Abstract
Systemic bone loss is associated with airway inflammatory diseases; yet, strategies to halt disease progression from inhalant exposures are not clear. Vitamin D might be a potentially protective approach against noxious respirable environmental exposures. We sought to determine whether vitamin D supplementation represents a viable lung and bone protective strategy following repetitive inhalant treatments with organic dust extract (ODE) or lipopolysaccharide (LPS) in mice. C57BL/5 mice were maintained on diets with low (1 IU/D/g) or high (10 IU/D/g) vitamin D for 5 weeks, and treated with ODE from swine confinement facilities, LPS, or saline daily for 3 weeks per established intranasal inhalation protocol. Lungs, hind limbs, and sera were harvested for experimental outcomes. Serum 25-hydroxy vitamin D levels were 10-fold different between low/high vitamin D treatment groups with no differences between inhalant agents/saline treatments. Serum calcium levels were not affected. There was no difference in the magnitude of ODE- or LPS-induced inflammatory cell influx or lung histopathology between high/low vitamin D treatment groups. However, high vitamin D treatment reversed the loss of bone mineral density, bone volume, and bone microarchitecture deterioration induced by ODE or LPS as determined by micro-CT analysis. Bone-resorbing osteoclasts were also reduced by high vitamin D treatment. In the low vitamin D treatment groups, ODE induced the greatest degree of airway inflammatory consequences, and LPS induced the greatest degree of bone loss. Collectively, high concentration vitamin D was protective against systemic bone loss, but not airway inflammation, resulting from ODE- or LPS-induced airway injury.
Keywords: animal models, preclinical, disease disorder, osteoporosis, systems biology, bone interactors-other
INTRODUCTION
Inhalant exposure to various environmental agents such as air pollution, particulate matter, organic dust, cigarette smoke, and microbial bioaerosols (e.g. LPS) can significantly contribute to the development of chronic inflammatory respiratory diseases, particularly chronic obstructive pulmonary disease (COPD) and asthma [1–6]. The extrapulmonary or systemic manifestations of airway inflammation are increasingly recognized to contribute to overall disease morbidity. Osteoporosis and fracture represent an important debilitating systemic feature of these inflammatory lung diseases [7–9]. Several risk factors for low BMD including low body mass index, female sex, age, select medications such as glucocorticoids, sedentary life-style, cigarette smoking, and nutritional status are well defined [8, 9]. However, recent studies demonstrate that low BMD and osteoporosis can occur independently of these established risk factors in the context of inflammatory lung disease [7, 8]. This observation suggests a pathogenic association between lung injury and reduced bone mineralization; yet effective mechanisms and strategies to prevent or treat osteoporosis in this setting are not known.
To provide mechanistic insights and develop future prophylactic and/or therapeutic strategies, we have utilized an animal inflammatory lung injury model to delineate the functional role of environmental biohazardous agents focused on complex, agriculture-based organic dusts and specific microbial cell wall components such as LPS [10, 11]. This is based on the observation that agricultural workers, particularly large animal confinement operation workers, have a high prevalence of chronic respiratory disease including COPD and asthma syndromes [2, 4]. Because these workers also have a very high prevalence of musculoskeletal disease (~90% lifetime prevalence [12], we considered an animal model to evaluate skeletal health consequences [11]. Importantly, this study demonstrates that intranasal inhalation of swine confinement facility organic dust extract (ODE), peptidoglycan, or LPS induced significant bone loss, quantified by micro-CT imaging [11]. Bone loss was greatest following ODE and LPS inhalation. However, strategies to prevent bone loss following lung injury induced by these inflammatory bioaerosols have not been investigated, which was the objective of this study.
A potential preventative and therapeutic intervention to target skeletal health and respiratory health is vitamin D. Vitamin D is a steroid hormone synthesized in the skin via sunlight exposure, or it can also be absorbed from dietary sources and supplements. Vitamin D plays an important role in maintaining skeletal health, primarily via regulation of calcium homeostasis and paracrine/autocrine effects on bone metabolism [13]. Vitamin D supplementation reduces fracture risk in humans [14,15], improves fracture healing in mice deficient in vitamin D [16], and prevents ethanol-induced bone loss in mice [17]. In addition, vitamin D displays immunomodulatory effects and anti-inflammatory properties [13]. Low vitamin D levels have been associated with COPD [18] and severe asthma [19], but interventional studies with vitamin D supplementation for airway inflammatory diseases (i.e. asthma) have been somewhat discouraging [20]. However, it remains possible that vitamin D might play a role against systemic bone loss induced by airway injury. In this study, we hypothesized that high concentration vitamin D would be a lung and bone protective strategy following repetitive inhalant treatments with ODE or LPS. We found that high concentration vitamin D supplementation was protective against systemic bone loss, but not airway inflammation, resulting from ODE- or LPS-induced airway injury.
METHODS
Organic dust extract (ODE) and LPS
Swine confinement facility organic dust was used due to its strong pro-inflammatory consequences in human and rodents. Aqueous ODE was collected and prepared as previously described [11, 21]. Briefly, settled surface dust samples (~3 feet off ground) from swine confinement animal feeding operations (~500–700 animals) were collected and 1 gm was placed into sterile Hank’s Balanced Salt Solution (10 ml; Sigma, St. Louis, MO), incubated for one hour at room temperature, and centrifuged for 20 minutes at 2000 x g. The final supernatant was filter sterilized (0.22 µm) in order to remove coarse particles and microorganisms, aliquotted, and stored at −20°C until use.
Stock ODE (100%) was diluted in sterile PBS (pH: 7.4; diluent) to a 12.5% concentration (vol/vol). The 12.5% ODE concentration in 50 µl volume has been previously shown to elicit optimal lung inflammation in mice and is otherwise well-tolerated [10]. Prior to extraction, the dust sample was analyzed using gas chromatography/mass spectrometry as previously described [22]. The concentrations detected of muramic acid (69.4 ng/mg), ergosterol (0.68 ng/mg), and 3-hydroxy fatty acids by carbon chain number (ng/ml; C8: 5.83; C9: 2.30; C10: 0.93; C12: 12.99; C13: 73.92; C14: 363.70; C15: 182.72; C16: 1159.40; C17: 1136.56; C18: 796.55) were similar to previous reports [22]. Diluted aqueous dust extracts contained approximately 4 mg/ml of total protein as measured by nanodrop spectrophotometry (NanoDrop Technologies, Wilmington, DE). The mean (SD) endotoxin concentration within 12.5% ODE was 146.6 (8.0) EU/ml as determined by the limulus amebocyte lysate assay according to manufacturer’s instructions (Lonza, Allendale, NJ). Comparison studies were conducted with LPS (100 ng, Escherichia coli (O55:B5), Sigma; approximation to LPS concentration within ODE) to remain consistent with previous work that showed a robust bone loss phenotype induced by inhalant LPS treatment [11], and to also broaden the applicability of our findings to other indoor and/or outdoor exposures.
Animal Model and Diet
Male C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 3–4 weeks of age. Upon arrival, mice were randomly assigned to a rodent chow (Land O’ Lakes-Purina; Minneapolis, MN) diet of a corn-based rodent supplemented with 1 IU/g or 10 IU/g of 1,25 hydroxyvitamin D. Manufacturer-provided samples of the specialty ordered diet were independently tested by N-P Analytical Laboratories (St. Louis, MO), which reported concentrations of 0.9 IU/gm and 9.2 IU/gm of 1,25-(OH)2D3 in the low and high concentration diets, respectively. After 5 weeks (chosen because the half-life of the active form 1,25-(OH)2D3 is approximately 7 days)[23] on respective diets, mice were treated with ODE (12.5%), LPS (100 ng) or sterile saline by daily intranasal inhalation daily for 5 days of the week for 3 weeks per established protocol [10]. Animals were maintained on respective high and low vitamin D supplemented diets. No mice exhibited respiratory distress or weight loss throughout the treatment period. Mice were euthanized 5 hours following the final exposure by intraperitoneal injection of 50 mg/kg of sodium pentobarbital (Nembutal; Abbott Labs, Chicago, IL). All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center. All experimental protocols were also conducted in accordance with the NIH guidelines for the appropriate use of rodents.
Serum
Whole blood was collected from mice at the time of sacrifice by cut down to the axillary artery. Serum 25-hydroxy vitamin D levels were quantified by an enzyme immunoassay (Immunodiagnostic Systems, Scottsdale, AZ). Serum calcium levels were quantified by a colorimetric calcium detection kit (Abcam, Cambridge, MA). Serum interleukin (IL)-6 was quantified according to manufacturer’s instructions using a Quantikine enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN) with lower limit of detection of 7.8 pg/ml.
