Abstract
Objective
Although 5-year survival for early-stage ovarian cancer is favorable, prognosis at recurrence is poor, necessitating appropriate initial management. We examined the patterns of care and the impact of the duration of chemotherapy on survival for women with early-stage ovarian cancer.
Methods
We used the SEER-Medicare database to identify women ≥65 years of age with stage I ovarian cancer diagnosed from 1992-2009. Patients were categorized as low-risk (non-clear cell histology, stage IA or IB, grade 1 or 2) or high-risk (clear cell histology, grade 3, or stage IC). We used multivariable logistic regression models to determine predictors of chemotherapy use and duration and Cox proportional hazards models to evaluate the effect of chemotherapy use and duration on survival.
Results
We identified 1394 patients. Among low-risk patients, 32.9% received adjuvant chemotherapy and the use of chemotherapy increased with time. Among high-risk patients, 71.9% received adjuvant chemotherapy; 44.2% had ≤ 3 months of treatment, and 55.8% had >3 months of treatment. Older patients were less likely to receive chemotherapy, while those with higher stage and grade were more likely to receive chemotherapy (P<0.05 for all). Among high-risk patients, the duration of chemotherapy did not impact overall (HR=0.93, 95% CI, 0.67-1.27) or cancer specific (HR=0.93; 95% CI, 0.61-1.42) survival.
Conclusions
Among early-stage ovarian cancer patients, practice patterns are widely divergent. Extended duration chemotherapy does not appear to impact survival for women with high-risk disease.
Introduction
Women with early-stage ovarian cancer have a favorable prognosis with five-year survival rates greater than 90% in some subgroups.1 Standard therapy for early-stage ovarian cancer consists of oophorectomy with surgical staging; prior reports have suggested that approximately 30% of patients with apparent ovary-confined disease have occult nodal, pelvic or abdominal metastases.2-4 Low socioeconomic status, advanced age, and minority race/ethnicity are associated with failure to receive recommended comprehensive surgical staging.5
Recommended adjuvant therapy for early-stage ovarian cancer depends on tumor sub-stage and grade. Two randomized controlled trials by the Gynecologic Oncology Group (GOG) demonstrated that adjuvant chemotherapy did not provide a survival benefit in patients with low-risk tumors (stage IA-IB, grade 1-2).6 In contrast, patients with high-risk (stage IA-IB grade 3, stage IC, stage II), early-stage ovarian cancer appear to benefit from adjuvant chemotherapy.1,7-10 The benefit of chemotherapy for subsets of patients with early-stage ovarian cancer has subsequently been confirmed in several trials.3,10,11
Although there is general consensus about the use of adjuvant chemotherapy in high-risk early-stage patients, there is debate about the optimal duration of chemotherapy. A randomized GOG trial comparing three versus six cycles of platinum and taxane-based chemotherapy showed no survival benefit for extended chemotherapy although this strategy was accompanied by increased toxicity.7 While the trial concluded that the optimal treatment for these patients is three cycles of chemotherapy, methodologic concerns have led to continued debate about the optimal duration of chemotherapy.1,9 While the risk of recurrence for stage I patients is lower, when patients do recur, treatment is palliative.1 Given these findings, appropriate initial management of early-stage ovarian cancer is paramount.
Given the controversy surrounding the management of early-stage ovarian cancer, we performed a population-based analysis to examine the quality of care and outcomes for women with early-stage ovarian cancer. Specifically, we explored the adherence to guideline-based recommendations for administration of adjuvant chemotherapy and analyzed the influence of duration of chemotherapy on survival for early-stage, high-risk patients.
Methods
Data Source
The Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database was used for analysis.12-14 SEER is a population-based cancer registry maintained by the National Cancer Institute that provides data on tumor histology, location, stage, treatment, and survival, as well as demographic and selected census tract-level information. The Medicare database includes information on patients with Medicare part A (inpatient) and part B (outpatient) including billed claims, and diagnoses. These two files are linked and provide data on initial services and all follow-up care. Exemption from the Columbia University Institutional Review Board was obtained.
Patient Selection
Women aged ≥ 65 years with stage I epithelial ovarian cancer diagnosed as their first or only cancer between January 1, 1992 and December 31, 2009 were analyzed. Only women who underwent primary cancer-directed surgery including oophorectomy were included.13 Women who did not have full coverage of both Medicare Parts A and B or were enrolled in a non-Medicare health maintenance organization from 12 months prior through 6 months after cancer diagnosis were excluded because the billing claims for these patients were not submitted to Medicare for reimbursement completely.15 Similarly, women who received chemotherapy prior to surgery were excluded and only those patients who survived for more than 6 months after cancer-directed surgery were included in the analysis. Patients were risk stratified based on previously published data: low-risk (stage IA or IB, grade 1 or 2, non-clear cell histology), high-risk (stage IA or IB grade 3, any stage clear cell histology, stage IC any grade) and unknown risk (insufficient data on grade available to further classify).6
Patient Characteristics
Age at diagnosis was categorized into 5-year intervals and race recorded as white, black, and other. Year of diagnosis was stratified into four time periods: 1992-1996, 1997-2001, 2002-2005, and 2006-2009. The SEER marital status variable was recorded as married, not married, and unknown. An aggregate socioeconomic status (SES) score was calculated from education, poverty level, and income data from the 2000 census tract data, as previously reported by Du and colleagues.16 Patients' scores were ranked on a scale of 1-5 by use of the formula that incorporated education, poverty, and income weighted equally, with 1 being the lowest value. To assess the prevalence of comorbid medical diseases, we used the Klabunde adaptation of the Charlson comorbidity index (i.e., the Klabunde–Charlson index).17,18 Medicare inpatient and outpatient claims were searched for diagnostic codes of the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM).19 Area of residence was categorized as metropolitan or nonmetropolitan and tumor grade grouped as well, moderately, or poorly differentiated or unknown. Tumor histology was classified as serous, mucinous, endometrioid, clear cell or other. Stage was captured using the American Joint Cancer Commission staging criteria.
