Abstract
What do animals hear? While it remains challenging to adequately assess sensory perception in animal models, it is important to determine perceptual abilities in model systems to understand how physiological processes and plasticity relate to perception, learning, and cognition. Here we discuss hearing in rodents, reviewing previous and recent behavioral experiments querying acoustic perception in rats and mice, and examining the relation between behavioral data and electrophysiological recordings from the central auditory system. We focus on measurements of critical bands, which are psychoacoustic phenomena that seem to have a neural basis in the functional organization of the cochlea and the inferior colliculus. We then discuss how behavioral training, brain stimulation, and neuropathology impact auditory processing and perception.
Keywords: audition, behavior, hearing, mouse, plasticity, rat
1. Introduction
The sensory nervous system provides information about the external world. Much is known about how sensory information is represented in neural circuits particularly in the cerebral cortex, including how recent sensory experience is used to update and modify previous representations via mechanisms of synaptic plasticity (Buonomano and Merzenich, 1998; Gilbert et al., 2001; Feldman and Brecht, 2005; Carcea and Froemke, 2013). However, less is understood about how neuronal responses, tuning curves, and patterns of network activity relate to sensory perception and behavioral decisions. This makes it challenging to understand the functional significance of synaptic modifications in sensory circuits and limits the application of nootropic therapies and interventions targeted to specific neural structures (Rubenstein and Merzenich, 2003; Kilgard, 2012; Ganguly and Poo, 2013; Nahum et al., 2013; Sale et al., 2014).
In part this is due to the difficulties in accurately assessing perceptual abilities in animals, which cannot report subjective experiences; thus experiments in animals on the neural bases of perception and cognition must rely on behavioral responses or physiological measures. Unfortunately, interpreting physiological response data in terms of perception is problematic. For example, adaptation at different stages throughout the nervous system could cause stimuli that are robustly represented in one area (e.g., auditory cortex) to fail to elicit responses in downstream target areas (e.g., frontal cortex). Likewise, failure to detect stimulus-evoked responses in brain regions related to motor control does not necessarily mean that animals have failed to perceive those stimuli. Additionally, lack of motivation can confound reliable behavioral response measurements, even if animals have been carefully trained or conditioned. It remains unclear both what animals perceive on specific trials of sensorimotor behavioral tasks, and what they can perceive, in terms of limitations of sensory systems.
Given these constraints, why study audition, and why use rodents to do so? To start, neurons in the auditory system can respond to sensory input with temporally precise spike firing and are able to follow modulations in stimulus statistics with high reliability – up to one kHz in the case of some single inner hair cells (Russell and Sellick, 1983). Phase locking and following rates decrease and responses generally become noisier further along the central auditory path, but auditory cortical responses to complex or natural sounds can be surprisingly reliable (Machens et al., 2004; Liu et al., 2006; Schnupp et al., 2006; Huetz et al., 2009). These observations motivate studies examining how excitatory and inhibitory synaptic inputs are coordinated to enforce spike timing precision, and suggest that the auditory system in particular is suitable for investigating the functional consequences of phenomena such as excitatory-inhibitory balance (Volkov and Galazjuk, 1991; Wehr and Zador, 2003; Tan and Wehr, 2009; Dorrn et al., 2010) and spike-timing-dependent plasticity (Froemke et al., 2005; Dahmen et al., 2008; Lee et al., 2009; Tzounopoulos and Kraus, 2009; Chen et al., 2013).
Adaptation, plasticity, and other forms of cellular and network-level memory are a fundamental part of auditory processing. Auditory objects are transient and usually persist for milliseconds to seconds, meaning that auditory scene analysis requires memory traces over multiple timescales (Bregman, 1994; Shamma et al., 2011). This may include recognition of novel or low-probability sounds, which likely involves adaptive processes such as short-term depression for filtering out behaviorally-irrelevant sensory information (Ulanovsky et al., 2003; 2004; Wehr and Zador, 2005). Mechanisms of longer-term plasticity could be involved in processing the behavioral significance of certain sounds (Froemke and Martins, 2011; Froemke et al., 2013), as indicated by studies in trained animals (Buonomano and Merzenich, 1998) and by examination of pup call representations in virgin female mice compared to dams (Liu et al., 2006; Bennur et al., 2013; Rothschild et al., 2013).
With all this in mind, the choice of animal model – particularly that of rodents – depends on a few key points. While a requirement for similarity to humans is not necessary for all fields or all questions, it becomes important in a context where human ability is inferred from animal results and where such results lay foundations for human therapies and technologies (e.g., cochlear implants). The structures and functions of the mammalian central auditory pathway are similar in rodents, carnivores, and primates, with some cochlear specializations for processing higher frequency sounds in smaller animals (Fay, 1988). Furthermore, like many species, many types of rodents use sound for social communication. Although the rodent auditory system can differ in some significant ways from that of humans (Felix, 2002), there is a large knowledge base provided by the decades of research in both auditory and non-auditory fields using mice and rats. Mice and rats are by far the most commonly used mammals in research (Malakoff, 2000) and according to Willott (2007), rodent auditory processing is arguably as well-understood as any other nonhuman species. Therefore, rodent models can be used to ask specific questions and uncover general principles, such as understanding the neural bases of critical bands or evaluating the function of auditory prostheses such as cochlear implants.
Finally, some details of contemporary experimental design make these studies more convenient in rodents, and rats in particular. Rats occupy an important niche in the fields of plasticity and behavior because of their low cost and ability to learn complex behavioral paradigms quickly, which allows them to be trained in large cohorts in combination with technically-challenging recording methods. Rats and mice provide a tremendous degree of experimental flexibility via cutting-edge tools such as optogenetics, in vivo whole-cell recording, two-photon imaging, or high-density array recording. As a result, these animals are important and tractable models for auditory research requiring in vivo recordings during behavior.
This review will discuss rodent auditory critical bands as a means for elucidating and understanding the limits of sensory resolution and perception. While critical bands have been observed in other sensory modalities (Marks, 1979; Jones et al., 1999; Nefs et al., 2003), they have been most thoroughly investigated in the auditory system. We review the rodent auditory critical band literature, describing the known anatomical and physiological basis for critical bands, as well as discussing the potential for plasticity throughout the central auditory system with respect to modifying critical bands and improving the limits of auditory perception.
2. Anatomy and Physiology of Critical Bands
How much of the limitations of sound perception – detection and recognition abilities in particular – are determined by the biophysics and organization of the auditory system? A major function of a sensory system is to detect stimuli. Detection abilities are largely defined by intensity thresholds across hearing ranges. Hearing range seems largely determined by cochlear anatomy and the physical characteristics of the head (Heffner and Heffner, 2007). Low-frequency hearing is limited by cochlear size and curvature (West, 1985; Manoussaki et al., 2008). In particular, there is a high inverse correlation between the low-end hearing limit and the ratio between the curvature radii of the cochlear base to apex (Manoussaki et al., 2008). By comparison, functional head size is defined as the time for sound to travel from one ear to the other, and is correlated with the extent of high frequency hearing (Heffner and Heffner, 2010) while setting a physical limit for sound localization abilities based on binaural cues (Phillips et al., 2012). There has been considerable progress on understanding how the mammalian auditory system uses interaural differences to compute the spatial location of sound sources (Brand et al., 2002; King et al., 2011). For example, the ability of rats to capitalize on interaural time and level differences has been described in Koka et al. (2008). Therefore here we will focus only on detection and recognition abilities instead of sound localization.
2.1. What are critical bands?
Critical bands are believed to impose a biophysical limit on possible frequency resolution. Originally defined by Fletcher in 1940, a critical band is a frequency range within which a tone will affect perception of a previous tone by masking. Tones within the frequency range of a critical band are thought to activate the same location of the basilar membrane (Schreiner et al., 2000). Critical bands contribute to the ability to discriminate sounds (Ehret and Merzenich, 1988; Schreiner and Langner, 1997; Egorova et al., 2006; Egorova and Ehret, 2008) and to detect sounds in background noise (Watson, 1963). Critical band filtering and integration are basic components of mammalian sound perception (Plomp, 1968; Scharf, 1970; Plomp, 1971; Greenwood, 1991; Roederer, 2008) and are active for any sound composed of complex frequencies, which is almost every natural sound (Scharf, 1970).