Bronchoalveolar lavage fluid
Bronchoalveolar lavage fluid (BALF) was collected by whole lung lavage with 3 × 1 ml of sterile PBS as previously described [24]. Total cells were enumerated and spun onto slides with Cytopro cytocentrifuge (Wescor, Logan, UT) and stained with DiffQuick (Dade Behring, Newar, DE). Cell counts were determined by the differential ratio of cell types in 200 cells per slide per animal.
Lung cytokine assays
Cytokine profiles were characterized in lung homogenates as previously described [25]. Briefly, after removal of BALF, the chest cavity was opened and the right ventricle was infused with 10 mL of sterile PBS with heparin to remove blood from the pulmonary vasculature. Lung homogenates were prepared by homogenizing ½ lung samples in 500 µl of sterile PBS, and 50 µl of cell-free homogenate was analyzed in duplicate by a custom protein multiplex immunoassay array for TNF-α, IL-6, IL-17A, IFN-γ, IL-10, IL-4, and IL-2 according to manufacturer’s instruction (BD Biosciences, San Jose, CA), with the lower limit of detection in pg/ml of 0.9, 1.4, 0.8, 0.5, 16.8, 0.03, and 0.1, respectively. IL-1β levels were quantified according to manufacturer’s instruction using a Quanitkine enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN) with the lower limit of detection of 12.5 pg/ml.
Lung histology
Whole lungs were excised after lavage and inflated to 20 cm H2O pressure with 10% formalin (Sigma) for 24 hours to preserve pulmonary architecture as previously described [10]. Lungs were processed, embedded in paraffin, and 4–5 µm sections were cut and stained with H&E. Slides were microscopically reviewed and semiquantitatively assessed by a pathologist blinded to the treatment assignment and scored using a previously published scoring system [10] for the degree and distribution of inflammatory changes reflected in the alveolar compartment, bronchiolar compartment, and lymphoid aggregate development.
Micro CT Analysis
Right hind limbs were excised and fixed in 4% paraformaldehyde at 4°C on a shaker for 48 hours, washed and stored in 70% ethanol for micro-CT scanning. The right knee joint, including the distal femur and proximal tibia was scanned using high-resolution micro-CT (Skyscan 1172; Skyscan, Aartselaar, Belgium). X-ray projection images were acquired at a resolution of 4.8 µm. The X-ray source was set at 60 kV and 167 µA with a 0.5 mm thick aluminum filter with an exposure time of 530 milliseconds. X-ray scanning was performed at 0.7° intervals and six average frames were obtained for each rotation. NRECON (Skyscan) software was used to reconstruct scanned images. Analysis was done on the stacked reconstructed images using CTAn (Skyscan) software and was focused on the trabecular bone (1.44 mm distance) in the metaphyseal region of the distal femur and proximal tibia using specific thresholds for each animal across the different groups. This approach utilizing long, weight-bearing bones differs from a prior study [11], in which the calcaneus (ankle bone, 0.36 mm distance) from mice fed a normal rodent chow diet consisting of 4.02 IU/gm of 1,25-(OH)2D3 was analyzed. Online Resource 1 describes the specific bone parameters of the femur and tibia from those mice previously described [11].
The position of each bone (femur and tibia) was corrected using Dataviewer (Skyscan) software to assure proper orientation along the longitudinal axis. Growth plates were identified as the reference point and mineralized cartilage was excluded from analysis by starting 75 slices distal to the established reference point. Final analysis in the distal metaphyseal femur and proximal metaphyseal tibia was done on a volume of interest (VOI) of 300 slides representing a distance of 1.44 mm (300 × 4.8 µm), for which an interpolated region of interest (ROI) was manually drawn to exclude the cortical shell. CT-Vox (Skyscan) and CT-Vol (Skyscan) software was used to construct two- or three-dimensional (2D/3D) images for the visual representation of the results.
The following 3D parameters were measured for trabecular bone in both distal femur and proximal tibia metaphysis: bone mineral density (g/mm3), percent bone volume (bone volume to tissue volume ratio, %), specific bone surface (bone surface to bone volume ratio, mm−1). Bone structural connectivity was analyzed by measuring trabecular pattern factor in 3D [26–27] and average object area/slice in 2D (measurement of small fragmented objects) [27]. Trabecular micro-architecture was studied using parameters including trabecular thickness (mm) trabecular number (mm−1), and trabecular separation (mm). Collectively, the quantitative and qualitative parameters that would indicate bone loss or deterioration include decreases in bone mineral density, percent bone volume, trabecular thickness, trabecular number and average area per object and specific bone surface. Increases in trabecular pattern factor and trabecular separation are associated with bone loss. Further detailed measurement methods for these bone parameters can be found on the Skyscan website (www.skyscan.be).
Bone Histology
Following high resolution micro-CT scanning, formalin fixed hind limbs were dissected to remove any soft tissue and tibia bone was collected to perform bone histology. Excised bones were decalcified in 15% ethylenediaminetetraacetic acid on a shaker at 4°C for approximately 3 weeks. The decalcification solution was changed every 2 days. After decalcification, the bones were processed, embedded, sectioned (4–5µm), and stained. To corroborate our micro-CT findings by histology, bone sections were H&E stained. To visualize the organic extracellular bone matrix (ECM), which is predominantly collagen type I (90%) in decalcified bones, a modified Masson trichrome (MMT) stained was utilized [28]. We also performed tartrate resistant acid phosphatase (TRAP) staining to identify bone-resorbing multinucleated TRAP+ osteoclasts [29]. Stained slides were scanned with an iScan Coreo digital pathology slide scanner (Ventana, Tucson, AZ) by the Tissue Sciences Facility at the Department of Pathology and Microbiology (University of Nebraska Medical Center, Omaha, NE) and converted into digital format.
Statistical Methods
Data are presented as the mean ± standard error of mean (SEM). To detect significant changes between groups, a one-way analysis of variance (ANOVA) was utilized and a post-hoc test (Tukey/LSD) was performed to account for multiple comparisons if the p value was less than 0.05. All statistical analysis was performed using SPSS software (SPSS, Chicago, IL, USA) and statistical significance accepted at p < 0.05.
RESULTS
Serum levels of vitamin D, but not calcium, differed between high and low vitamin D treatment groups
Animals fed a high vitamin D (~10 IU D/gm) supplemented rodent chow diet demonstrated an approximate 10-fold increase in serum 25-hydroxy vitamin D levels compared to animals fed a low vitamin D (~1 IU D/gm) supplemented diet (Table 1). There were no differences in serum calcium levels across treatment groups. These studies indicate that a substantial difference was achieved in serum vitamin D levels between high versus low vitamin D treatment groups, but that treatment with ODE and LPS did not alter serum vitamin D levels. Importantly, there was no evidence of adverse changes in calcium homeostasis.
Table 1.
Serum calcium and 25-hydroxyvitamin D levels in mice fed a low (1 IU D/g) and high (10 IU D/g) vitamin D rodent chow diet. Animals placed on respective diet for 5 weeks after weaning and maintained on diet during daily saline, organic dust extract (ODE) or lipopolysaccharide (LPS) intranasal inhalation treatments for 3 weeks.
| Serum Calcium µg/mL |
Serum Vitamin D ng/mL |
|
|---|---|---|
| Low Vitamin D Diet + Saline Treatment | 0.83 (0.08) | 6.95 (0.34) |
| High Vitamin D Diet+ Saline Treatment | 0.99 (0.17) | 83.0 (7.78)*** |
| Low Vitamin D Diet + ODE Treatment | 1.09 (0.21) | 7.44 (0.41) |
| High Vitamin D Diet + ODE Treatment | 0.83 (0.46) | 74.63 (7.03)*** |
| Low Vitamin D Diet + LPS Treatment | 0.96 (0.15) | 5.4 (0.27) |
| High Vitamin D Diet + LPS Treatment | 1.0 (0.26) | 62.48 (3.53)*** |
Mean ± SEM are shown. Asterisks (***p<0.001) denote statistical significance between respective low vs. high vitamin D treatment groups. No difference in serum calcium levels between groups. Saline is N=12 mice/group, ODE is N=8 mice/group, and LPS is N=4 mice/group combined from independent studies.