Treatments
Data on chemotherapy use was extracted from the Medicare files by searching the Level II Healthcare Common Procedure Coding System, Current Procedural Terminology (CPT) codes, ICD-9-CM diagnostic and procedure codes, and revenue center codes from physician claims files, the hospital outpatient claims files, or the Medicare provider review files. If a patient had at least one claim for chemotherapy within 6 months of surgery, she was coded as having received chemotherapy. A second analysis was performed to determine the influence of the duration of chemotherapy on outcomes for high-risk patients.12,20 To exclude patients with recurrent and progressive disease, only high-risk patients who received <8 months of continuous chemotherapy were included. Patients were stratified based on the duration of chemotherapy as ≤3 months and 4-8 months.
To assess adequacy of surgical staging, we evaluated lymphadenectomy. A patient with any pathologic nodal assessment as defined by SEER was considered to have undergone lymphadenectomy.
Statistical Analysis
Frequency distributions between categorical variables were compared using χ2 tests. The Cochran-Armitage test was used to examine changes in the use of chemotherapy over time. Multivariable logistic regression models were developed to determine predictors for chemotherapy use and duration. Separate models were developed for low and high-risk patient groups. Survival was calculated from the date of diagnosis to the date of death. The effect of chemotherapy on survival was examined using the Kaplan-Meier method and the results compared with the log-rank test. To examine the association between chemotherapy use and survival while controlling for other clinical and demographic variables, Cox proportional hazards models were developed. Separate analyses were performed for overall and cancer-specific survival. All analyses were conducted with SAS, version 9.4 (SAS Institute, Cary, NC). All statistical tests were two-sided. A P-value of <0.05 was considered statistically significant.
Results
We identified a total of 1394 women with stage I epithelial ovarian cancer. The median follow-up time for the cohort was 73 months. The cohort included 477 patients with low-risk tumors, 754 patients with high-risk early-stage tumors and 163 patients classified as unknown risk. The clinical and demographic characteristics of the low-risk patients are displayed (Table 1). Overall, 32.9% of women with low-risk tumors received chemotherapy. Chemotherapy use increased over time for low-risk patients from 29.4% (95% CI, 7.8-51.1%) in 1992 to 36.0% (95% CI, 17.2-54.8%) in 2009 (P=0.0001) (Figure 1). Lymph node sampling was performed in 223 (46.8%) low-risk patients. Chemotherapy was administered to 88 (56.1%) of patients who underwent lymphadenectomy compared to 69 (44.0%) of those who did not have nodal sampling (P=0.004).
Table 1. Clinical and demographic characteristics of the cohort stratified by risk and receipt of adjuvant chemotherapy for low risk patients.
| Low-risk | |||||
|---|---|---|---|---|---|
|
|
|||||
| No chemotherapy | Chemotherapy | ||||
|
| |||||
| N | (%) | N | (%) | P-value | |
| 320 | (67.1) | 157 | (32.9) | ||
| Age (years) | 0.002 | ||||
| 65-69 | 69 | (21.6) | 51 | (32.5) | |
| 70-74 | 83 | (25.9) | 42 | (26.8) | |
| 75-79 | 76 | (23.8) | 42 | (26.8) | |
| ≥80 | 92 | (28.8) | 22 | (14.0) | |
| Race | 0.14 | ||||
| White | 274 | (85.6) | 142 | (90.5) | |
| Black/other/unknown | 46 | (14.4) | 15 | (9.6) | |
| Year of diagnosis | 0.002 | ||||
| 1992-1996 | 89 | (27.8) | 21 | (13.4) | |
| 1997-2001 | 89 | (27.8) | 41 | (26.1) | |
| 2002-2005 | 68 | (21.3) | 48 | (30.6) | |
| 2006-2009 | 74 | (23.1) | 47 | (29.9) | |
| Marital status | 0.09 | ||||
| Married | 125 | (39.1) | 74 | (47.1) | |
| Unmarried/unknown | 195 | (60.9) | 83 | (52.9) | |
| SEER registry | 0.47 | ||||
| Eastern | 64 | (20.0) | 38 | (24.2) | |
| Midwest | 146 | (45.6) | 72 | (45.9) | |
| West | 110 | (34.4) | 47 | (29.9) | |
| Socioeconomic status | 0.23 | ||||
| Lowest (first) quintile | 41 | (12.8) | 12 | (7.6) | |
| Second quintile | 80 | (25.0) | 31 | (19.8) | |
| Third quintile | 71 | (22.2) | 40 | (25.5) | |
| Fourth quintile | 59 | (18.4) | 35 | (22.3) | |
| Highest (fifth) | 69 | (21.6) | 39 | (24.8) | |
| quintile/unknown | |||||
| Comorbidity score | 0.003 | ||||
| 0 | 184 | (57.5) | 113 | (72.0) | |
| 1 | 96 | (30.0) | 25 | (15.9) | |
| ≥2 | 40 | (12.5) | 19 | (12.1) | |
| Lymphadenectomy | 0.004 | ||||
| No/unknown | 185 | (57.8) | 69 | (44.0) | |
| Yes | 135 | (42.2) | 88 | (56.1) | |
| Histology | 0.001 | ||||
| Serous | 71 | (22.2) | 38 | (24.2) | |
| Mucinous | 116 | (36.3) | 31 | (19.8) | |
| Endometrioid/other | 133 | (41.6) | 88 | (56.1) | |
| Clear cell | - | - | - | - | |
| Grade | <0.001 | ||||
| 1 | 162 | (50.6) | 46 | (29.3) | |
| 2 | 158 | (49.4) | 111 | (70.7) | |
| 3 | - | - | - | - | |
| Unknown | - | - | - | - | |
| Stage | 0.01 | ||||
| IA | 301 | (94.1) | 137 | (87.3) | |
| IB | 19 | (5.9) | 20 | (12.7) | |
| IC | - | - | - | - | |
| INOS | - | - | - | - | |
Figure 1. Trends in percentage of early-stage ovarian cancer patients receiving chemotherapy, by risk group and year.