Direct determination of critical bands is time consuming and thus these measurements exist for few animals (Fay, 1988). The alternative is an indirect measure of frequency selectivity, the critical ratio, which is much simpler to obtain because it only requires a single masked threshold measurement (Yost and Shofner, 2009). The critical ratio is a signal-to-noise ratio, where the signal is the power of the tone at masked threshold and the noise is simply the power of the noise per unit bandwidth (Yost and Shofner, 2009). Fletcher (1940) developed both concepts – critical bands and critical ratios – when he showed that noise-masked tone thresholds were affected by a certain “critical band” of frequencies centered on a tone that was to be detected (Fig. 1A). Essentially, noise masking did not affect thresholds until critical bands were reached where frequencies within these bands lowered the masked tone threshold, approaching the unmasked threshold. Following two fundamental assumptions about critical bands, Fletcher derived the critical ratio as an indirect measure of critical bands (Fletcher, 1940; Yost and Shofner, 2009).
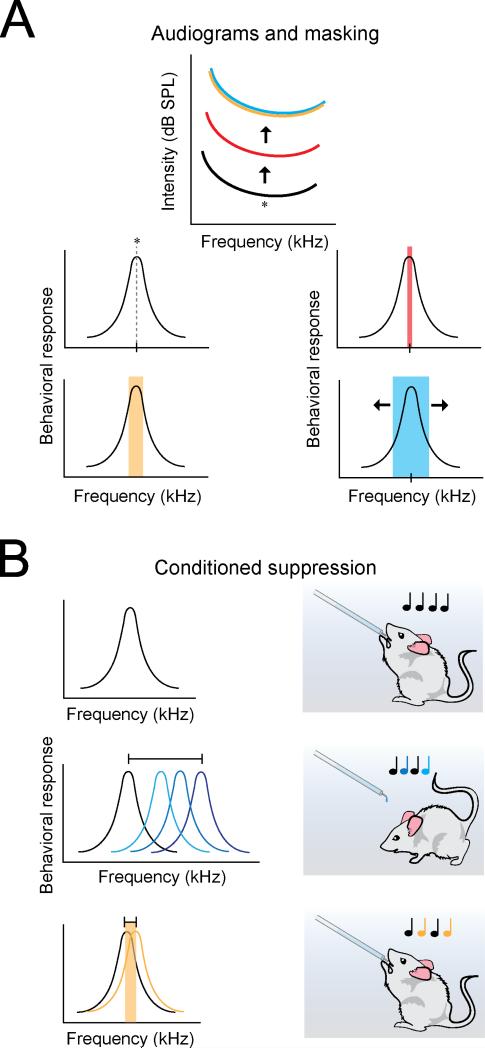
Figure 1.
Measurement of rodent critical bands. A, Tone masking by narrowband center frequency-centered noise (Ehret, 1976). Top: audiogram thresholds across tones of increasing frequency. Panels below: pure tone of a certain center frequency (asterisk), with increasingly large noisebands centered at the center frequency (red to orange to blue bands). Tone thresholds increase in the presence of a noiseband centered around the center frequency (red) and continue to increase up to a critical point (orange), at which larger noisebands result in no further increase in noise-masked tone thresholds (blue). B, Conditioned suppression in response to tones of a different frequency from target tone (Heffner and Masterton, 1980). Animals were trained to lick during presentation of a target tone of a specific frequency and to suppress licking for tones of a different frequency. Tones of varying frequency were presented to measure frequency discrimination.
Critical band and critical ratios have a few key features that overlap and allow critical ratios to be used as an estimate of critical bands. One caveat is that more recent data now suggests that critical ratios may be poor indicators of critical bands, especially in animals (Yost and Shofner, 2009). Both critical bands and critical ratios depend on frequency but are mostly independent of sound intensity, although absolute bandwidths for the two measures may differ significantly (Fletcher, 1940; Hawkins and Stevens, 1950; Scharf, 1970; Pickles, 1975; Seaton and Trahiotis, 1975; Ehret, 1976; Pickles, 1979). The absolute bandwidths at any given center frequency can vary by factors of 1 to 2.5, depending on the measurement method used, though the critical ratio is usually equal to or smaller than the critical band (Ehret and Schreiner, 1997).
Critical ratio measures for several non-human species are much larger than those for humans, implying that humans have sharper critical bands, i.e., better frequency selectivity, than other species (Yost and Shofner, 2009). Measurements of critical bands in different rodent species indicates that they are consistently much lower than critical ratios and not very different from direct frequency selectivity measurement in humans; additionally, critical ratios in animal models were consistently much higher than in humans (Evans et al., 1992; Niemiec et al., 1992; May et al., 2006). These larger critical ratios indicate that rodents may be inefficient at detecting a tone in a noise background; however, since rodent critical band sizes are similar to humans, these species may have similar frequency discrimination abilities. Yost and Schofner (2009) therefore postulated that rodents use a wide-band detection strategy, while humans use narrow-band detection. As a result, critical ratios and critical bands should be treated separately in the rodent, so we will focus here on critical bands rather than critical ratios.
The description of critical bands arose out of a rich literature on human psychoacoustics, followed by the development of parallel studies in various animals, including rodents. Many tests on both humans and other animals have flushed out the details and properties of critical bands (Scharf, 1970; Moore, 2013). Broadly, these can be categorized as studies on tone masking, e.g., by center-frequency noise bands (Fletcher, 1940; Ehret, 1976; Greenwood, 1991), by notched noise bands (Patterson, 1976; Weber, 1977), ripple noise (Houtgast, 1977; Pick, 1980), loudness summation within a band (Scharf, 1959), sound lateralization within bands (Scharf et al., 1976), or other approaches (Plomp and Levelt, 1965; Schorer, 1986).
In animals, thresholds have been measured as pure tone audiograms. While behavioral methods ultimately must be used to determine perceptual abilities, some animals can be difficult to rapidly and reliably train. This is especially challenging in young animals, as some early postnatal developmental processes occur more rapidly than the duration required for training (Dorrn et al., 2010; Froemke and Jones, 2011). Additionally, tone-evoked responses may not generalize to more complex stimuli that make up vocalizations and other natural sounds; conventional tuning curves reflect single tone measurements, while critical bands filter the interactions of two or more spectrally complex sounds (Ehret and Merzenich, 1988; Egorova et al., 2006). Single-tone tuning curve shapes differ significantly from those derived for complex sounds (Ehret and Schreiner, 1997). While critical bands depend both on measurement method and species tested (Ehret and Schreiner, 1997), critical bandwidths have been established across techniques and species (Fay, 1988). The striking similarities between critical bands of humans and those of other mammals contextualizes human psychoacoustics in a wide animal literature and supports the hypothesis that the neural basis of critical bands is similar in many mammalian species from rodents to humans.
Critical bands have three general features (Ehret and Merzenich, 1988; Ehret, 1995). First, frequency dependence- frequency bandwidths increase with increasing critical band center frequency (Scharf, 1970; Fay, 1988; Evans et al., 1992; Ehret and Schreiner, 1997). Second, intensity independence- up to about 80 dB sound pressure level (SPL), each frequency bandwidth around a separate center frequency is largely independent of sound intensity (Scharf, 1970; Scharf et al., 1976; Scharf and Meiselman, 1977; Ehret and Schreiner, 1997). Third, critical band filters are linear, such that sound type and energy do not affect energy summation within a single critical band (Ehret and Schreiner, 1997).
2.2. What structures support critical bands?
A neuronal theory of critical bands should account for these three major psychophysical properties. The logarithmic scale of critical band frequency dependence implicated the cochlea in determining this feature, since cochlear maps are also logarithmic (Braun, 1997). Intensity independence and linearity are not present peripherally, however, and instead are thought to be represented more centrally, perhaps by midbrain structures (Ehret and Merzenich, 1985; 1988; Egorova and Ehret, 2008).