Effects of high dietary vitamin D supplementation on airway inflammatory consequences following repeated exposures to ODE or LPS
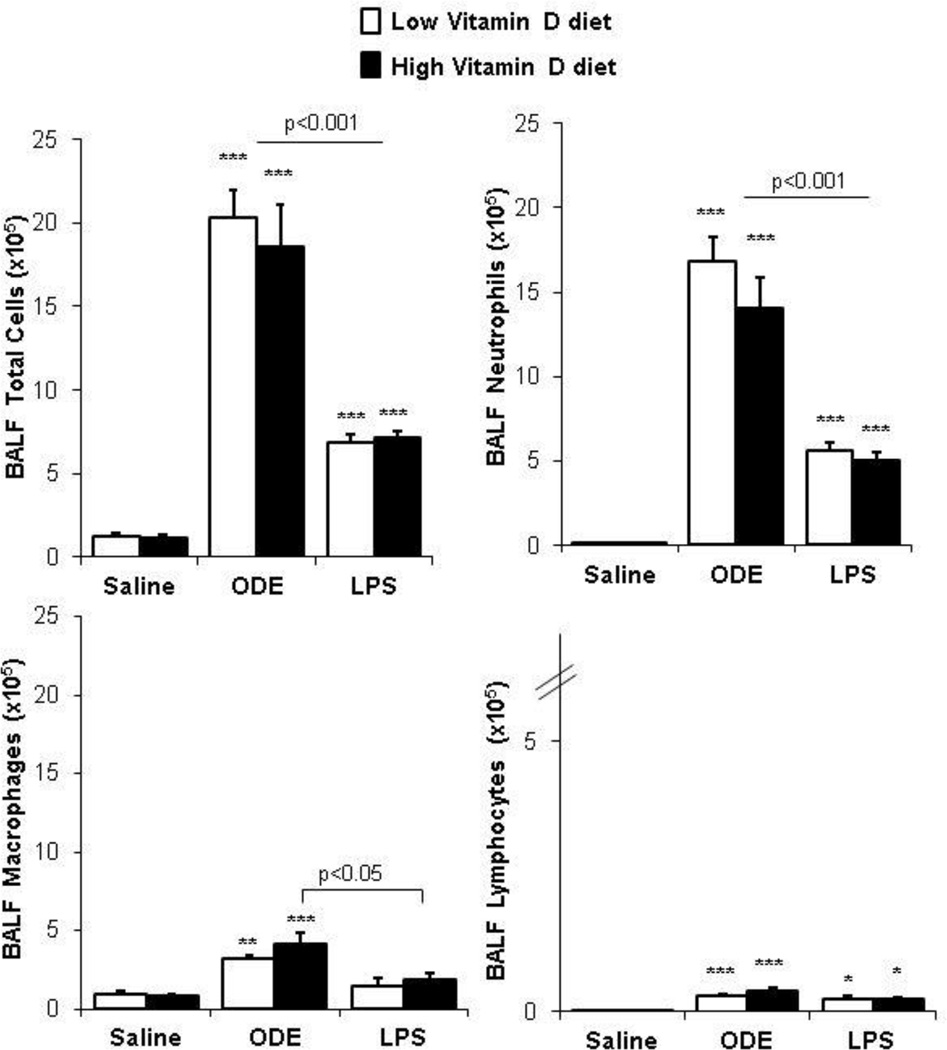
Previous studies demonstrated that supplemental dietary vitamin D reduced airway inflammatory neutrophil influx and neutrophil chemoattractant production following an acute or one-time exposure to ODE in mice [30]. In the present study, we investigated whether high dietary supplementation with vitamin D would reduce airway inflammatory responses following three weeks of daily, repetitive inhalant exposure to ODE or LPS. Consistent with previous work [10, 21], repetitive ODE treatment resulted in significant increases in total cell influx including neutrophils, macrophages, and lymphocytes (Figure 1). Repetitive treatment with LPS also resulted in significant increases in total cell influx including neutrophils, and lymphocytes as compared to saline, but to a significantly lesser magnitude as compared to ODE treatment (Figure 1). However, there was no difference in the magnitude of ODE- or LPS-induced inflammatory cell influx with high vitamin D as compared to low vitamin D treatment groups, respectively (p>0.05).
Figure 1. High vitamin D did not reduce airway cellular influx induced following inhalant organic dust extract (ODE) or lipopolysaccharide (LPS) treatment.
Results represent (mean±SEM) of bronchoalveolar lavage (BALF) total cell counts and differential. Statistical significant differences denoted by asterisks (*p<0.05, **p<0.01, ***p<0.001) as compared to representative saline control. Statistical significant differences between ODE and LPS treatment groups as indicated by line. N=mice/group: saline, 12; ODE, 8; and LPS, 4.
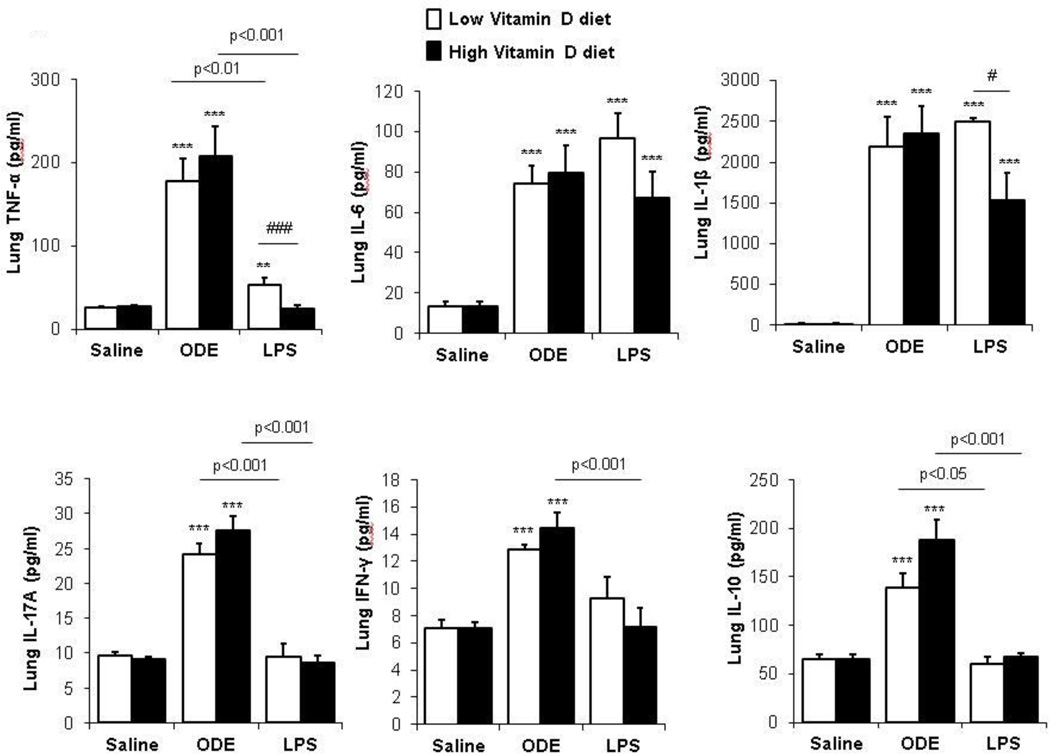
Next, we determined whether high supplemental vitamin D would modulate the lung cytokine responses following repetitive saline, ODE, and LPS inhalant treatment. Consistent with prior work [25], repetitive ODE treatment induced a Th1/Th17 lung cytokine milieu marked by increased TNF-α, IL-6, IL-1β, IL-17A, and IFN-γ as compared to saline control treated animals (Figure 2). However, there were no significant differences in these cytokine levels between the ODE-treated high versus low vitamin D treatment groups (Figure 2). Repetitive treatment with LPS demonstrated different findings in comparison to ODE treatment and between the high and low vitamin D supplemented diets (Figure 2). Namely, repetitive LPS treatment induced lung TNF-α, IL-6, IL-1β production, which was most pronounced in the low vitamin D treatment group. The high vitamin D plus LPS treatment group demonstrated a diminished TNF-α and IL-1β response as compared to the low vitamin D plus LPS treatment group. Repetitive LPS treatment did not significantly increase IL-17A or IFN-γ production. Repetitive ODE, but not LPS, treatment induced IL-10 production, but there were no significant differences between high versus low vitamin D diet treatment groups. IL-2 and IL-4 levels were at the lower limit of the assay detection in all treatment groups (data not shown).
Figure 2. Effect of high vitamin D on lung cytokine responses induced following inhalant organic dust extract (ODE) or lipopolysaccharide (LPS) treatment.
Cytokine profiles associated with T cell subsets from cell-free supernatants of half lung homogenates by protein multiplex immunoassay are shown. Results represent (mean±SEM) with statistical significant differences denoted by asterisks (***p<0.01, ***p<0.001) versus respective saline control and number signs (#p<0.05, ###p<0.001) demonstrate differences between respective low vs. high vitamin D diet. Statistical significant differences between ODE and LPS treatment groups as indicated by line. N=mice/group: saline, 12; ODE, 8; and LPS, 4.