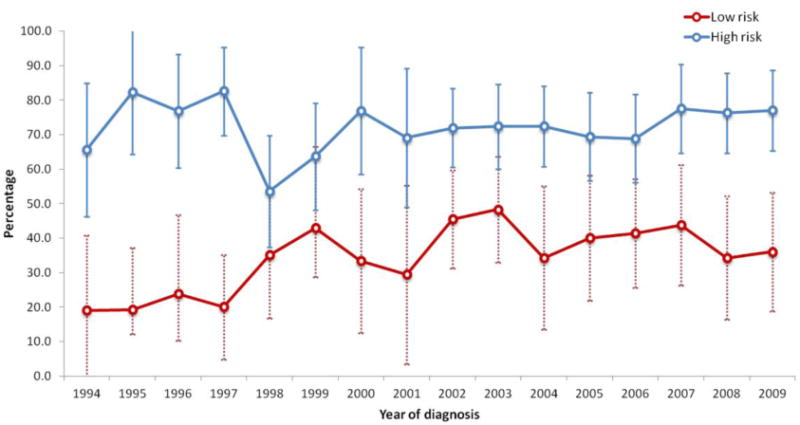
In a multivariable model of factors associated with receipt of chemotherapy for women with low-risk tumors, year of diagnosis was the strongest predictor of use of chemotherapy. Compared to patients treated in 1992-1996, those diagnosed in 1997-2001 (OR=2.36; 95% CI, 1.19-4.66), in 2002-2005 (OR=3.14; 95% CI, 1.58-6.25) and those treated in 2006-2009 (OR=3.31; 95% CI, 1.63-6.70) were more likely to receive chemotherapy. Patients with grade 2 tumors (vs. grade 1) (OR=2.28; 95% CI, 1.43-3.63) and patients with stage IB (vs. IA) (OR=2.69; 95% CI, 1.26-5.74) neoplasms were also more likely to receive chemotherapy. Use of chemotherapy decreased with advancing age (OR=0.31; 95% CI 0.16-0.60 for ≥80 compared to 65-69 years of age). While race was not associated with receipt of chemotherapy, patients with a comorbidity score of 1 were less likely to receive chemotherapy than those without comorbidities.
Among women with high-risk tumors, chemotherapy was administered to 71.9% of patients (Table 2). Among women who received chemotherapy, 54.6% underwent lymphadenectomy, while 36.3% of those who did not receive chemotherapy had a node dissection. Chemotherapy use increased from 62.5% (95% CI, 43.1-81.9%) in 1992 to 77.1% (95% CI 66.5-87.6%) in 2009 (P=0.17) (Figure 1). For patients with high-risk tumors, advancing stage and higher grade were associated with receipt of chemotherapy (Table 3). In contrast, older women were less likely to receive chemotherapy (P<0.05 for all).
Table 2. Univariate analysis of use and duration of use of chemotherapy for high-risk patients.
| High-risk | Duration of chemotherapy for high-risk patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| No chemotherapy | Chemotherapy | ≤3 months | 4-8 months | |||||||
|
| ||||||||||
| N | (%) | N | (%) | P-value | N | (%) | N | (%) | P-value | |
| 212 | (28.1) | 542 | (71.9) | 215 | (44.2) | 271 | (55.8) | |||
| Age (years) | <0.001 | 0.45 | ||||||||
| 65-69 | 32 | (15.1) | 172 | (31.7) | 64 | (29.8) | 94 | (34.7) | ||
| 70-74 | 39 | (18.4) | 187 | (34.5) | 79 | (36.7) | 84 | (31.0) | ||
| 75-79 | 45 | (21.2) | 105 | (19.4) | 40 | (18.6) | 57 | (21.0) | ||
| ≥80 | 96 | (45.3) | 78 | (14.4) | 32 | (14.9) | 36 | (13.3) | ||
| Race | 0.10 | 0.24 | ||||||||
| White | 184 | (86.8) | 498 | (91.9) | 200 | (93.0) | 246 | (90.8) | ||
| Black | 11 | (5.2) | 19 | (3.5) | * | * | * | * | ||
| Other/unknown | 17 | (8.0) | 25 | (4.6) | * | * | * | * | ||
| Year of diagnosis | 0.68 | 0.20 | ||||||||
| 1992-1996 | 43 | (20.3) | 101 | (18.6) | 32 | (14.9) | 60 | (22.1) | ||
| 1997-2001 | 54 | (25.5) | 126 | (23.3) | 54 | (25.1) | 59 | (21.8) | ||
| 2002-2005 | 57 | (26.9) | 143 | (26.4) | 62 | (28.8) | 67 | (24.7) | ||
| 2006-2009 | 58 | (27.4) | 172 | (31.7) | 67 | (31.2) | 85 | (31.4) | ||