2.2.1. Auditory periphery: cochlea and auditory nerve
The first stages of auditory processing occur in the cochlea, which is organized as a frequency map. Sensory hair cells are specific distances from the base of the cochlea and are best stimulated by the frequency that corresponds to the place where they reside. This frequency-place map is related to the stiffness change of the basilar membrane on which the hair cells sit- the base is stiffer and thicker, while the apex is flexible and wider (Ehret and Frankenreiter, 1977; Ehret, 1978). The cochlear tonotopy that this organization produces establishes the faithful encoding of frequency and selectivity for the rest of the auditory system. In fact, the frequency tuning of basilar membrane displacement is as sharp as the tuning of single auditory nerve fibers (Khanna and Leonard, 1982; 1986). Greenwood (1961) demonstrated that critical bandwidths are representations of constant distance intervals on the basilar membrane, approximating one millimeter each. Thus the auditory periphery can reliably represent discrete frequency bands; however, the cochlea also contributes to masking functions characteristic of sound representations, whereas the auditory nerve does not.
The inner and outer hair cells of the cochlea interact to determine absolute and noise-masked auditory thresholds, respectively. Inner hair cell loss increases absolute auditory threshold, while loss of outer hair cells has no effect on absolute auditory threshold. Outer hair cell damage does affect masked thresholds in the frequency range in which they are damaged; damage of outer hair cells results in decreased noise-masked thresholds, indicating that masking is a function of these cells in cats (Dolan et al., 1974), chinchillas (Ward and Duvall, 1971), guinea pigs (Wersäll et al., 1973) and mice (Ehret, 1979).
2.2.2. Subcortical processing: brainstem and midbrain
Properties of spiral ganglion cells and auditory nerve fibers seem to be only minimally involved in masking or determining the intensity-independence of critical bands. Instead, these cells act as nonlinear filters for sound intensities >30 dB SPL above the tone response threshold (Evans, 1974) (Pickles, 1975; Nienhuys and Clark, 1979; Pickles, 1979; Ehret and Moffat, 1984; Ehret and Merzenich, 1985; 1988). These properties therefore are thought to depend on processing further along in the auditory feedforward pathway (Ehret and Merzenich, 1988).
The lowest subcortical relay that demonstrates filtering properties consistent with critical bands is the inferior colliculus (Pickles, 1975; Ehret, 1976; Pickles, 1979; Ehret and Moffat, 1984; Ehret and Merzenich, 1985; 1988). This is supported by extensive single neuron recordings in the central nucleus of the inferior colliculus of the cat, whose laminar structure and extensive lateral inhibition properties are precisely organized along critical bands (Ehret and Merzenich, 1985; Langner and Schreiner, 1987; Ehret and Merzenich, 1988). Additionally, human evoked brainstem potentials also suggest the critical bands arise in the midbrain (Zerlin, 1986). Conversely, neuronal responses within the cochlear nucleus, which provides the major feedforward input to the inferior colliculus, do not tend to exhibit relationships to critical band properties (Greenwood and Goldberg, 1970; Spirou and Young, 1991).
The three-dimensional structure of the central nucleus of the inferior colliculus is fairly complex, but has been finely mapped in cats, rats, and mice. The lower auditory brainstem nuclei send more than ten input projections to partially overlapping areas in the central nucleus (Brunso-Bechtold et al., 1981; Malmierca, 2004; Winer and Schreiner, 2005). Sound representations in the lower nuclei are transmitted to frequency-band laminae in the central nucleus, which are comprised of major and minor frequency gradients (Schreiner and Langner, 1997). These laminae are determined by the major gradient, formed by the tonotopic distribution of characteristic frequencies. The ventromedial area contains neurons tuned to high frequencies while neurons with lower characteristic frequencies are found within the dorsolateral area. The minor gradients run within each frequency-band lamina, with high frequency characteristic frequencies across the ventrolateral area and neurons with lower characteristic frequencies found dorsomedial. Strikingly, the spectral distance between frequency-band laminae corresponds to one critical band; frequencies spanned by a single lamina are also separated by one critical band (Schreiner and Langner, 1997). Thus in the cat, the anatomical and physiological characteristics of the central nucleus provide a spatial tonotopy organized in terms of critical bands (Egorova and Ehret, 2008). Furthermore, spectral bandwidths, inhibition and correlated firing within the central nucleus of the inferior colliculus are all on the order of one critical band (1/3 octave) in cat (Rodriguez et al., 2010; Chen et al., 2012). It has also been demonstrated that rats have a comparable tonotopic map in the inferior colliculus, with each individual lamina spanning a single critical band of approximately 0.3 octaves (Malmierca et al., 2008). Analogous studies in the mouse midbrain have demonstrated similar gradients in rodents as in carnivores (Willard and Ryugo, 1983; Stiebler and Ehret, 1985; Meininger et al., 1986; Romand and Ehret, 1990).
While critical bands themselves are psychoacoustic phenomena, the neuronal representations of critical band properties are referred to as ‘neural critical bands’. Neural critical bandwidths are 3/8 to 1/3 of an octave around the respective excitatory characteristic frequency, and are asymmetrically centered closer to the high-frequency boundary. However, neural critical bandwidth boundaries are not the same as the extents of excitatory receptive fields, but are instead defined by lateral inhibition (Ehret and Merzenich, 1988; Egorova et al., 2006). The organization of excitation and inhibition in the inferior colliculus also contributes to the balance of spectral and temporal modulation sensitivity at this central relay; as sensitivity to one increases, sensitivity to the other decreases, across the collicular tonotopic gradient (Rodriguez et al., 2010). Finally, in his model of collicular processing, Ehret (1995) has demonstrated that excitatory, facilitatory and inhibitory inputs within and across laminae can produce constant, intensity-independent neural critical bands.
2.2.3. Cortical refinement: auditory cortex
Some neurons in primary auditory cortex (AI) also seem to represent critical bands. These neurons are found in central and ventral AI, as neurons in dorsal AI neurons have properties that are incompatible with critical band features (Ehret and Schreiner, 1997). This may be related to the distinct forms of auditory processing in these subdivisions of AI. Spectral information is represented in central and ventral AI while dorsal AI may be involved in discriminating sound relevance.
In cat ventral and central AI, a large range of spectral bandwidth sensitivities can be found (Ehret and Schreiner 1997, Read et al., 2001, Atencio and Schreiner, 2012). Similar to the inferior colliculus, (Rodriguez et al., 2010) excitatory bandwidths in AI scale with suppressive bandwidth (Atencio and Schreiner, 2012). In agreement, the tuning properties of excitatory and inhibitory synaptic inputs to AI neurons in adult cat, rat, and mouse are balanced (Dorrn et al., 2010; Froemke et al., 2013; Tan et al., 2007; Wehr and Zador, 2003; Tan and Wehr, 2009). This means that frequency tuning and intensity tuning profiles of excitation and inhibition are usually (but not always) similar, with GABAergic inhibitory responses scaling proportionally with the magnitude of tone-evoked excitation. Interestingly, this balanced inhibition is not observed in young animals, but requires developmental auditory experience (Chang et al., 2005; Dorrn et al., 2010; Jones and Froemke, 2011). These data support the notion that critical bandwidth properties are constructed and refined through a combination of co-tuned excitation and inhibition in central auditory stations.
In contrast, neural responses in dorsal AI are not characterized by the traditional critical band features: they are not dependent on characteristic frequency, they are not intensity independent, and they are quite broad (Ehret and Schreiner, 1997). Further, the general sound processing strategy of dorsal AI is unusual and neuronal tuning curves here have multiple peaks (Sutter and Schreiner, 1991) and poor frequency resolution (Ehret and Schreiner, 1997). Dorsal AI may be involved in discriminating sound relevance – analogous areas in the bat participate in echolocation (Suga, 1988) and anatomical homologs in the mouse contribute to social communication (Stiebler et al., 1997).