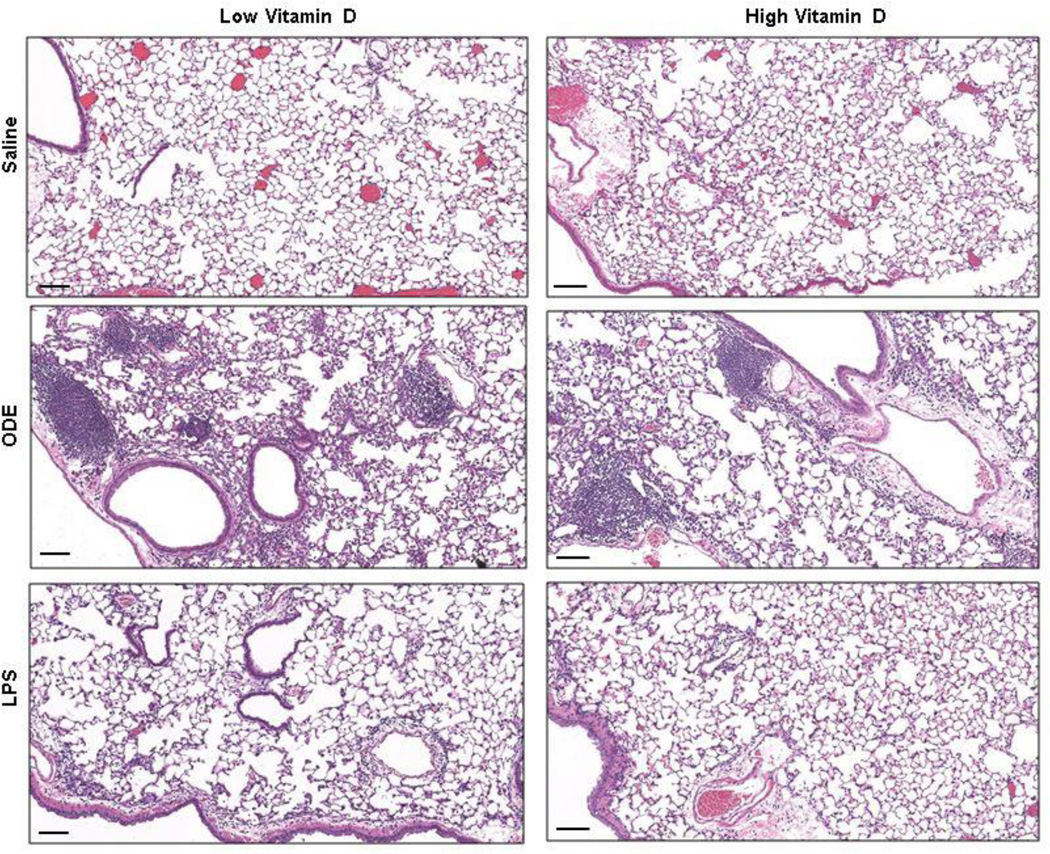
Finally, lung histopathology from the treatment groups was reviewed and scored. It has been well established that repetitive ODE treatment induces significant murine lung histopathologic changes marked by the development of lymphoid aggregates and significant increases in bronchiolar and alveolar compartment inflammation [10, 11]. Here, we also found that repetitive ODE-induced increased lung parenchymal histopathology (Figure 3). However, there was no difference by microscopic review between the low vitamin D plus ODE and high vitamin D plus ODE treatment groups. In addition, semi-quantitative inflammatory scoring revealed no difference in ODE-induced lymphoid aggregates, bronchiolar compartment, or alveolar compartment inflammation between low versus high supplemental vitamin D dietary treatment groups (data not shown). It has been previously demonstrated that repetitive LPS intranasal inhalation treatment at this same concentration results in subtle, but non-significant, increases in alveolar and bronchiolar inflammation [11]. In these studies, we again found that repetitive LPS inhalant treatment (100 ng) did not result in a robust increase in lung parenchymal histopathologic effects (Figure 3).
Figure 3. Murine lung histopathology with low versus high vitamin D diet following inhalant treatment with saline, organic dust extract (ODE), and lipopolysaccharide (LPS).
A representative 4- to 5-µm-thick section (H&E stained) of one mouse per treatment group (N-mice/group: saline 12; ODE, 8, LPS, 4) is shown at 10X magnification with line scale representing 100 µm.
Systemic bone loss induced by inhalant lung injury is protected by high vitamin D supplementation
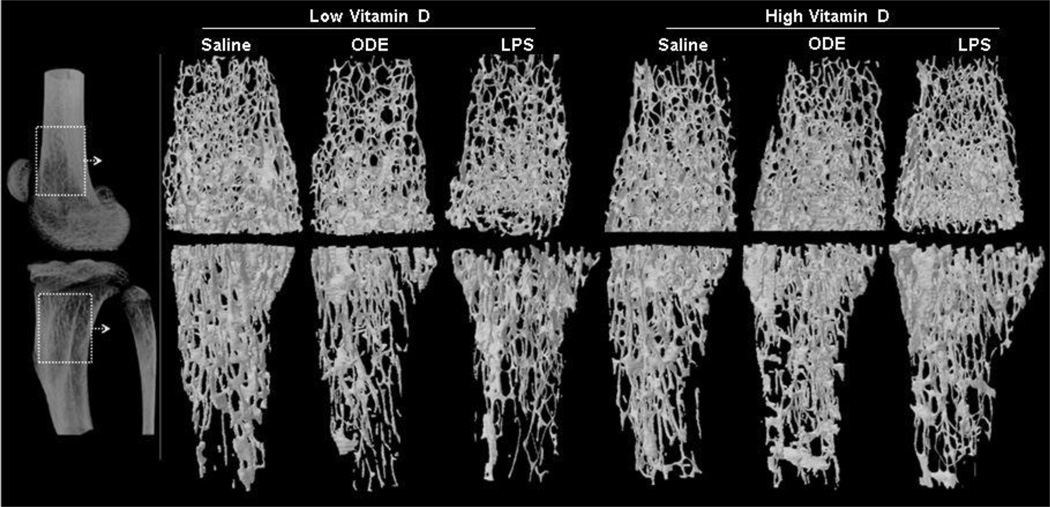
The femur and tibia from the same treated animals described above were investigated for bone health consequences. In the low, but not high, vitamin D treatment groups, inhalation of ODE and LPS resulted in substantial bone loss. Figure 4 shows a representative 3D reconstructed image of the region of interest (ROI) of the knee joint from each treatment group. This ROI was utilized to quantify differences in specific bone parameters of the femur and tibia, and overall, findings were similar for the femur and tibia across treatment groups (Table 2). Focusing on the femur data (Table 2), there was a decrease in the BMD following ODE or LPS treatment as compared to the saline control treatment group in mice fed a low vitamin D diet. Animals fed the high vitamin D diet had significant increases in BMD as compared to the low vitamin D diet across exposure groups: saline (p = 0.016), ODE (p = 0.027) and LPS (p = 0.005). The greatest mean percent increase in BMD for high dose relative to low dose vitamin D fed animals was demonstrated in the LPS treatment group. There was also a significant decrease in the volumetric content of mineralized bone tissue as shown by percent bone volume in ODE (p = 0.025) and LPS (p = 0.023) treatment groups as compared to saline treated mice. High supplementation with vitamin D improved the bone volume loss, which was evident by significant increases in the percent bone volume for ODE (p = 0.029, 15% increase) and LPS (p< 0.001, 30% increase) treatment groups as compared to saline control.