| Marital status | <0.001 | 0.17 | ||||||||
| Married | 78 | (36.8) | 277 | (51.1) | 119 | (55.4) | 133 | (49.1) | ||
| Unmarried/unknown | 134 | (63.2) | 265 | (48.9) | 96 | (44.7) | 138 | (50.9) | ||
| Area of residence | 0.15 | 0.98 | ||||||||
| Metropolitan | 184 | (86.8) | 490 | (90.4) | 195 | (90.7) | 246 | (90.8) | ||
| Non-metropolitan | 28 | (13.2) | 52 | (9.6) | 20 | (9.3) | 25 | (9.2) | ||
| SEER registry | 0.41 | 0.35 | ||||||||
| Eastern | 43 | (20.3) | 130 | (24.0) | 45 | (20.9) | 72 | (26.6) | ||
| Midwest | 90 | (42.5) | 234 | (43.2) | 97 | (45.1) | 114 | (42.1) | ||
| West | 79 | (37.3) | 178 | (32.8) | 73 | (34.0) | 85 | (31.4) | ||
| Socioeconomic status | 0.002 | 0.02 | ||||||||
| Lowest (first) quintile | 34 | (16.0) | 55 | (10.2) | 15 | (7.0) | 31 | (11.4) | ||
| Second quintile | 47 | (22.2) | 89 | (16.4) | 35 | (16.3) | 44 | (16.2) | ||
| Third quintile | 51 | (24.1) | 126 | (23.3) | 62 | (28.8) | 49 | (18.1) | ||
| Fourth quintile | 45 | (21.2) | 115 | (21.2) | 38 | (17.7) | 68 | (25.1) | ||
| Highest (fifth) | 35 | (16.5) | 157 | (29.0) | 65 | (30.2) | 79 | (29.2) | ||
| quintile/unknown | ||||||||||
| Comorbidity score | 0.001 | 0.14 | ||||||||
| 0 | 127 | (59.9) | 377 | (69.6) | 142 | (66.1) | 193 | (71.2) | ||
| 1 | 46 | (21.7) | 119 | (22.0) | 57 | (26.5) | 52 | (19.2) | ||
| ≥2 | 39 | (18.4) | 46 | (8.5) | 16 | (7.4) | 26 | (9.6) | ||
| Lymphadenectomy | <0.001 | 0.001 | ||||||||
| No/unknown | 135 | (63.7) | 246 | (45.4) | 79 | (36.7) | 139 | (51.3) | ||
| Yes | 77 | (36.3) | 296 | (54.6) | 136 | (63.3) | 132 | (48.7) | ||
| Omentectomy | <0.001 | 0.60 | ||||||||
| No | 90 | (42.5) | 150 | (27.7) | 55 | (25.6) | 75 | (27.7) | ||
| Yes | 122 | (57.6) | 392 | (72.3) | 160 | (74.4) | 196 | (72.3) | ||
| Histology | 0.20 | 0.75 | ||||||||
| Serous | 61 | (28.8) | 196 | (36.2) | 78 | (36.3) | 101 | (37.3) | ||
| Mucinous | 24 | (11.3) | 60 | (11.1) | 21 | (9.8) | 32 | (11.8) | ||
| Endometrioid | 45 | (21.2) | 119 | (22.0) | 44 | (20.5) | 61 | (22.5) | ||
| Clear cell | 54 | (25.5) | 118 | (21.8) | 49 | (22.8) | 55 | (20.3) | ||
| Other | 28 | (13.2) | 49 | (9.0) | 23 | (10.7) | 22 | (8.1) | ||
| Grade | 0.13 | 0.83 | ||||||||
| 1 | 22 | (10.4) | 39 | (7.2) | 14 | (6.5) | 21 | (7.8) | ||
| 2 | 29 | (13.7) | 102 | (18.8) | 37 | (17.2) | 52 | (19.2) | ||
| 3 | 116 | (54.7) | 308 | (56.8) | 129 | (60.0) | 152 | (56.1) | ||
| Unknown | 45 | (21.2) | 93 | (17.2) | 35 | (16.3) | 46 | (17.0) | ||
| Stage | <0.001 | 0.003 | ||||||||
| IA | 100 | (47.2) | 164 | (30.3) | 84 | (39.1) | 65 | (24.0) | ||
| IB | * | * | 29 | (5.4) | * | * | * | * | ||
| IC | 98 | (46.2) | 336 | (62.0) | 119 | (55.4) | 181 | (66.8) | ||
| INOS | * | * | 13 | (2.4) | * | * | * | * | ||
Cell ≤10.
Table 3. Multivariable logistic regression model of predictors of receipt of chemotherapy.