The auditory cortex usually plays a prominent role in processing frequency and critical band information when stimuli acquire behavioral significance or are linked to attentional processes. For example, in the monkey visual system, attention improves task performance by decreasing interneuronal correlation and modifying the sensitivity of a neuronal population, rather than affecting properties of single neurons (Cohen and Maunsell, 2009). In auditory perception, some studies suggest that attention modulates features of secondary auditory cortex, while others demonstrate a role for attention in primary auditory cortex. Attention seems to increase both cortical gain as well as frequency selectivity, possibly by lateral inhibition (Kauramäki et al., 2007). This has been demonstrated in trained ferrets where selective attention refines cortical receptive fields to focus on the attended frequencies via a center excitatory-surround inhibitory mechanism (Fritz et al., 2003). Additionally, Atiani et al. (2009) show that task performance correlates with the magnitude of change in auditory cortex where neurons sensitive to the background frequencies are suppressed and those representing the attended frequencies are enhanced.
3. Plasticity of Critical Bands
3.1. Plasticity in the central auditory system
The nervous system is sensitive and vulnerable to both intrinsic and extrinsic influences, especially during formation (e.g., gestation) and development (e.g., critical periods). Neural circuits are then continually plastic and adaptive throughout life, allowing experiences and activities to modify brain function and impact perceptual and cognitive skills. Since critical bands depend on both peripheral and central processes, it is reasonable to expect that variability in the development, maintenance and/or modification of each auditory relay can affect critical band organization and auditory perception.
3.1.1. Development of critical bands and the auditory system
There has been limited work on the development of critical bands and their modification before adulthood. (Schneider et al., 1990) showed that critical bandwidth does not significantly change with age in human infants and adults, at least not enough to account for developmental changes in masked and absolute thresholds. Adult thresholds are lower than those in infants by 8-15 dB SPL (Bull et al., 1981; Trehub et al., 1981; Nozza and Wilson, 1984). Over the same time frame, critical bandwidths are fairly constant (Olsho, 1985; Irwin et al., 1986). Even with a 50% larger critical bandwidth in infants, this would only account for a 1.75 dB SPL higher threshold. The rest of the threshold drop over development is unlikely to be due to differential motivation or attentional states (Schneider et al., 1990), and thus must result from other central modifications rather than from critical band development per se.
The central auditory system develops, matures and changes during life as a result of both intrinsic factors and experience. For example, the rodent auditory cortex is particularly plastic during early postnatal life, with hearing onset beginning at around postnatal day (P) 10 (Froemke and Jones, 2011). Beginning at hearing onset, a series of staggered critical periods progress over the first month of life for the development of characteristic frequency, bandwidth, direction selectivity, and excitatory-inhibitory balance (de Villers-Sidani et al., 2007; Insanally et al., 2009; Dorrn et al., 2010). Sensorimotor development and expression of auditory reflexes in pups can be accelerated if mothers are housed in an enriched environment during gestation (Cárdenas et al., 2015). Motherhood also modifies auditory representation and perception: auditory responses of AI parvalbumin-expressing neurons have recently been found to be centered around higher frequencies in lactating mother mice compared to virgins, which is appropriate for registering pup ultrasonic vocalizations (Cohen and Mizrahi, 2015). Other forms of behaviorally-relevant sound exposure affect auditory processing as well: while music-related neuroplasticity was originally thought to be a cortical phenomenon (Patel, 2003), music and language experience can also tune subcortical representations of complex sounds (Wong et al., 2007; Bidelman and Krishnan, 2010; Bidelman et al., 2011; Parbery-Clark et al., 2012).
3.1.2. Auditory pathology
Pathology – congenital, perinatal, or environmentally-induced – dramatically alters structural and functional aspects of the auditory brain. The most straightforward example of pathology affecting auditory development is deafness. There is an extensive literature enumerating the effects of hearing loss and congenital deafness on anatomy, physiology, and behavior in animal models (Kral et al., 2000; Salvi et al., 2000; Kral et al., 2002; Kral, 2007) and in humans (Bauer et al., 2006; Moore, 2008; Sharma et al., 2009). Other causes of perinatal period auditory pathology can include thyroid hormone imbalance, where both hypo- and hyperthyroidism can produce nervous system dysfunction; hypothyroid animals demonstrate middle and inner ear malformation as well as delayed onset of cochlear activity and elevated brainstem response thresholds (Hébert et al., 1985). These cochlear and brainstem effects would be expected to influence the frequency-dependence and intensity-independence of critical bands, respectively.
Disruption of normative patterns of acoustic experience can also impair development of the central auditory system. Several studies using noise (Zhang et al., 2002; Chang and Merzenich, 2003; Speechley et al., 2007), excessive loud or single tone exposure, or patterned stimulation (Nakahara et al., 2004) have provided the groundwork for observing changes in auditory maps and discrimination ability. For example, rat pups exposed to trains of 5 kHz pure tones show larger than normal regions of AI tuned to 5 kHz (Han et al., 2007). Surprisingly, adult rats previously exposed to 5 kHz tones have impaired discrimination around 5 kHz but enhanced discrimination at other target frequencies (Han et al., 2007). Thus at least in some cases, over-representations of certain frequencies after developmental exposure can reduce auditory discrimination. Other studies of late effects of early noise exposure demonstrate morphological changes in multiple auditory relays, including the inferior colliculus, medial geniculate body and auditory cortex (Ouda et al., 2014). Tuning curves in the inferior colliculus broaden and intensity coding is impacted (Grécová et al., 2009; Bures et al., 2010). While critical bandwidths may be relatively stable throughout life, it is clear that critical period plasticity can dramatically influence auditory processing that depends on subcortical and cortical effects.
3.2. Experimentally-induced plasticity
Instead of impairing performance, some forms of plasticity might be beneficial and lead to improvements in auditory perception. Why might it be attractive to improve plasticity and perceptual limits? Generally, the ability to experimentally manipulate neural circuits can provide a deeper understanding of how specific circuit elements might be related to behavior or psychoacoustic phenomena. Moreover, understanding the rules and mechanisms of experience-dependent plasticity in the mammalian central auditory system should be useful for the development and refinement of prosthetic devices, including cochlear implants or auditory brainstem/midbrain implants.
3.2.1. Behavioral training
Frequency recognition limens and just-noticeable differences are often much closer than suggested by critical bandwidths. Heffner and Masterton (1980) measured frequency discrimination in rodents via conditioned suppression (Fig. 1B). They found that rats have frequency discrimination limens of around 1-2%, while mice have frequency limens of around 2-5%, in agreement with an earlier study by Ehret (1975). These values are comparable to a recent study by de Hoz and Nelken (2014). Across mice, the just-noticeable difference was a frequency separation of 4-7%. When a different cohort of animals was pre-exposed to a certain frequency in the absence of an aversive air puff, they were slower to be conditioned to tones of that frequency. Interestingly, this latent inhibition generalized to tones over a much broader frequency range than was determined for frequency discrimination. These broader, asymmetrical generalization ranges of 20-40% are closer to nominal critical bandwidth (~33%), indicating that different neural circuits might be involved in sound frequency generalization as opposed to frequency recognition and discrimination. Specifically, stimulus generalization might be more influenced by the filtering properties of subcortical neurons, whereas the effects of training to recognize or discriminate stimuli may shape circuits in the cortex to improve frequency resolution for behaviorally-relevant stimuli.
3.2.2. Stimulation and neuromodulation
One of the first attempts to more specifically induce cortical plasticity and relate changes in cortical tuning curves to psychophysical abilities was performed by Talwar and Gerstein (2001). Adult rats were trained on one of two different frequency discrimination tasks. Intracortical microstimulation was then used to shift frequency tuning curves in a robust and reliable manner. However, little to no effect was observed on behavioral performance on either task (Talwar and Gerstein, 2001).