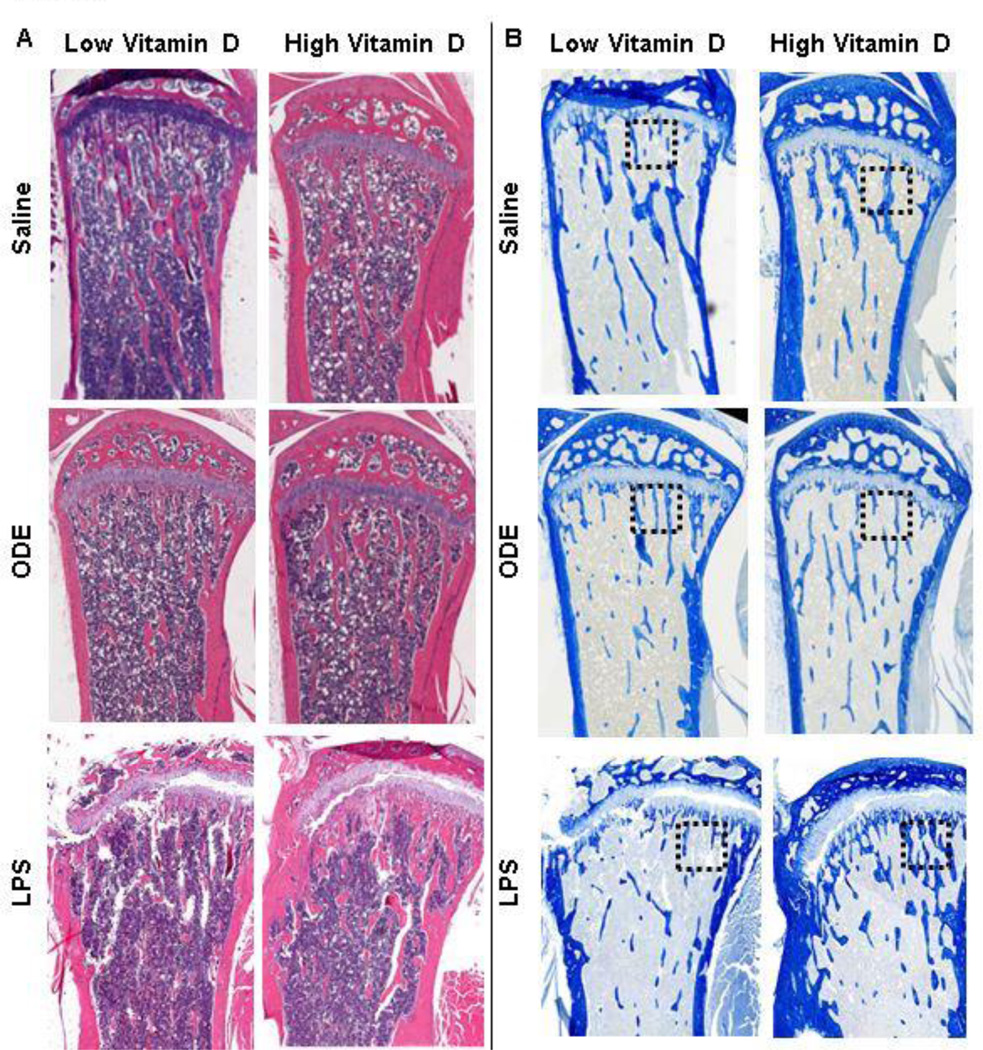
Figure 4. High vitamin D protects against inhalant ODE and LPS induced bone loss.
Left panel represents 2D image of distal femur and proximal tibia with dotted rectangle indicating region of interest where 3D reconstructed images and micro-CT analysis was performed. Main panel shows a representative 3D reconstructed image of distal femur and proximal tibia from one mouse per treatment group (saline 12; ODE 8; LPS 4). Note the substantial loss of trabecular and cortical bone in the low vitamin D plus ODE or LPS treatment groups.
Table 2.
Quantitative overview of trabecular bone in distal femur and proximal tibia using micro-CT analysis in mice treated with intranasal saline, 12.5% organic dust extract (ODE), or 100 ng lipopolysaccharide (LPS) for 3 weeks while maintained on low versus high vitamin D (VD) diet.
| Parameter | Saline Low VD |
Saline High VD |
ODE Low VD |
ODE High VD |
LPS Low VD |
LPS High VD |
p- value |
Mean % delta of Low vs. High VD |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Saline | ODE | LPS | ||||||||
| Femur | ||||||||||
| Bone mineral density, mg/mm3 | 317 ± 3.05 | 330 ±3.53# | 304 ± 2.20* | 320 ± 7.30# | 309 ± 4.41 | 332 ± 6.62# | 0.001 | 4.10 | 5.26 | 7.44 |
| Bone volume, % | 8.54 ±0.20 | 9.22 ±0.25 | 7.50±0.15* | 8.63±0.51# | 7.40±0.34* | 9.65±0.55# | 0.001 | 7.96 | 15.06 | 30.40 |
| Specific bone surface, mm−1 | 102.7±1.5 | 101.3±1.2 | 105.4±2.5 | 101.7±2.9 | 114.7±2.2*† | 99.9±5.3# | 0.006 | −1.43 | −3.50 | −12.88 |
| Trabecular pattern factor, mm−1 | 29.15±0.55 | 27.95±0.47 | 30.74±0.64 | 29.16±1.26 | 31.69±0.84* | 26.57±1.5# | 0.006 | −4.12 | −5.12 | −16.16 |
| Average object area/slice, µm−2 | 2700 ± 80 | 2800 ± 80 | 2500 ± 110 | 2700 ± 140 | 2100 ± 90* | 2900±330# | 0.010 | 3.70 | 8.00 | 38.10 |
| Trabecular thickness, µm | 35.4 ± 0.75 | 35.1 ± 0.32 | 34.6 ± 1.0 | 35.3 ± 1.0 | 31.9 ± 0.44 | 37.2 ± 2.23 | 0.054 | −0.85 | 2.02 | 16.61 |
| Trabecular number, µm−1 | 242 ± 37 | 263 ± 61# | 217± 28* | 243 ± 87# | 232 ± 83 | 260 ± 58# | 0.001 | 8.68 | 12.00 | 12.07 |
| Trabecular separation, µm | 202 ± 2.33 | 195 ± 3.40 | 211 ±2.24* | 201 ± 3.0# | 201 ± 3.44 | 194 ± 2.93 | 0.011 | −3.47 | −4.74 | −3.48 |
| Tibia | ||||||||||
| Bone mineral density, mg/mm3 | 294 ± 3.70 | 303 ±2.13# | 287 ± 1.71 | 299± 4.80# | 282 ± 3.10* | 306± 6.50# | 0.001 | 3.06 | 4.18 | 8.51 |
| Percent bone volume, % | 6.16 ± 0.27 | 6.82±0.16# | 5.64 ± 0.26 | 6.67±0.28# | 5.65 ± 0.30 | 7.30±0.42# | 0.002 | 10.71 | 18.26 | 29.20 |
| Specific bone surface, mm−1 | 111.4±2.4 | 107.5±1.2 | 115.9±2.4 | 111.1±2.0 | 111.7±2.4 | 101.5±4.0#† | 0.011 | −3.55 | −4.12 | −9.17 |
| Trabecular pattern factor, mm−1 | 36.07±1.15 | 34.01±0.42 | 36.61±0.62 | 33.94±1.05 | 36.67±1.05 | 33.06±1.11 | 0.054 | −5.71 | −7.30 | −9.84 |
| Average object area/slice, µm2 | 1700 ± 90 | 1800 ± 40 | 1500 ± 40 | 1700 ± 60 | 1700 ± 70 | 2000 ± 30 | 0.034 | 5.88 | 13.33 | 17.65 |
| Trabecular thickness, µm | 44.2 ± 0.8 | 44.3 ± 0.5 | 45.2 ± 1.2 | 43.7 ± 0.9 | 43.4 ± 0.7 | 47.5± 2.0 | 0.152 | 0.23 | −3.32 | 9.45 |
| Trabecular number, µm−1 | 140 ± 50 | 154 ± 42# | 125 ± 73 | 153 ± 71# | 130 ± 63 | 154 ± 65# | 0.004 | 10.00 | 22.40 | 18.46 |
| Trabecular separation, µm | 233 ± 3.70 | 230 ± 4.25 | 232 ± 6.73 | 219 ± 3.47 | 253 ± 5.48*† | 225± 6.78# | 0.007 | −1.29 | −5.60 | −11.07 |
Mean ± SEM of treatment groups with N=4–8 mice/group combined from independent studies. P-value denotes statistical significance (ANOVA) among treatment groups.
Statistical difference between groups denoted by asterisks (* p<0.05) as compared to respective saline,
number sign (#) compares respective low vs. high vitamin D,
dagger sign († p<0.05) compares respective ODE and LPS.
Representing bone resorption, specific bone surface (BS/BV, mm−1) was increased in the LPS treated group as compared to ODE (p = 0.019) and saline (p= 0.001). High dietary vitamin D supplementation led to a minor improvement in bone resorption in saline and ODE groups, whereas in the LPS treated group, high vitamin D supplementation resulted in an significant (p = 0.001, 13%) decrease in bone resorption. Within the LPS treatment group, vitamin D supplementation also improved changes in structural connectivity. Specifically, inhalation of LPS resulted in significant increases in the trabecular pattern factor (p= 0.041) and decreases in the smaller fragmented objects (average object area/slice in 2D, p = 0.005) as compared to saline. High vitamin D supplementation improved the structural connectivity by decreasing the trabecular pattern disconnects (p= 0.001, 16 % decrease) and fragments (p = 0.001, 38 % increase) within the trabecular architecture. There was no difference in the ODE treatment groups for these structural connectivity bone parameters.