| Low risk patients | High risk patients | High risk patients who received chemotherapy, duration >3 months | |
|---|---|---|---|
| Age (years) | |||
| 65-69 | Referent | Referent | Referent |
| 70-74 | 0.73 (0.41-1.31) | 0.90 (0.52-1.55) | 0.76 (0.47-1.23) |
| 75-79 | 0.70 (0.38-1.27) | 0.44 (0.25-0.76)* | 1.14 (0.64-2.01) |
| ≥80 | 0.31 (0.16-0.60)* | 0.14 (0.08-0.24)* | 0.74 (0.38-1.45) |
| Race | |||
| White | Referent | Referent | Referent |
| Black | 0.78 (0.29-2.08) | 0.92 (0.36-2.33) | 0.53 (0.17-1.64) |
| Other/unknown | 0.89 (0.33-2.40) | 0.74 (0.34-1.60) | 2.30 (0.79-6.71) |
| Year of diagnosis | |||
| 1992-1996 | Referent | Referent | Referent |
| 1997-2001 | 2.36 (1.19-4.66)* | 0.93 (0.53-1.63) | 0.47 (0.25-0.88)* |
| 2002-2005 | 3.14 (1.58-6.25)* | 1.27 (0.72-2.25) | 0.50 (0.27-0.93)* |
| 2006-2009 | 3.31 (1.63-6.70)* | 1.10 (0.63-1.91) | 0.67 (0.37-1.23) |
| Marital status | |||
| Married | Referent | Referent | Referent |
| Unmarried | 0.88 (0.56-1.38) | 0.82 (0.56-1.20) | 1.34 (0.89-2.03) |
| Unknown | 0.49 (0.11-2.23) | 1.66 (0.55-5.06) | 1.38 (0.50-3.77) |
| Area of residence | |||
| Metropolitan | Referent | Referent | Referent |
| Non-metropolitan | 0.31 (0.12-0.82)* | 1.16 (0.61-2.21) | 1.03 (0.48-2.21) |
| SEER registry | |||
| Eastern | Referent | Referent | Referent |
| Midwest | 0.95 (0.53-1.71) | 0.83 (0.50-1.40) | 0.68 (0.40-1.16) |
| West | 0.69 (0.37-1.28) | 0.73 (0.44-1.22) | 0.62 (0.36-1.07) |
| Socioeconomic status | |||
| Lowest (first) quintile | Referent | Referent | Referent |
| Second quintile | 1.10 (0.46-2.62) | 0.73 (0.37-1.43) | 0.53 (0.22-1.27) |
| Third quintile | 1.35 (0.56-3.25) | 1.01 (0.52-1.95) | 0.28 (0.12-0.68)* |
| Fourth quintile | 1.38 (0.56-3.43) | 0.83 (0.42-1.66) | 0.69 (0.28-1.68) |
| Highest (fifth) quintile/unknown | 1.10 (0.44-2.72) | 1.65 (0.82-3.32) | 0.48 (0.21-1.13) |
| Comorbidity score | |||
| 0 | Referent | Referent | Referent |
| 1 | 0.42 (0.24-0.74)* | 1.19 (0.76-1.87) | 0.70 (0.43-1.13) |
| ≥2 | 0.89 (0.46-1.76) | 0.39 (0.22-0.67)* | 1.23 (0.59-2.55) |
| Lymphadenectomy | |||
| No | Referent | Referent | Referent |
| Yes | 1.19 (0.75-1.89) | 1.47 (0.99-2.17) | 0.51 (0.34-0.79)* |
| Unknown | 0.65 (0.19-2.16) | 0.58 (0.26-1.27) | 0.97 (0.36-2.64) |
| Histology | |||
| Serous | Referent | Referent | Referent |
| Mucinous | 0.71 (0.37-1.35) | 0.91 (0.47-1.77) | 0.96 (0.48-1.91) |
| Endometrioid | 1.19 (0.67-2.11) | 0.75 (0.45-1.27) | 1.17 (0.68-2.01) |
| Clear cell | - | 0.75 (0.42-1.36) | 1.06 (0.56-2.01) |
| Other | 1.43 (0.52-3.92) | 0.58 (0.31-1.09) | 0.45 (0.21-0.93)* |
| Grade | |||
| 1 | Referent | Referent | Referent |
| 2 | 2.28 (1.43-3.63)* | 2.13 (1.00-4.54)* | 1.05 (0.44-2.47) |
| 3 | - | 2.31 (1.14-4.68)* | 1.21 (0.54-2.73) |
| Unknown | - | 1.51 (0.69-3.29) | 1.20 (0.47-3.06) |
| Stage | |||
| IA | Referent | Referent | Referent |
| IB | 2.69 (1.26-5.74)* | 1.46 (0.64-3.36) | 2.07 (0.77-5.52) |
| IC | - | 2.52 (1.59-3.97)* | 2.22 (1.37-3.59)* |
| INOS | - | 4.14 (1.04-16.58)* | 4.86 (1.19-19.87)* |
P<0.05
Use of chemotherapy was not associated with survival for women with low-risk tumors. In a multivariable Cox proportional hazards model, chemotherapy use was not associated with improved cancer-specific (HR=1.62; 95% CI, 0.74-3.56) or overall (HR=0.93; 95% CI, 0.65-1.33) survival (Table 4). Among high-risk patients, administration of chemotherapy was associated with improved overall survival (HR=0.70; 95% CI, 0.53-0.91) but not cancer-specific survival (HR=0.89; 95% CI, 0.59-1.35). Figure 2A displays a Kaplan-Meier analysis of overall survival for high-risk patients stratified by receipt of chemotherapy (log-rank P<0.001).
Table 4. Impact of use of chemotherapy in low-risk and high-risk patients and duration of chemotherapy in high-risk patients on survival.
| Overall survival | Cancer-specific survival | |
|---|---|---|
| Use of chemotherapy in low-risk patients | 0.93 (0.65-1.33) | 1.62 (0.74-3.56) |
| Use of chemotherapy in high-risk patients | 0.70 (0.53-0.91)* | 0.89 (0.59-1.35) |
| Long versus short duration chemotherapy in high-risk patients | 0.93 (0.67-1.27) | 0.93 (0.61-1.42) |
Hazard ratio (95% confidence interval).
P<0.05
Adjusted for age, race, year of diagnosis, marital status, area of residence, SEER registry, SES, comorbidity, lymphadenectomy, histology, grade, and stage.