An alternative approach exemplified by the classic studies of Bakin and Weinberger (1996) demonstrated that pairing a pure tone with electrical stimulation of the cholinergic nucleus basalis can shift frequency tuning in anesthetized adult rats. Repetitive pairing in this manner induces long-term forms of synaptic modifications that collectively serve to shift cortical tuning curves to have new preference for paired inputs (Bakin and Weinberger, 1996; Froemke et al., 2007) and to reorganize auditory cortical maps (Kilgard and Merzenich, 1998). Behavioral modification occurs in conjunction with physiological changes: nucleus basalis pairing can improve frequency recognition performance (Reed et al., 2011) and improve detection of quiet sounds in background noise (Froemke et al., 2013).
These studies of cortical plasticity indicate that auditory perception in general- and critical band limitations in particular- can be changed and improved by behavioral training and/or manipulations of receptive field properties within the central auditory system. It is unlikely that basic biophysical properties of the cochlea are modified by training, although the functions of hair cells can be modulated by the olive (Darrow et al., 2007; Elgoyhen et al., 2009). Instead, it is more plausible that at least some of the behaviorally-determined limits of auditory perception arise within central structures. This is because neuromodulatory-based plasticity of cortical tuning curves can improve performance beyond the levels achieved in highly trained animals (Froemke et al., 2013). It remains to be determined to what degree these gains in performance are due to alterations of motivation or other internal state variables, acceleration of nominally-slower plastic processes engaged by training, or actual boosts in auditory processing capabilities within the central nervous system.
4. Conclusions
Over the last century, there has been intense effort devoted to understanding the mechanisms of auditory transduction and relating these mechanisms to auditory processing and perception, ultimately in hopes of restoring hearing to the profoundly deaf. Examination of critical band phenomena has been an essential and productive part of these studies. Critical bands denote the spectral bandwidth where more-or-less simultaneously presented tones interfere with and perception of each individual tone, and as such provide a tractable approach examining how the auditory system responds to complex acoustic stimuli. Classically, critical bands are thought to arise from cochlear biophysics, and thus the central auditory system is thought to inherit these fundamental limitations to auditory perception from the earliest stages of peripheral transduction. Directly testing this hypothesis requires robust behavioral paradigms for assessing auditory perception in animal models amenable to careful and invasive anatomical and physiological measurement techniques.
Rodents can be rapidly and reliably trained to report sensory stimuli, in a way that enables direct study of perceptual, cognitive, and memory systems. Behavioral readouts are ultimately required to query sensory perception in animals, and there are straightforward and reliable methods for performing these sorts of experiments in mice and rats. Although there are some anatomical and biophysical limitations on critical bands and other psychoacoustic phenomena, some failures in cognitive processing seem to be due to failures in synaptic transmission, motivation, attention, or other aspects of neural circuits that can be remediated via additional training or brain stimulation methods. However, to accurately modify synaptic strength and network function, such methods must be carefully applied and take into consideration the extant abilities of each animal, much like training procedures themselves. A major goal of future research is to understand how conditioning, training and learning recruit various neuromodulatory systems in a coordinated manner for control and optimization of behavioral performance.
Highlights.
- Auditory perception in rodents is measured behaviorally and physiologically.
- Behavioral training leads to physiological and perceptual modifications.
- Critical bands impose limits on frequency perception due to cochlear biophysics.
Acknowledgements
We thank L. de Hoz and I. Nelken for comments. This work was funded by NIDCD (DC009635 and DC012557) and a Sloan Research Fellowship (R.C.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atencio CA, Schreiner CE. Spectrotemporal processing in spectral tuning modules of cat primary auditory cortex. PLoS One. 2012;7:e31537. doi: 10.1371/journal.pone.0031537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atiani S, Elhilali M, David SV, Fritz JB, Shamma SA. Task difficulty and performance induce diverse adaptive patterns in gain and shape of primary auditory cortical receptive fields. Neuron. 2009;61:467–480. doi: 10.1016/j.neuron.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PW, Sharma A, Martin K, Dorman M. Central Auditory Development in Children With Bilateral Cochlear Implants. Arch Otolaryngol Head Neck Surg. 2006;132:1133–1136. doi: 10.1001/archotol.132.10.1133. [DOI] [PubMed] [Google Scholar]
- Bennur S, Tsunada J, Cohen YE, Liu RC. Understanding the neurophysiological basis of auditory abilities for social communication: a perspective on the value of ethological paradigms. Hear Res. 2013;305:3–9. doi: 10.1016/j.heares.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidelman GM, Gandour JT, Krishnan A. Cross-domain effects of music and language experience on the representation of pitch in the human auditory brainstem. J Cogn Neurosci. 2011;23:425–434. doi: 10.1162/jocn.2009.21362. [DOI] [PubMed] [Google Scholar]
- Bidelman GM, Krishnan A. Effects of reverberation on brainstem representation of speech in musicians and non-musicians. Brain Res. 2010;1355:112–125. doi: 10.1016/j.brainres.2010.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417:543–547. doi: 10.1038/417543a. [DOI] [PubMed] [Google Scholar]
- Braun M. Frequency spacing of multiple spontaneous otoacoustic emissions shows relation to critical bands: a large-scale cumulative study. Hear Res. 1997;114:197–203. doi: 10.1016/s0378-5955(97)00160-3. [DOI] [PubMed] [Google Scholar]
- Bregman AS. Auditory scene analysis: The perceptual organization of sound. Bradford Books; Cambridge, MA: 1994. [Google Scholar]
- Brunso-Bechtold JK, Thompson GC, Masterton RB. HRP study of the organization of auditory afferents ascending to central nucleus of inferior colliculus in cat. J Comp Neurol. 1981;197:705–722. doi: 10.1002/cne.901970410. [DOI] [PubMed] [Google Scholar]
- Bull D, Schneider BA, Trehub SE. The masking of octave-band noise by broad-spectrum noise: a comparison of infant and adult thresholds. Percept Psychophys. 1981;30:101–106. doi: 10.3758/bf03204466. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Bures Z, Grécová J, Popelá J, Syka J. Noise exposure during early development impairs the processing of sound intensity in adult rats. Eur J Neurosci. 2010;32:155–164. doi: 10.1111/j.1460-9568.2010.07280.x. [DOI] [PubMed] [Google Scholar]