To further investigate the structural changes in trabecular morphology, we measured trabecular thickness, number, and separation across treatment groups (Table 2). Remaining focused on the femur, there was no significant difference in the trabecular thickness (p = 0.054) as compared to saline, but the greatest decline in trabecular thickness was found in the LPS treatment group fed a low vitamin D diet. The trabecular number was also decreased for animals fed a low vitamin D diet and treated with ODE (p = 0.007) and LPS (p = 0.054) as compared to the respective saline group. Trabecular number was significantly improved in animals fed a high vitamin D diet within saline (p = 0.006), ODE (p = 0.009) and LPS (p = 0.012) treatment groups. However, there was an increase in the gap between individual trabeculae, as measured by trabecular separation parameter. Interestingly, as compared to the saline treatment group, only ODE (p = 0.041) demonstrated a significant increase in trabecular separation in the low vitamin D treatment group.
It was previously reported that intranasal inhalation of ODE or LPS in animals fed a regular rodent chow diet with approximately 4 IU/g of 1,25 hydroxyvitamin D resulted in bone loss in the calcaneus [11]. In this present study, we investigated the distal femur and proximal tibia of those previously described mice and confirmed that there was also an overall deterioration of the trabecular bone quantity and quality in these long, weight-bearing bones (Online Resource 1).
Bone Histology
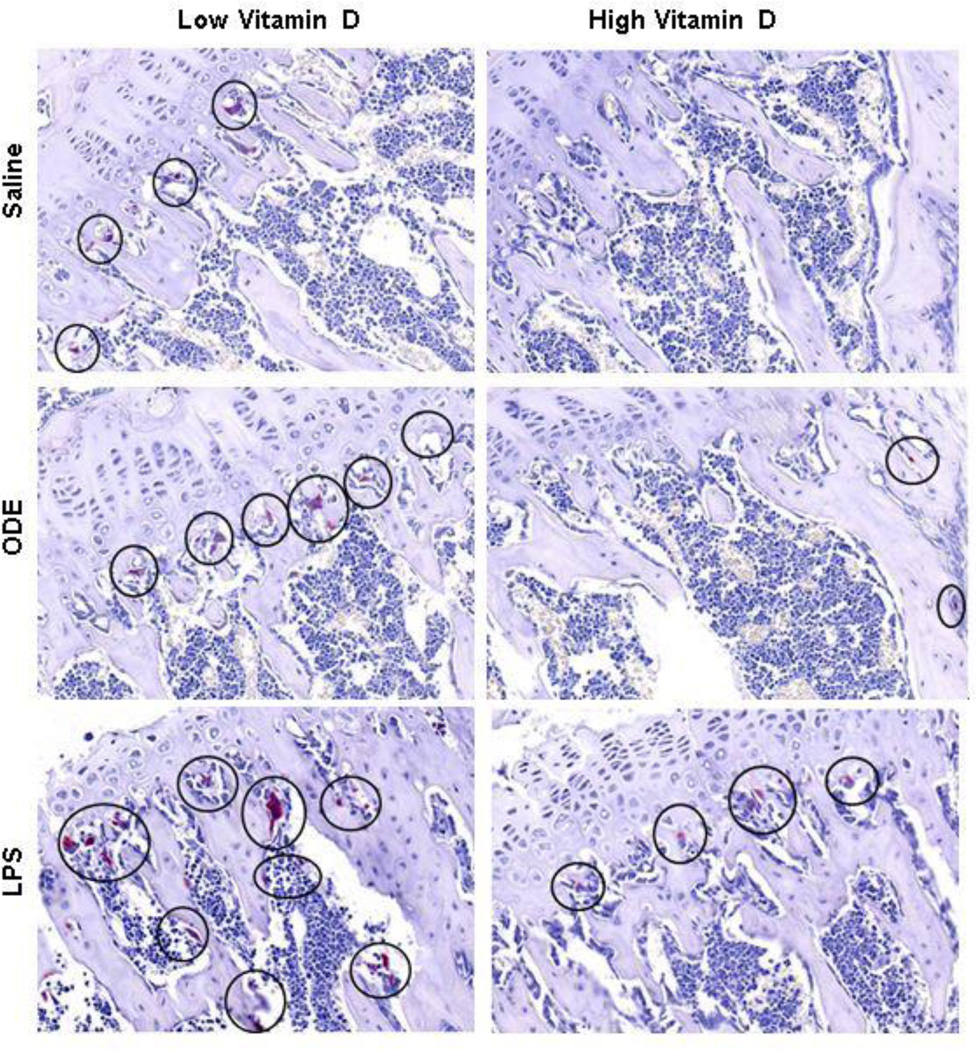
To complement the micro-CT results, we analyzed the proximal tibia for bone histology. Consistent with the micro-CT scan results, in animals fed the low vitamin D diet, trabecular bone volume (Figure 5A) and type I collagen in the extracellular matrix (Figure 5B) was decreased as compared to mice fed the high vitamin D diet. To determine whether osteoclasts were present, bone sections were stained for TRAP (Figure 6). TRAP+ osteoclasts were observed in the low vitamin D supplemented groups, and none or very few TRAP+ cells were found in the high vitamin D supplemented groups. This finding suggests that osteoclastic bone resorption in the ODE and LPS treatment groups might be eliminated or nearly eliminated by high vitamin D supplementation.
Figure 5. Bone histopathology effects of high versus low vitamin D diet following inhalant treatment with ODE and LPS.
Sections of one mouse per treatment group (saline 12; ODE 8; LPS 4) were stained with H&E (A) and modified Masson’s (B) and shown at 2X magnification. Note the loss of blue color staining indicative of type I collagen in the extracellular matrix of animals fed a low vitamin D diet. Black dotted box represents region of staining for TRAP+ osteoclasts shown in Figure 6.
Figure 6. High vitamin D supplementation reduces TRAP+ multinucleated, bone-resorbing osteoclasts.
Sections of one mouse per treatment group were stained for tartrate resistant acid phosphatase (TRAP), which gives the characteristic magenta color. TRAP+ osteoclasts are circled.
Serum IL-6 levels increased in the ODE and LPS treatment groups
To determine whether systemic mediators might play a role in the cross-talk between lung inflammation and bone loss, serum IL-6 was measured due to its established role in promoting osteoclastogenesis.(31) Repetitive intranasal inhalant treatments with ODE and LPS induced increased serum levels of IL-6 as compared to saline treated animals (p<0.001), but there was no significant difference between low versus high vitamin D treatment (p>0.05). There was also no significant difference between ODE and LPS treatment (p>0.05). Specifically, mean (SEM) serum IL-6 levels were as follows: low vitamin D diet + saline treatment: 0.55 (0.3); high vitamin D + saline treatment 0.4 (0.3); low vitamin D diet + ODE treatment 11.1 (3.6); high vitamin D diet + ODE 7.5 (2.3); low vitamin D diet + LPS 12.6 (1.7); and high vitamin D diet + LPS 8.8 (1.7). TNF-α has also been implicated in osteoclastogenesis [31], but there was no discernible ODE or LPS treatment induced increase in serum TNF-α levels (data not shown).
DISCUSSION
Using our animal model system, we investigated the role for high vitamin D supplementation on potentially modulating the adverse airway and skeletal health effects induced by inhalation treatment with ODE and LPS. This study demonstrated that bone deterioration induced by repetitive inhalational exposure to ODE or LPS was prevented by high dietary vitamin D supplementation. The beneficial effect of the high vitamin D diet was related to the extent of skeletal disease induced by ODE or LPS. However, high vitamin D supplementation did not significantly reduce the majority of airway inflammatory consequences induced following these repetitive inhalation exposures. This is the first animal study to our knowledge demonstrating a beneficial role for vitamin D in protecting against potential systemic skeletal health consequences induced by potent environmental inhalant bioaerosol exposures. Our findings might have musculoskeletal implications for the farming industry or endotoxin-enriched occupational settings, whereby shift workers employed in these indoor/enclosed settings may lack sunlight exposure or dietary vitamin D fortification. Prophylactic dietary supplementation of vitamin D might possibly be a reasonable recommendation to promote skeletal health of at-risk workers.