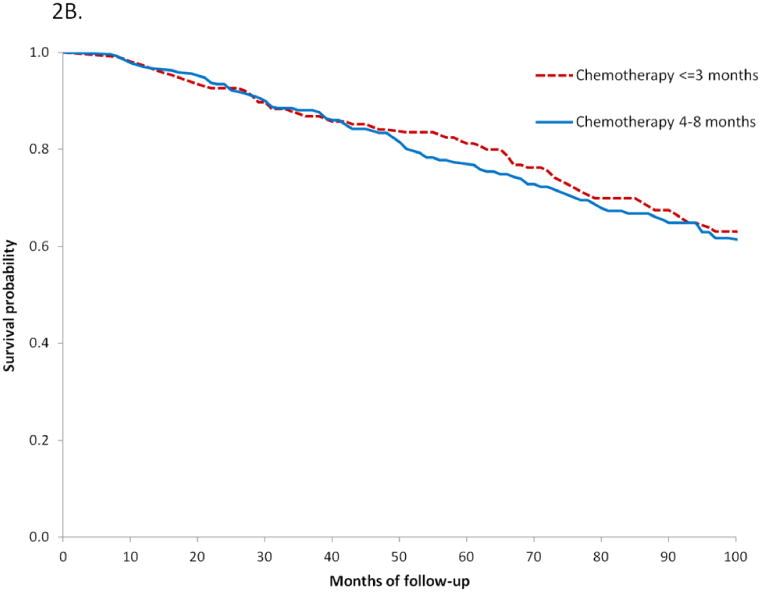
Figure 2.
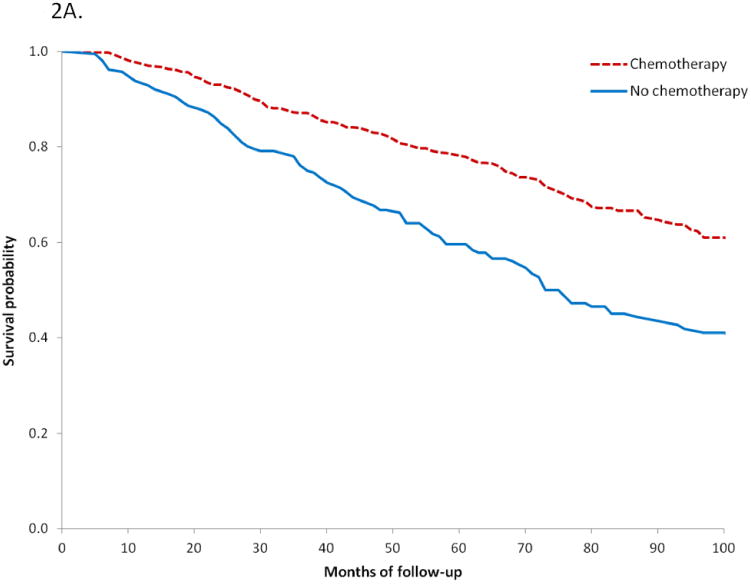
A. Kaplan-Meier analysis of overall survival for high-risk women based on receipt of chemotherapy (P<0.0001), red line chemotherapy, blue line no chemotherapy.
B. Kaplan-Meier analysis of overall survival based on duration of chemotherapy for high-risk women (P=0.14), red line chemotherapy ≤3 months, blue line chemotherapy 4-8 months.
When duration of chemotherapy was analyzed among high-risk patients, we noted that 215 (44.2%) women received ≤3 months of treatment while 271 (55.8%) received 4-8 months of chemotherapy. Advanced stage was the strongest predictor of extended duration chemotherapy. Women with stage IC tumors were more likely to receive chemotherapy for 4-8 months than women with stage IA tumors (OR=2.22; 95% CI, 1.37-3.59). In contrast, those who underwent lymphadenectomy (OR=0.51; 95% CI, 0.34-0.79) were less likely to receive longer duration chemotherapy. Among high-risk women who received chemotherapy and after adjustment for clinical and oncologic characteristics, the duration of chemotherapy had no effect on either cancer-specific (HR=0.93; 95% CI, 0.61-1.42) or overall (HR=0.93; 95% CI, 0.67-1.27) survival. Likewise, in a Kaplan-Meier analysis, duration of chemotherapy had no effect on survival (P=0.76).
Discussion
Our findings demonstrate wide spread variation in practice patterns for elderly women with early-stage ovarian cancer. Fewer than half of the women analyzed underwent lymphadenectomy as part of comprehensive surgically staging. More concerning, 28% of women for whom chemotherapy was indicated did not receive treatment, while nearly a third of patients with low-risk tumors who are unlikely to derive benefit from chemotherapy were treated with chemotherapy.
These data add to a growing body of literature that suggests that women with early-stage ovarian cancer often receive treatment that is discordant with evidence-based recommendations.21,22 Prior studies have shown that comprehensive surgical staging is commonly omitted for women with ovarian cancer.5,22-26 In a study of 4057 early-stage ovarian cancer patients from the Health Care Cost and Utilization Project, only 53% underwent lymph node sampling.22 Similar to population-based studies in the United States and Europe, our findings demonstrate a lack of adherence to comprehensive surgical staging guidelines in early-stage ovarian cancer, with omission of lymphadenectomy occurring in the majority of patients.22,27,28 Our study adds to prior analyses by focusing on presumed early-stage disease, a population in whom surgical staging significantly impacts adjuvant treatment planning and survival. In our cohort, only 46.3% of women underwent lymphadenectomy. While performance of lymphadenectomy had no impact on receipt of chemotherapy for both low and high-risk patients, high-risk women who underwent lymphadenectomy were less likely to receive chemotherapy for >3 months. Further, one would expect that approximately 30% of the patients in our series would have been upstaged had they undergone lymphadenectomy.24
Women with low-risk tumors are unlikely to derive benefit from chemotherapy.6 Current recommendations by the National Comprehensive Cancer Center (NCCN) recommend observation for patients with stage IA/IB grade 1 tumors and observation or chemotherapy for grade 2 neoplasms 29. Consistent with these data, we found no improvement in survival for low-risk patients treated with chemotherapy. We did however note that use of chemotherapy was not only common for women with low-risk tumors, but appeared to be increasing over time.