- Carcea I, Froemke RC. Cortical plasticity, excitatory-inhibitory balance, and sensory perception. Prog Brain Res. 2013;207:65–90. doi: 10.1016/B978-0-444-63327-9.00003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas L, García-García F, Santiago-Roque I, Martínez AJ, Coria-Ávila GA, Corona-Morales AA. Enriched environment restricted to gestation accelerates the development of sensory and motor circuits in the rat pup. Int J Dev Neurosci. 2015;41:68–73. doi: 10.1016/j.ijdevneu.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- Chang EF, Bao S, Imaizumi K, Schreiner CE, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Proc Natl Acad Sci U S A. 2005 Nov 8;102(45):16460–16465. doi: 10.1073/pnas.0508239102. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Read HL, Escabi M. Precise feature based time scales and frequency decorrelation lead to a sparse auditory code. J Neurosci. 2012;32:8454–8468. doi: 10.1523/JNEUROSCI.6506-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-Y, Lonjers P, Lee C, Chistiakova M, Volgushev M, Bazhenov M. Heterosynaptic plasticity prevents runaway synaptic dynamics. J Neurosci. 2013;33:15915–15929. doi: 10.1523/JNEUROSCI.5088-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Mizrahi A. Plasticity during Motherhood: Changes in Excitatory and Inhibitory Layer 2/3 Neurons in Auditory Cortex. Journal of Neuroscience. 2015;35:1806–1815. doi: 10.1523/JNEUROSCI.1786-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JHR. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009;12:1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmen JC, Hartley DEH, King AJ. Stimulus-timing-dependent plasticity of cortical frequency representation. J Neurosci. 2008;28:13629–13639. doi: 10.1523/JNEUROSCI.4429-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC. Selective removal of lateral olivocochlear efferents increases vulnerability to acute acoustic injury. J Neurophysiol. 2007;97:1775–1785. doi: 10.1152/jn.00955.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoz L, Nelken I. Vicario DS, editor. Frequency tuning in the behaving mouse: different bandwidths for discrimination and generalization. PLoS ONE. 2014;9:e91676. doi: 10.1371/journal.pone.0091676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007;27:180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan TR, Ades HW, Bredberg G, Neff WD. Inner Ear Damage And Hearing Loss After Exposure To Tones Of High Intensity. Acta Otolaryngol. 1974;80:343–352. doi: 10.3109/00016487509121336. [DOI] [PubMed] [Google Scholar]
- Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Nature. 2010;465:932–936. doi: 10.1038/nature09119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova M, Ehret G. Tonotopy and inhibition in the midbrain inferior colliculus shape spectral resolution of sounds in neural critical bands. Eur J Neurosci. 2008;28:675–692. doi: 10.1111/j.1460-9568.2008.06376.x. [DOI] [PubMed] [Google Scholar]
- Egorova M, Vartanyan I, Ehret G. Frequency response areas of mouse inferior colliculus neurons: II. Critical bands. Neuroreport. 2006;17:1783–1786. doi: 10.1097/01.wnr.0000239966.29308.fb. [DOI] [PubMed] [Google Scholar]
- Ehret G. Critical bands and filter characteristics in the ear of the housemouse (Mus musculus). Biol Cybern. 1976;24:35–42. doi: 10.1007/BF00365592. [DOI] [PubMed] [Google Scholar]
- Ehret G. Stiffness gradient along the basilar membrane as a basis for spatial frequency analysis within the cochlea. J Acoust Soc Am. 1978;64:1723–1726. doi: 10.1121/1.382153. [DOI] [PubMed] [Google Scholar]
- Ehret G. Correlations between cochlear hair cell loss and shifts of masked and absolute behavioral auditory thresholds in the house mouse. Acta Otolaryngol. 1979;87:28–38. doi: 10.3109/00016487909126384. [DOI] [PubMed] [Google Scholar]
- Ehret G. Auditory frequency resolution in mammals: from neuronal representation to perception. In: Manley GA, Klump GM, Koppl C, Fastl H, Oeckinghaus H, editors. Advances in Hearing Research. World Scientific; Singapore: 1995. pp. 387–397. [Google Scholar]
- Ehret G, Frankenreiter M. Quantitative analysis of cochlear structures in the house mouse in relation to mechanisms of acoustical information processing. J Comp Physiol A. 1977;122:65–85. [Google Scholar]
- Ehret G, Merzenich MM. Auditory midbrain responses parallel spectral integration phenomena. Science. 1985;227:1245–1247. doi: 10.1126/science.3975613. [DOI] [PubMed] [Google Scholar]
- Ehret G, Merzenich MM. Complex sound analysis (frequency resolution, filtering and spectral integration) by single units of the inferior colliculus of the cat. Brain Res. 1988;472:139–163. doi: 10.1016/0165-0173(88)90018-5. [DOI] [PubMed] [Google Scholar]
- Ehret G, Moffat AJ. Noise masking of tone responses and critical ratios in single units of the mouse cochlear nerve and cochlear nucleus. Hear Res. 1984;14:45–57. doi: 10.1016/0378-5955(84)90068-6. [DOI] [PubMed] [Google Scholar]
- Ehret G, Schreiner CE. Frequency resolution and spectral integration (critical band analysis) in single units of the cat primary auditory cortex. J Comp Physiol A. 1997;181:635–650. doi: 10.1007/s003590050146. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Katz E, Fuchs PA. The nicotinic receptor of cochlear hair cells: a possible pharmacotherapeutic target? Biochem Pharmacol. 2009;78:712–719. doi: 10.1016/j.bcp.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EF. Auditory Frequency Selectivity and the Cochlear Nerve. In: Cazals Y, Demany L, Horner K, editors. Auditory Physiology and Perception. Pergamon; Oxford: 1974. pp. 159–169. [Google Scholar]
- Evans EF, Pratt SR, Spenner H. Comparisons of physiological and behavioural properties: Auditory frequency selectivity. Auditory Physiology and . 1992 [Google Scholar]
- Fay RR. Comparative psychoacoustics. Hear Res. 1988;34:295–305. doi: 10.1016/0378-5955(88)90009-3. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Felix H. Anatomical differences in the peripheral auditory system of mammals and man. A mini review. Adv Otorhinolaryngol. 2002;59:1–10. doi: 10.1159/000059235. [DOI] [PubMed] [Google Scholar]
- Fletcher H. Auditory Patterns. Rev Mod Phys. 1940;12:47–65. [Google Scholar]
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Carcea I, Barker AJ, Yuan K, Seybold BA, Martins ARO, Zaika N, Bernstein H, Wachs M, Levis PA, Polley DB, Merzenich MM, Schreiner CE. Long-term modification of cortical synapses improves sensory perception. Nat Neurosci. 2013;16:79–88. doi: 10.1038/nn.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Jones BJ. Development of auditory cortical synaptic receptive fields. Neurosci Biobehav Rev. 2011;35:2105–2113. doi: 10.1016/j.neubiorev.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Martins ARO. Spectrotemporal dynamics of auditory cortical synaptic receptive field plasticity. Hear Res. 2011;279:149–161. doi: 10.1016/j.heares.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Poo M-M, Dan Y. Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature. 2005;434:221–225. doi: 10.1038/nature03366. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Poo M-M. Activity-dependent neural plasticity from bench to bedside. Neuron. 2013;80:729–741. doi: 10.1016/j.neuron.2013.10.028. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001;31:681–697. doi: 10.1016/s0896-6273(01)00424-x. [DOI] [PubMed] [Google Scholar]
- Greenwood DD. Critical Bandwidth and the Frequency Coordinates of the Basilar Membrane. J Acoust Soc Am. 1961;33:1344–1356. [Google Scholar]
- Greenwood DD. Critical bandwidth and consonance: their operational definitions in relation to cochlear nonlinearity and combination tones. Hear Res. 1991;54:209–246. doi: 10.1016/0378-5955(91)90118-s. [DOI] [PubMed] [Google Scholar]
- Greenwood DD, Goldberg JM. Response of neurons in the cochlear nuclei to variations in noise bandwidth and to tone-noise combinations. J Acoust Soc Am. 1970;47:1022–1040. doi: 10.1121/1.1912002. [DOI] [PubMed] [Google Scholar]
- Grécová J, Bures Z, Popelá J, Suta D, Syka J. Brief exposure of juvenile rats to noise impairs the development of the response properties of inferior colliculus neurons. Eur J Neurosci. 2009;29:1921–1930. doi: 10.1111/j.1460-9568.2009.06739.x. [DOI] [PubMed] [Google Scholar]
- Han YK, Köver H, Insanally MN, Semerdjian JH, Bao S. Early experience impairs perceptual discrimination. Nat Neurosci. 2007;10:1191–1197. doi: 10.1038/nn1941. [DOI] [PubMed] [Google Scholar]