These data demonstrated that high vitamin D supplementation in animals prevented the adverse skeletal health consequences induced following inhalant ODE or LPS treatments. The high vitamin D supplementation diet resulted in serum vitamin D levels that are obtainable in terms of human supplementation, whereby some experts recommend aiming for serum vitamin D levels above 40 ng/ml [32]. The beneficial effect of vitamin D might be explained by a reduction in bone resorption, possibly through reducing osteoclasts. The salutary effects on bone health with supplementation of high vitamin D, particularly in lieu of normal serum calcium levels, suggest a role for vitamin D on maintaining normal osseous tissue homeostasis (i.e. inducing mineralization and deposition). Recent human and animal studies support this explanation by showing that active vitamin D compounds increase bone mass and decrease fracture risk, primarily by suppression of bone resorption [33,34]. Vitamin D is an osteotropic hormone involved in regulating bone homeostasis, which is a tightly regulated physiologic process between bone-forming osteoblasts and bone-resorbing osteoclasts. Vitamin D has been described to inhibit osteoclasts through several mechanisms. Vitamin D can inhibit osteoclastogenesis indirectly by either decreasing expression of receptor activator of nuclear factor κ B ligand (RANKL) from osteoblasts [34, 35] or by increasing the expression of osteoprotegerin [36], an inhibitor of osteoclast maturation. Vitamin D can also act by suppressing various transcription factors required for osteoclast maturation including c-fos [37] and nuclear factor of activated T cells, cytoplasmic 1 (NFATc1) [38]. Our studies would support an inhibitory role for vitamin D on osteoclasts because animals fed the high vitamin D diet had little evidence of TRAP+ osteoclasts, which were instead, prominently found in animals fed the low vitamin D diet and challenged with ODE or LPS (Figure 6).
It has been previously reported that non-LPS components within the dust such as bacterial peptidoglycans are important drivers of large animal confinement ODE-induced airway inflammatory consequences [22, 39, 40]. Others have made similar conclusions based on studies focused on dust sample analysis (culture-independent approaches) [41, 42], rodent modeling [43], human genetic polymorphism studies [44], and human inhalation challenge studies [45], However, LPS remains present in complex organic dust [22], and as reported by Dusad et al., the degree of lung injury and bone loss is discordant between ODE and LPS [11]. Namely, inhalant LPS induced the greatest bone loss with minimal induction of adverse lung histopathology, whereas inhalant ODE resulted in worsened lung histopathology and significant bone loss. Similar findings were again demonstrated in this present study, which might suggest that various inhalant exposures might regulate the airway injury-bone disease inflammatory axis through differing mechanisms. Although we speculate that inhalant ODE is indirectly causing bone loss through a robust airway inflammatory response, it is possible that LPS is escaping into the circulation to directly affect bone health, which will be explored in future studies. Interestingly, supplementation with high concentration vitamin D protected against bone loss following either ODE or LPS inhalant treatments.
High vitamin D supplementation failed to significantly reduce airway inflammation following repetitive inhalation treatment to ODE or LPS. The explanation for why vitamin D did not reduce airway inflammatory outcomes may be several-fold. Most likely it is that the immunomodulatory immune effects described for vitamin D were unable to overcome the strong and repeated inflammatory stimulus of potent environmental inflammatory agents. It is possible that there could be a role for vitamin D supplementation following less potent, respirable environmental inflammatory agent exposures. Others have shown that an important immunomodulatory effect of vitamin D is by increasing T-regulatory cells and IL-10 production [46], which has been, in part, hypothesized for a potential role for vitamin D and difficult-to-control asthma. Although non-significant, our studies found a slight increase in lung IL-10 levels in animals fed a high vitamin D diet and treated with ODE. Thus, it might also be warranted to determine whether vitamin D supplementation plays a role in post-airway inflammatory homeostasis. Nonetheless, our findings of bone protection without evidence of major airway disease protection with vitamin D treatment might have important implications for future airway inflammatory disease studies. Whereas several studies have found associations with low vitamin D levels and either adverse asthma or COPD outcomes [18, 19], interventional studies with add-on vitamin D treatment have been largely disappointing to date [47]. For example, a relatively large, randomized, placebo-controlled human interventional study found no significant improvement for vitamin D3 supplementation in reducing treatment failures or exacerbations in adults with asthma and vitamin D insufficiency [20]. The study authors’ conclusions were that their findings did not support a strategy of therapeutic vitamin D supplementation in symptomatic asthma. Based upon our animal study findings, there still might be an important role for vitamin D in patients with symptomatic and/or chronic airway diseases as a bone protective strategy. A role for vitamin D in other extrapulmonary manifestation could also be considered.
In conclusion, high vitamin D supplementation protected mice against bone loss, but not airway inflammation, following inhalant ODE or LPS treatments. These findings suggest that vitamin D supplementation might have implications for skeletal health consequences following inflammatory bioaerosol-induced lung diseases. Furthermore, it might be warranted for future studies investigating a role for vitamin D in chronic airway diseases to include bone mineral investigations.
Supplementary Material
Acknowledgments
Grant support: This material is the result of work supported with resources and the use of facilities at the VA Nebraska-Western Iowa Health Care System, Omaha, NE. Study supported by grants from the American College of Rheumatology/Rheumatology Research Foundation, Department of Internal Medicine, University of Nebraska Medical Center, Nebraska Arthritis Outcomes Research Center, National Institute of Arthritis and Musculoskeletal Diseases, and National Institute of Environmental Health Sciences. This work was also supported in part by the Central States Center for Agricultural Safety and Health (CS-CASH).
REFERENCES
- 1.Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L. Definition, epidemiology and natural history of COPD. Eur Respir J. 2007 Nov;30(5):993–1013. doi: 10.1183/09031936.00082507. [DOI] [PubMed] [Google Scholar]
- 2.Poole JA. Farming-associated environmental exposures and effect on atopic diseases. Ann Allergy Asthma Immunol. 2012 Aug;109(2):93–98. doi: 10.1016/j.anai.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, et al. Chronic obstructive pulmonary disease phenotypes: The future of COPD. Am J Respir Crit Care Med. 2010 Sep 1;182(5):598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eduard W, Pearce N, Douwes J. Chronic bronchitis, COPD, and lung function in farmers: The role of biological agents. Chest. 2009 Sep;136(3):716–725. doi: 10.1378/chest.08-2192. [DOI] [PubMed] [Google Scholar]
- 5.Alexis NE, Eldridge MW, Peden DB. Effect of inhaled endotoxin on airway and circulating inflammatory cell phagocytosis and CD11b expression in atopic asthmatic subjects. J Allergy Clin Immunol. 2003 Aug;112(2):353–361. doi: 10.1067/mai.2003.1651. [DOI] [PubMed] [Google Scholar]
- 6.Kauffmann F, Demenais F. Gene-environment interactions in asthma and allergic diseases: Challenges and perspectives. J Allergy Clin Immunol. 2012 Dec;130(6):1229. doi: 10.1016/j.jaci.2012.10.038. 40; quiz 1241-2. [DOI] [PubMed] [Google Scholar]
- 7.Jung JW, Kang HR, Kim JY, Lee SH, Kim SS, Cho SH. Are asthmatic patients prone to bone loss? Ann Allergy Asthma Immunol. 2014 May;112(5):426–431. doi: 10.1016/j.anai.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Graat-Verboom L, Smeenk FW, van den Borne BE, Spruit MA, Donkers-van Rossum AB, Aarts RP, et al. Risk factors for osteoporosis in caucasian patients with moderate chronic obstructive pulmonary disease: A case control study. Bone. 2012 Jun;50(6):1234–1239. doi: 10.1016/j.bone.2012.02.638. [DOI] [PubMed] [Google Scholar]
- 9.Lehouck A, Boonen S, Decramer M, Janssens W. COPD, bone metabolism, and osteoporosis. Chest. 2011 Mar;139(3):648–657. doi: 10.1378/chest.10-1427. [DOI] [PubMed] [Google Scholar]