For women with high-risk early-stage ovarian cancer, clinical trials have demonstrated the benefits of adjuvant chemotherapy.3,10,11 We noted similar results among our cohort of elderly women with ovarian cancer. Among patients with high-risk, early-stage ovarian cancer, chemotherapy was associated with improved overall survival. In this population the survival benefit was modest and limited to overall survival. Despite the benefits of chemotherapy in this population of women, we noted that 28% of high-risk patients did not receive adjuvant chemotherapy.
Although there is consensus about the benefit of adjuvant chemotherapy for high-risk patients, the optimal duration of chemotherapy remains controversial. While a clinical trial comparing three versus six cycles of chemotherapy demonstrated no survival benefit with six cycles of therapy, these data were only powered to detect a >30% difference in survival.7 Post-hoc analysis of the GOG data suggested that some groups of women, particularly those with serous histology, derive benefit from longer duration chemotherapy.30 Our findings are in line with the GOG's data in that there was no improvement in survival for high-risk, early-stage patients treated with longer duration chemotherapy.
While our report benefits from the inclusion of a large sample size, we recognize a number of important limitations. As with any study using administrative data, we cannot control for unmeasured confounding factors that undoubtedly influenced not only the decision to utilize chemotherapy, but also the duration of chemothearpy. Using Medicare billing data, it is difficult to precisely determine the number of cycles of chemotherapy administered. To overcome this limitation, we used duration of chemotherapy as a surrogate for cycles of treatment as has been previously described.31 Similarly, over the timespan of the study, the therapeutic agents for ovarian cancer and the way these drugs are delivered have evolved. We used a permissive definition of chemotherapy to include treatment with any cytotoxic agent. A priori we also recognize that a large number of patients did not undergo comprehensive staging and may have had occult disease. While this is a limitation in that the reported stage is based on incomplete pathologic assessment, these data capture a “real world” scenario of how patients are managed surgically and how available pathologic data is used to make decisions regarding adjuvant treatment. For some of the subset analysis, particularly for low-risk patients, our sample size and power were limited to detect small differences in survival. Lastly, our data only includes elderly women and may not be generalizable to younger patients.
Our findings demonstrate that the management of early-stage ovarian cancer in clinical practice is widely divergent from evidence-based guidelines in elderly women. Many factors likely contributed to these deviations from standard of care and further efforts should be directed toward exploring why recommended care is not delivered.32,33 Given the poor prognosis of recurrent ovarian cancer, initiatives to optimize the management of women with early stage ovarian cancer are clearly warranted..
Research Highlights.
-Among early-stage ovarian cancer patients, practice patterns are widely divergent
-Extended duration chemotherapy does not appear to impact survival for women with high-risk disease
Acknowledgments
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01CA134964) are recipients of grants from the National Cancer Institute. Dr. Tergas is the recipient of a fellowship from the NCI (NCI R25 CA094061-11).
Footnotes
The authors have no conflicts of interest or disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chan JK, Tian C, Teoh D, et al. Survival after recurrence in early-stage high-risk epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecologic oncology. 2010;116:307–11. doi: 10.1016/j.ygyno.2009.10.074. [DOI] [PubMed] [Google Scholar]
- 2.NCCN Ovarian Cancer Treatment Guidelines. Accessed at http://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf.)
- 3.Trimbos B, Timmers P, Pecorelli S, et al. Surgical staging and treatment of early ovarian cancer: long-term analysis from a randomized trial. Journal of the National Cancer Institute. 2010;102:982–7. doi: 10.1093/jnci/djq149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harlan LC, Clegg LX, Trimble EL. Trends in surgery and chemotherapy for women diagnosed with ovarian cancer in the United States. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:3488–94. doi: 10.1200/JCO.2003.01.061. [DOI] [PubMed] [Google Scholar]
- 5.Goff BA, Matthews BJ, Larson EH, et al. Predictors of comprehensive surgical treatment in patients with ovarian cancer. Cancer. 2007;109:2031–42. doi: 10.1002/cncr.22604. [DOI] [PubMed] [Google Scholar]
- 6.Young RC, Walton LA, Ellenberg SS, et al. Adjuvant therapy in stage I and stage II epithelial ovarian cancer. Results of two prospective randomized trials. N Engl J Med. 1990;322:1021–7. doi: 10.1056/NEJM199004123221501. [DOI] [PubMed] [Google Scholar]
- 7.Bell J, Brady MF, Young RC, et al. Randomized phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: a Gynecologic Oncology Group study. Gynecologic oncology. 2006;102:432–9. doi: 10.1016/j.ygyno.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Chan JK, Tian C, Monk BJ, et al. Prognostic factors for high-risk early-stage epithelial ovarian cancer: a Gynecologic Oncology Group study. Cancer. 2008;112:2202–10. doi: 10.1002/cncr.23390. [DOI] [PubMed] [Google Scholar]