- Hawkins JE, Jr, Stevens SS. The Masking of Pure Tones and of Speech by White Noise. J Acoust Soc Am. 1950;22:6–13. [Google Scholar]
- Heffner H, Masterton B. Hearing in Glires: Domestic rabbit, cotton rat, feral house mouse, and kangaroo rat. J Acoust Soc Am. 1980;68:1584–1599. [Google Scholar]
- Heffner HE, Heffner RS. Hearing ranges of laboratory animals. J Am Assoc Lab Anim Sci. 2007;46:20–22. [PubMed] [Google Scholar]
- Heffner R, Heffner H. Explaining high-frequency hearing. Anat Rec (Hoboken) 2010;293:2080–2082. doi: 10.1002/ar.21292. [DOI] [PubMed] [Google Scholar]
- Hébert R, Langlois J-M, Dussault JH. Permanent defects in rat peripheral auditory function following perinatal hypothyroidism: Determination of a critical period. Developmental Brain Research. 1985;23:161–170. doi: 10.1016/0165-3806(85)90037-9. [DOI] [PubMed] [Google Scholar]
- Houtgast T. Auditory-filter characteristics derived from direct-masking data and pulsation-threshold data with a rippled-noise masker. J Acoust Soc Am. 1977;62:409–415. doi: 10.1121/1.381541. [DOI] [PubMed] [Google Scholar]
- Huetz C, Philibert B, Edeline J-M. A spike-timing code for discriminating conspecific vocalizations in the thalamocortical system of anesthetized and awake guinea pigs. J Neurosci. 2009;29:334–350. doi: 10.1523/JNEUROSCI.3269-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insanally MN, Köver H, Kim H, Bao S. Feature-dependent sensitive periods in the development of complex sound representation. J Neurosci. 2009;29:5456–5462. doi: 10.1523/JNEUROSCI.5311-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RJ, Stillman JA, Schade A. The width of the auditory filter in children. Journal of Experimental Child Psychology. 1986;41:429–442. doi: 10.1016/0022-0965(86)90003-2. [DOI] [PubMed] [Google Scholar]
- Jones LA, Hunter IW, Irwin RJ. Are there critical bands in kinesthesia? Percept Psychophys. 1999;61:508–514. doi: 10.3758/bf03211969. [DOI] [PubMed] [Google Scholar]
- Kauramäki J, Jääskeläinen IP, Sams M. Harris J, editor. Selective attention increases both gain and feature selectivity of the human auditory cortex. PLoS ONE. 2007;2:e909. doi: 10.1371/journal.pone.0000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna SM, Leonard DG. Basilar membrane tuning in the cat cochlea. Science. 1982;215:305–306. doi: 10.1126/science.7053580. [DOI] [PubMed] [Google Scholar]
- Khanna SM, Leonard DG. Measurement of basilar membrane vibrations and evaluation of the cochlear condition. Hear Res. 1986;23:37–53. doi: 10.1016/0378-5955(86)90174-7. [DOI] [PubMed] [Google Scholar]
- Kilgard MP. Harnessing plasticity to understand learning and treat disease. Trends Neurosci. 2012;35:715–722. doi: 10.1016/j.tins.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- King AJ, Dahmen JC, Keating P, Leach ND, Nodal FR, Bajo VM. Neural circuits underlying adaptation and learning in the perception of auditory space. Neurosci Biobehav Rev. 2011;35:2129–2139. doi: 10.1016/j.neubiorev.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka K, Read HL, Tollin DJ. The acoustical cues to sound location in the rat: Measurements of directional transfer functions. J Acoust Soc Am. 123(6):4297–4309. doi: 10.1121/1.2916587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A. Unimodal and cross-modal plasticity in the “deaf” auditory cortex. Int J Audiol. 2007;46:479–493. doi: 10.1080/14992020701383027. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Congenital auditory deprivation reduces synaptic activity within the auditory cortex in a layer-specific manner. Cerebral Cortex. 2000;10:714–726. doi: 10.1093/cercor/10.7.714. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Hearing after congenital deafness: central auditory plasticity and sensory deprivation. Cerebral Cortex. 2002;12:797–807. doi: 10.1093/cercor/12.8.797. [DOI] [PubMed] [Google Scholar]
- Langner G, Schreiner CE. Topology of functional parameters in the inferior colliculus of the cat. In: Elsner N, Creutzfeldt OD, editors. New frontiers in brain research. Thieme; Stuttgart: 1987. p. 122. [Google Scholar]
- Lee S, Sen K, Kopell N. Graham LJ, editor. Cortical gamma rhythms modulate NMDAR-mediated spike timing dependent plasticity in a biophysical model. PLoS Comput Biol. 2009;5:e1000602. doi: 10.1371/journal.pcbi.1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RC, Linden JF, Schreiner CE. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur J Neurosci. 2006;23:3087–3097. doi: 10.1111/j.1460-9568.2006.04840.x. [DOI] [PubMed] [Google Scholar]
- Machens CK, Wehr MS, Zador AM. Linearity of cortical receptive fields measured with natural sounds. J Neurosci. 2004;24:1089–1100. doi: 10.1523/JNEUROSCI.4445-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakoff D. The rise of the mouse, biomedicine's model mammal. Science. 2000;288:248–253. doi: 10.1126/science.288.5464.248. [DOI] [PubMed] [Google Scholar]
- Malmierca MS. The Inferior Colliculus: A Center for Convergence of Ascending and Descending Auditory Information. Neuroembryol and Aging. 2004;3:215–229. [Google Scholar]
- Malmierca MS, Izquierdo MA, Cristaudo S, Hernandez O, Perez-Gonzalez D, Covey E, Oliver DL. A discontinuous tonotopic organization in the inferior colliculus of the rat. J. Neurosci. 2008;28(18):4767–4776. doi: 10.1523/JNEUROSCI.0238-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoussaki D, Chadwick RS, Ketten DR, Arruda J, Dimitriadis EK, O'Malley JT. The influence of cochlear shape on low-frequency hearing. Proc Natl Acad Sci USA. 2008;105:6162–6166. doi: 10.1073/pnas.0710037105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks LE. Summation of vibrotactile intensity: an analog to auditory critical bands? Sens Processes. 1979;3:188–203. [PubMed] [Google Scholar]
- May BJ, Kimar S, Prosen CA. Auditory filter shapes of CBA/CaJ mice: behavioral assessments. J Acoust Soc Am. 2006;120:321–330. doi: 10.1121/1.2203593. [DOI] [PubMed] [Google Scholar]
- Meininger V, Pol D, Derer P. The inferior colliculus of the mouse. A Nissl and Golgi study. Neuroscience. 1986;17:1159–1179. doi: 10.1016/0306-4522(86)90085-0. [DOI] [PubMed] [Google Scholar]
- Moore BC. Cochlear Hearing Loss: Physiological, pscyhological and technical issues. John Wiley & Sons, Ltd.; West Sussex: 2008. [Google Scholar]
- Moore BC. An Introduction to the Psychology of Hearing. Koninklijke Brill NV; Leiden: 2013. [Google Scholar]
- Nahum M, Lee H, Merzenich MM. Principles of neuroplasticity-based rehabilitation. Prog Brain Res. 2013;207:141–171. doi: 10.1016/B978-0-444-63327-9.00009-6. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Zhang LI, Merzenich MM. Specialization of primary auditory cortex processing by sound exposure in the “critical period.”. Proc Natl Acad Sci USA. 2004;101:7170–7174. doi: 10.1073/pnas.0401196101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefs HT, Kappers AML, Koenderink JJ. Detection of amplitude modulation and frequency modulation in tactual gratings: a critical bandwidth for active touch. Perception. 2003;32:1259–1271. doi: 10.1068/p3366. [DOI] [PubMed] [Google Scholar]
- Niemiec AJ, Yost WA, Shofner WP. Behavioral measures of frequency selectivity in the chinchilla. J Acoust Soc Am. 1992;92:2636–2649. doi: 10.1121/1.404380. [DOI] [PubMed] [Google Scholar]
- Nienhuys TG, Clark GM. Critical bands following the selective destruction of cochlear inner and outer hair cells. Acta Otolaryngol. 1979;88:350–358. doi: 10.3109/00016487909137179. [DOI] [PubMed] [Google Scholar]
- Nozza RJ, Wilson WR. Masked and unmasked pure-tone thresholds of infants and adults: development of auditory frequency selectivity and sensitivity. J Speech Hear Res. 1984;27:613–622. doi: 10.1044/jshr.2704.613. [DOI] [PubMed] [Google Scholar]