- 10.Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, et al. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol. 2009 Apr 24;296(6):L1085–L1095. doi: 10.1152/ajplung.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dusad A, Thiele GM, Klassen LW, Gleason AM, Bauer C, Mikuls TR, et al. Organic dust, lipopolysaccharide, and peptidoglycan inhalant exposures result in bone loss/disease. Am J Respir Cell Mol Biol. 2013 Nov;49(5):829–836. doi: 10.1165/rcmb.2013-0178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osborne A, Blake C, Fullen BM, Meredith D, Phelan J, McNamara J, et al. Prevalence of musculoskeletal disorders among farmers: A systematic review. Am J Ind Med. 2012 Feb;55(2):143–158. doi: 10.1002/ajim.21033. [DOI] [PubMed] [Google Scholar]
- 13.Holick MF. Vitamin D deficiency. N Engl J Med. 2007 Jul 19;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 14.Bischoff-Ferrari HA, Shao A, Dawson-Hughes B, Hathcock J, Giovannucci E, Willett WC. Benefit-risk assessment of vitamin D supplementation. Osteoporos Int. 2010 Jul;21(7):1121–1132. doi: 10.1007/s00198-009-1119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorter EA, Hamdy NA, Appelman-Dijkstra NM, Schipper IB. The role of vitamin D in human fracture healing: A systematic review of the literature. Bone. 2014 Jul;64:288–297. doi: 10.1016/j.bone.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 16.St-Arnaud R, Naja RP. Vitamin D metabolism, cartilage and bone fracture repair. Mol Cell Endocrinol. 2011 Dec 5;347(1-2):48–54. doi: 10.1016/j.mce.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Mercer KE, Wynne RA, Lazarenko OP, Lumpkin CK, Hogue WR, Suva LJ, et al. Vitamin D supplementation protects against bone loss associated with chronic alcohol administration in female mice. J Pharmacol Exp Ther. 2012 Nov;343(2):401–412. doi: 10.1124/jpet.112.197038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Persson LJ, Aanerud M, Hiemstra PS, Hardie JA, Bakke PS, Eagan TM. Chronic obstructive pulmonary disease is associated with low levels of vitamin D. PLoS One. 2012;7(6):e38934. doi: 10.1371/journal.pone.0038934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010 Apr 1;181(7):699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: The VIDA randomized clinical trial. JAMA. 2014 May;311(20):2083–2091. doi: 10.1001/jama.2014.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poole JA, Anderson L, Gleason AM, West WW, Romberger DJ, Wyatt TA. Pattern recognition scavenger receptor A/CD204 regulates airway inflammatory homeostasis following organic dust extract exposures. J Immunotoxicol. 2014 Feb 3; doi: 10.3109/1547691X.2014.882449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poole JA, Dooley GP, Saito R, Burrell AM, Bailey KL, Romberger DJ, et al. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J Toxicol Environ Health A. 2010 Jan;73(10):684–700. doi: 10.1080/15287390903578539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith JE, Goodman DS. The turnover and transport of vitamin D and of a polar metabolite with the properties of 25-hydroxycholecalciferol in human plasma. J Clin Invest. 1971 Oct;50(10):2159–2167. doi: 10.1172/JCI106710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poole JA, Thiele GM, Alexis NE, Burrell AM, Parks C, Romberger DJ. Organic dust exposure alters monocyte-derived dendritic cell differentiation and maturation. Am J Physiol Lung Cell Mol Physiol. 2009 Jul;31:L767–L776. doi: 10.1152/ajplung.00107.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole JA, Gleason AM, Bauer C, West WW, Alexis N, Reynolds SJ, et al. Alphabeta T cells and a mixed Th1/Th17 response are important in organic dust-induced airway disease. Ann Allergy Asthma Immunol. 2012 Oct;109(4) doi: 10.1016/j.anai.2012.06.015. 266,273.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn M, Vogel M, Pompesius-Kempa M, Delling G. Trabecular bone pattern factor--a new parameter for simple quantification of bone microarchitecture. Bone. 1992;13(4):327–330. doi: 10.1016/8756-3282(92)90078-b. [DOI] [PubMed] [Google Scholar]
- 27.Vermeirsch H, Biermans R, Salmon PL, Meert TF. Evaluation of pain behavior and bone destruction in two arthritic models in guinea pig and rat. Pharmacol Biochem Behav. 2007 Aug-Sep;87(3):349–359. doi: 10.1016/j.pbb.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Paschalis EP, Shane E, Lyritis G, Skarantavos G, Mendelsohn R, Boskey AL. Bone fragility and collagen cross-links. J Bone Miner Res. 2004 Dec;19(12):2000–2004. doi: 10.1359/JBMR.040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi N, Udagawa N, Kobayashi Y, Suda T. Generation of osteoclasts in vitro, and assay of osteoclast activity. Methods Mol Med. 2007;135:285–301. doi: 10.1007/978-1-59745-401-8_18. [DOI] [PubMed] [Google Scholar]
- 30.Golden GA, Wyatt TA, Romberger DJ, Reiff D, McCaskill M, Bauer C, et al. Vitamin D treatment modulates organic dust-induced cellular and airway inflammatory consequences. J Biochem Mol Toxicol. 2013 Jan;27(1):77–86. doi: 10.1002/jbt.21467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schett G. Review: Immune cells and mediators of inflammatory arthritis. Autoimmunity. 2008 Apr;41(3):224–229. doi: 10.1080/08916930701694717. [DOI] [PubMed] [Google Scholar]
- 32.Cannell JJ, Hollis BW, Zasloff M, Heaney RP. Diagnosis and treatment of vitamin D deficiency. Expert Opin Pharmacother. 2008 Jan;9(1):107–118. doi: 10.1517/14656566.9.1.107. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi N, Udagawa N, Suda T. Vitamin D endocrine system and osteoclasts. Bonekey Rep. 2014 Feb 5;3:495. doi: 10.1038/bonekey.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harada S, Mizoguchi T, Kobayashi Y, Nakamichi Y, Takeda S, Sakai S, et al. Daily administration of eldecalcitol (ED-71), an active vitamin D analog, increases bone mineral density by suppressing RANKL expression in mouse trabecular bone. J Bone Miner Res. 2012 Feb;27(2):461–473. doi: 10.1002/jbmr.555. [DOI] [PubMed] [Google Scholar]
- 35.van Driel M, van Leeuwen JP. Vitamin D endocrine system and osteoblasts. Bonekey Rep. 2014 Feb 5;3:493. doi: 10.1038/bonekey.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldock PA, Thomas GP, Hodge JM, Baker SU, Dressel U, O'Loughlin PD, et al. Vitamin D action and regulation of bone remodeling: Suppression of osteoclastogenesis by the mature osteoblast. J Bone Miner Res. 2006 Oct;21(10):1618–1626. doi: 10.1359/jbmr.060714. [DOI] [PubMed] [Google Scholar]
- 37.Takasu H, Sugita A, Uchiyama Y, Katagiri N, Okazaki M, Ogata E, et al. c-fos protein as a target of anti-osteoclastogenic action of vitamin D, and synthesis of new analogs. J Clin Invest. 2006 Feb;116(2):528–535. doi: 10.1172/JCI24742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakai S, Takaishi H, Matsuzaki K, Kaneko H, Furukawa M, Miyauchi Y, et al. 1-alpha, 25-dihydroxy vitamin D3 inhibits osteoclastogenesis through IFN-beta-dependent NFATc1 suppression. J Bone Miner Metab. 2009;27(6):643–652. doi: 10.1007/s00774-009-0084-4. [DOI] [PubMed] [Google Scholar]
- 39.Poole JA, Wyatt TA, Kielian T, Oldenburg P, Gleason AM, Bauer A, et al. Toll-like receptor 2 regulates organic dust-induced airway inflammation. Am J Respir Cell Mol Biol. 2011 Oct;45(4):711–719. doi: 10.1165/rcmb.2010-0427OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauer C, Kielian T, Wyatt TA, Romberger DJ, West WW, Gleason AM, et al. Myeloid differentiation factor 88-dependent signaling is critical for acute organic dust-induced airway inflammation in mice. Am J Respir Cell Mol Biol. 2013 Jun;48(6):781–789. doi: 10.1165/rcmb.2012-0479OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nehme B, Letourneau V, Forster RJ, Veillette M, Duchaine C. Culture-independent approach of the bacterial bioaerosol diversity in the standard swine confinement buildings, and assessment of the seasonal effect. Environ Microbiol. 2008 Mar;10(3):665–675. doi: 10.1111/j.1462-2920.2007.01489.x. [DOI] [PubMed] [Google Scholar]
- 42.Nehme B, Gilbert Y, Letourneau V, Forster RJ, Veillette M, Villemur R, et al. Culture-independent characterization of archaeal biodiversity in swine confinement building bioaerosols. Appl Environ Microbiol. 2009 Sep;75(17):5445–5450. doi: 10.1128/AEM.00726-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charavaryamath C, Juneau V, Suri SS, Janardhan KS, Townsend H, Singh B. Role of tolllike receptor 4 in lung inflammation following exposure to swine barn air. Exp Lung Res. 2008 Jan;34(1):19–35. doi: 10.1080/01902140701807779. [DOI] [PubMed] [Google Scholar]
- 44.Gao Z, Dosman JA, Rennie DC, Schwartz DA, Yang IV, Beach J, et al. Association of toll-like receptor 2 gene polymorphisms with lung function in workers in swine operations. Ann Allergy Asthma Immunol. 2013 Jan;110(1) doi: 10.1016/j.anai.2012.11.003. 44,50.e1. [DOI] [PubMed] [Google Scholar]
- 45.Sundblad BM, von Scheele I, Palmberg L, Olsson M, Larsson K. Repeated exposure to organic material alters inflammatory and physiological airway responses. Eur Respir J. 2009 Jul;34(1):80–88. doi: 10.1183/09031936.00105308. [DOI] [PubMed] [Google Scholar]
- 46.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006 Jan;116(1):146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014 Jan;2(1):76–89. doi: 10.1016/S2213-8587(13)70165-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.