- 9.Kolomainen DF, A'Hern R, Coxon FY, et al. Can patients with relapsed, previously untreated, stage I epithelial ovarian cancer be successfully treated with salvage therapy? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:3113–8. doi: 10.1200/JCO.2003.06.119. [DOI] [PubMed] [Google Scholar]
- 10.Trimbos JB, Parmar M, Vergote I, et al. International Collaborative Ovarian Neoplasm trial 1 and Adjuvant ChemoTherapy In Ovarian Neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. Journal of the National Cancer Institute. 2003;95:105–12. [PubMed] [Google Scholar]
- 11.Colombo N, Guthrie D, Chiari S, et al. International Collaborative Ovarian Neoplasm trial 1: a randomized trial of adjuvant chemotherapy in women with early-stage ovarian cancer. J Natl Cancer Inst. 2003;95:125–32. doi: 10.1093/jnci/95.2.125. [DOI] [PubMed] [Google Scholar]
- 12.Wright J, Doan T, McBride R, Jacobson J, Hershman D. Variability in chemotherapy delivery for elderly women with advanced stage ovarian cancer and its impact on survival. Br J Cancer. 2008;98:1197–203. doi: 10.1038/sj.bjc.6604298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright JD, Herzog TJ, Neugut AI, et al. Effect of radical cytoreductive surgery on omission and delay of chemotherapy for advanced-stage ovarian cancer. Obstet Gynecol. 2012;120:871–81. doi: 10.1097/AOG.0b013e31826981de. [DOI] [PubMed] [Google Scholar]
- 14.Wright JD, Neugut AI, Wilde ET, et al. Physician characteristics and variability of erythropoiesis-stimulating agent use among Medicare patients with cancer. J Clin Oncol. 2011;29:3408–18. doi: 10.1200/JCO.2010.34.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hershman DL, Wilde ET, Wright JD, et al. Uptake and economic impact of first-cycle colony-stimulating factor use during adjuvant treatment of breast cancer. J Clin Oncol. 2012;30:806–12. doi: 10.1200/JCO.2011.37.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110:660–9. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Sax FL, MacKenzie CR, Fields SD, Braham RL, Douglas RG., Jr Assessing illness severity: does clinical judgment work? J Chronic Dis. 1986;39:439–52. doi: 10.1016/0021-9681(86)90111-6. [DOI] [PubMed] [Google Scholar]
- 18.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 19.International Classification of Disease 9th revision. 2008 [Google Scholar]
- 20.Neugut AI, Matasar M, Wang X, et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. J Clin Oncol. 2006;24:2368–75. doi: 10.1200/JCO.2005.04.5005. [DOI] [PubMed] [Google Scholar]
- 21.Bristow RE, Powell MA, Al-Hammadi N, et al. Disparities in Ovarian Cancer Care Quality and Survival According to Race and Socioeconomic Status. Journal of the National Cancer Institute. 2013 doi: 10.1093/jnci/djt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goff BA, Matthews BJ, Wynn M, Muntz HG, Lishner DM, Baldwin LM. Ovarian cancer: patterns of surgical care across the United States. Gynecologic oncology. 2006;103:383–90. doi: 10.1016/j.ygyno.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Trope C, Kaern J. Adjuvant chemotherapy for early-stage ovarian cancer: review of the literature. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:2909–20. doi: 10.1200/JCO.2007.11.1013. [DOI] [PubMed] [Google Scholar]
- 24.Young RC, Decker DG, Wharton JT, et al. Staging laparotomy in early ovarian cancer. Jama. 1983;250:3072–6. [PubMed] [Google Scholar]
- 25.Le T, Adolph A, Krepart GV, Lotocki R, Heywood MS. The benefits of comprehensive surgical staging in the management of early-stage epithelial ovarian carcinoma. Gynecologic oncology. 2002;85:351–5. doi: 10.1006/gyno.2002.6636. [DOI] [PubMed] [Google Scholar]
- 26.Timmers PJ, Zwinderman K, Coens C, Vergote I, Trimbos JB. Lymph node sampling and taking of blind biopsies are important elements of the surgical staging of early ovarian cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2010;20:1142–7. doi: 10.1111/igc.0b013e3181ef8e03. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe CD, Tilling K, Raju KS. Management and survival of ovarian cancer patients in south east England. Eur J Cancer. 1997;33:1835–40. doi: 10.1016/s0959-8049(97)00192-5. [DOI] [PubMed] [Google Scholar]
- 28.Tingulstad S, Skjeldestad FE, Hagen B. The effect of centralization of primary surgery on survival in ovarian cancer patients. Obstetrics and gynecology. 2003;102:499–505. doi: 10.1016/s0029-7844(03)00579-9. [DOI] [PubMed] [Google Scholar]
- 29.Alberts DS, Dorr RT. New Perspectives on an Old Friend: Optimizing Carboplatin for the Treatment of Solid Tumors. Oncologist. 1998;3:15–34. [PubMed] [Google Scholar]
- 30.Chan JK, Tian C, Fleming GF, et al. The potential benefit of 6 vs. 3 cycles of chemotherapy in subsets of women with early-stage high-risk epithelial ovarian cancer: an exploratory analysis of a Gynecologic Oncology Group study. Gynecologic oncology. 2010;116:301–6. doi: 10.1016/j.ygyno.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 31.Thrall MM, Gray HJ, Symons RG, Weiss NS, Flum DR, Goff BA. Trends in treatment of advanced epithelial ovarian cancer in the Medicare population. Gynecol Oncol. 2011;122:100–6. doi: 10.1016/j.ygyno.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Earle CC, Schrag D, Neville BA, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. Journal of the National Cancer Institute. 2006;98:172–80. doi: 10.1093/jnci/djj019. [DOI] [PubMed] [Google Scholar]
- 33.Schrag D, Earle C, Xu F, et al. Associations between hospital and surgeon procedure volumes and patient outcomes after ovarian cancer resection. Journal of the National Cancer Institute. 2006;98:163–71. doi: 10.1093/jnci/djj018. [DOI] [PubMed] [Google Scholar]


