in natural anticoagulant levels during allogeneic bone marrow transplantation: a prospective study in 27 patients. Bone marrow transplantation. 1993;11:299–305. [PubMed] [Google Scholar]
- 35.Matsuda Y, Hara J, Osugi Y, et al. Serum levels of soluble adhesion molecules in stem cell transplantation-related complications. Bone marrow transplantation. 2001;27:977–982. doi: 10.1038/sj.bmt.1703026. [DOI] [PubMed] [Google Scholar]
- 36.Labrador J, Lopez-Anglada L, Perez-Lopez E, et al. Analysis of incidence, risk factors and clinical outcome of thromboembolic and bleeding events in 431 allogeneic hematopoietic stem cell transplantation recipients. Haematologica. 2013;98:437–443. doi: 10.3324/haematol.2012.069559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esch FS, Shimasaki S, Mercado M, et al. Structural characterization of follistatin: a novel follicle-stimulating hormone release-inhibiting polypeptide from the gonad. Molecular endocrinology. 1987;1:849–855. doi: 10.1210/mend-1-11-849. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura T, Takio K, Eto Y, Shibai H, Titani K, Sugino H. Activin-binding protein from rat ovary is follistatin. Science. 1990;247:836–838. doi: 10.1126/science.2106159. [DOI] [PubMed] [Google Scholar]
- 39.de Kretser DM, O’Hehir RE, Hardy CL, Hedger MP. The roles of activin A and its binding protein, follistatin, in inflammation and tissue repair. Molecular and cellular endocrinology. 2012;359:101–106. doi: 10.1016/j.mce.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Gavino MA, Wenemoser D, Wang IE, Reddien PW. Tissue absence initiates regeneration through Follistatin-mediated inhibition of Activin signaling. eLife. 2013;2:e00247. doi: 10.7554/eLife.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones KL, Mansell A, Patella S, et al. Activin A is a critical component of the inflammatory response, and its binding protein, follistatin, reduces mortality in endotoxemia. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16239–16244. doi: 10.1073/pnas.0705971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dohi T, Ejima C, Kato R, et al. Therapeutic potential of follistatin for colonic inflammation in mice. Gastroenterology. 2005;128:411–423. doi: 10.1053/j.gastro.2004.11.063. [DOI] [PubMed] [Google Scholar]
- 43.Zhu J, Li Y, Lu A, et al. Follistatin improves skeletal muscle healing after injury and disease through an interaction with muscle regeneration, angiogenesis, and fibrosis. The American journal of pathology. 2011;179:915–930. doi: 10.1016/j.ajpath.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Enninga EA, Nevala WK, Creedon DJ, Markovic SN, Holtan SG. Fetal Sex-Based Differences in Maternal Hormones, Angiogenic Factors, and Immune Mediators During Pregnancy and the Postpartum Period. American journal of reproductive immunology. 2014 doi: 10.1111/aji.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phan C, McMahon AW, Nelson RC, Elliott JF, Murray AG. Activated lymphocytes promote endothelial cell detachment from matrix: a role for modulation of endothelial cell beta 1 integrin affinity. Journal of immunology. 1999;163:4557–4563. [PubMed] [Google Scholar]
- 46.Almici C, Skert C, Verardi R, et al. Changes in circulating endothelial cells count could become a valuable tool in the diagnostic definition of acute graft-versus-host disease. Transplantation. 2014;98:706–712. doi: 10.1097/TP.0000000000000385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Association of Ang2 and sEng with response to acute graft-versus-host disease therapy at day 28. Asterisk denotes p<0.05 by Wilcoxon signed rank.
Abbreviations: Ang2=angiopoietn-2, CR=complete response, NR=no response, NS=not significant, PR=partial response
Table S1. Differences in 6 AF at onset of aGVHD compared to controls.
Table S2. Correlation of 6 AF with time to onset of aGVHD. Lower levels of VEGF-A and EGF were associated with the development of aGVHD earlier post-HCT in CTN 0302, while higher levels of Ang2 were associated with earlier development of aGVHD in CTN 0802.
Table S3. Association of 6 AF and aGVHD severity. PlGF was associated aGVHD severity, with higher levels observed in less severe disease at onset and at day 28 in CTN 0302.
Table S4. Association of 6 AF and severity of organ involvement. We evaluated whether levels of any of the 6 AF were associated with severity of organ involvement at onset and at day 28 after initial aGVHD therapy. Associations of severity of organ involvement were observed only in CTN 0302. At aGVHD onset, patients in CTN 0302 with stage 1 liver aGVHD had significantly elevated FS (median 1,250 pg/ml for stage 1, compared 671 pg/ml for stage 0, 438 pg/ml for stage 1, 458 pg/ml for stage 3, p=0.004). Also at onset, sEng was significantly lower in patients with upper GI aGVHD (median 827 pg/ml versus 1,162 pg/ml, p=0.02). At day 28, patients with stage 3 liver aGVHD had the highest Ang2 (median 3,807 pg/ml for stage 3, compared to 2,359 pg/ml for stage 0, 2,072 pg/ml for stage 1, and 1,746 for stage 2, p=0.02).