- Olsho LW. Infant auditory perception: Tonal masking. Infant Behavior and Development. 1985;8:371–384. [Google Scholar]
- Ouda L, Burianová J, Balogová Z, Lu HP, Syka J. Structural changes in the adult rat auditory system induced by brief postnatal noise exposure. Brain Struct Funct. 2014:1–13. doi: 10.1007/s00429-014-0929-z. [DOI] [PubMed] [Google Scholar]
- Parbery-Clark A, Anderson S, Hittner E, Kraus N. Musical experience offsets age-related delays in neural timing. Neurobiol Aging. 2012;33:1483, e1–e4. doi: 10.1016/j.neurobiolaging.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Patel AD. Language, music, syntax and the brain. Nat Neurosci. 2003;6:674–681. doi: 10.1038/nn1082. [DOI] [PubMed] [Google Scholar]
- Patterson RD. Auditory filter shapes derived with noise stimuli. J Acoust Soc Am. 1976;59:640–654. doi: 10.1121/1.380914. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Quinlan CK, Dingle RN. Stability of central binaural sound localization mechanisms in mammals, and the Heffner hypothesis. Neurosci Biobehav Rev. 2012;36:889–900. doi: 10.1016/j.neubiorev.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Pick GF. Level dependence of psychophysical frequency resolution and auditory filter shape. J Acoust Soc Am. 1980;68:1085–1095. doi: 10.1121/1.384979. [DOI] [PubMed] [Google Scholar]
- Pickles JO. Normal critical bands in the cat. Acta Otolaryngol. 1975;80:245–254. doi: 10.3109/00016487509121325. [DOI] [PubMed] [Google Scholar]
- Pickles JO. Psychophysical frequency resolution in the cat as determined by simultaneous masking and its relation to auditory-nerve resolution. J Acoust Soc Am. 1979;66:1725–1732. doi: 10.1121/1.383645. [DOI] [PubMed] [Google Scholar]
- Plomp R. Pitch, timbre, and hearing theory. Int J Audiol. 1968;7:322–344. [Google Scholar]
- Plomp R. Old and new data on tone perception. Contrib Sens Physiol. 1971;5:179–216. doi: 10.1016/b978-0-12-151805-9.50011-0. [DOI] [PubMed] [Google Scholar]
- Plomp R, Levelt WJ. Tonal consonance and critical bandwidth. J Acoust Soc Am. 1965;38:548–560. doi: 10.1121/1.1909741. [DOI] [PubMed] [Google Scholar]
- Read HL, Winer JA, Schreiner CE. Modular organization of intrinsic connections associated with spectral tuning in cat auditory cortex. Proc Natl Acad Sci U S A. 2001 Jul 3;98(14):8042–8047. doi: 10.1073/pnas.131591898. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A, Riley J, Carraway R, Carrasco A, Perez C, Jakkamsetti V, Kilgard MP. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron. 2011;70:121–131. doi: 10.1016/j.neuron.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Rodriguez FA, Read HL, Escabi MA. Spectral and temporal modulation tradeoff in the inferior colliculus. J Neurophysiol. 2010;103:887–903. doi: 10.1152/jn.00813.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer JG. The Physics and Psychophysics of Music. Springer Science & Business Media; New York, NY: 2008. [Google Scholar]
- Romand R, Ehret G. Development of tonotopy in the inferior colliculus. I. Electrophysiological mapping in house mice. Brain Res Dev Brain Res. 1990;54:221–234. doi: 10.1016/0165-3806(90)90145-o. [DOI] [PubMed] [Google Scholar]
- Rothschild G, Cohen L, Mizrahi A, Nelken I. Elevated correlations in neuronal ensembles of mouse auditory cortex following parturition. J Neurosci. 2013;33:12851–12861. doi: 10.1523/JNEUROSCI.4656-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JLR, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell IJ, Sellick PM. Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J Physiol (Lond) 1983;338:179–206. doi: 10.1113/jphysiol.1983.sp014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale A, Berardi N, Maffei L. Environment and brain plasticity: towards an endogenous pharmacotherapy. Physiol Rev. 2014;94:189–234. doi: 10.1152/physrev.00036.2012. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Scharf B. Critical Bands and the Loudness of Complex Sounds Near Threshold. J Acoust Soc Am. 1959;31:365–370. [Google Scholar]
- Scharf B. Critical bands. In: Tobias J, editor. Foundations of modern auditory theory. Vol. 1. Academic Press; New York: 1970. pp. 157–202. [Google Scholar]
- Scharf B, Florentine M, Meiselman CH. Critical band in auditory lateralization. Sens Processes. 1976;1:109–126. [PubMed] [Google Scholar]
- Scharf B, Meiselman CH. Critical bandwidth at high intensities. In: Evans EF, Wilson JP, editors. Psychophysics and physiology of hearing. Academic Press; London: 1977. pp. 221–232. [Google Scholar]
- Schneider BA, Morrongiello BA, Trehub SE. Size of critical band in infants, children, and adults. J Exp Psychol Hum Percept Perform. 1990;16:642–652. doi: 10.1037//0096-1523.16.3.642. [DOI] [PubMed] [Google Scholar]
- Schnupp JWH, Hall TM, Kokelaar RF, Ahmed B. Plasticity of temporal pattern codes for vocalization stimuli in primary auditory cortex. J Neurosci. 2006;26:4785–4795. doi: 10.1523/JNEUROSCI.4330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorer E. Critical modulation frequency based on detection of AM versus FM tones. J Acoust Soc Am. 1986;79:1054–1057. doi: 10.1121/1.393377. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Langner G. Laminar fine structure of frequency organization in auditory midbrain. Nature. 1997;388:383–386. doi: 10.1038/41106. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Read HL, Sutter ML. Modular organization of frequency integration in primary auditory cortex. Annu Rev Neurosci. 2000;23:501–529. doi: 10.1146/annurev.neuro.23.1.501. [DOI] [PubMed] [Google Scholar]
- Seaton WH, Trahiotis C. Comparison of critical ratios and critical bands in the monaural chinchilla. J Acoust Soc Am. 1975;57:193–199. doi: 10.1121/1.380414. [DOI] [PubMed] [Google Scholar]
- Shamma SA, Elhilali M, Micheyl C. Temporal coherence and attention in auditory scene analysis. Trends Neurosci. 2011;34:114–123. doi: 10.1016/j.tins.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Nash AA, Dorman M. Cortical development, plasticity and re-organization in children with cochlear implants. J Commun Disord. 2009;42:272–279. doi: 10.1016/j.jcomdis.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speechley WJ, Hogsden JL, Dringenberg HC. Continuous white noise exposure during and after auditory critical period differentially alters bidirectional thalamocortical plasticity in rat auditory cortex in vivo. Eur J Neurosci. 2007;26:2576–2584. doi: 10.1111/j.1460-9568.2007.05857.x. [DOI] [PubMed] [Google Scholar]
- Spirou GA, Young ED. Organization of dorsal cochlear nucleus type IV unit response maps and their relationship to activation by bandlimited noise. J Neurophysiol. 1991;66:1750–1768. doi: 10.1152/jn.1991.66.5.1750. [DOI] [PubMed] [Google Scholar]
- Stiebler I, Ehret G. Inferior colliculus of the house mouse. I. A quantitative study of tonotopic organization, frequency representation, and tone-threshold distribution. Journal of Comparative Neurology. 1985;238:65–76. doi: 10.1002/cne.902380106. [DOI] [PubMed] [Google Scholar]
- Stiebler I, Neulist R, Fichtel I, Ehret G. The auditory cortex of the house mouse: left-right differences, tonotopic organization and quantitative analysis of frequency representation. J Comp Physiol A. 1997;181:559–571. doi: 10.1007/s003590050140. [DOI] [PubMed] [Google Scholar]
- Suga N. Comparative Neuroscience and Neurobiology. Birkhäuser Boston; Boston, MA: 1988. Echolocation. pp. 30–33. [Google Scholar]
- Sutter ML, Schreiner CE. Physiology and topography of neurons with multipeaked tuning curves in cat primary auditory cortex. J Neurophysiol. 1991;65:1207–1226. doi: 10.1152/jn.1991.65.5.1207. [DOI] [PubMed] [Google Scholar]
- Talwar SK, Gerstein GL. Reorganization in awake rat auditory cortex by local microstimulation and its effect on frequency-discrimination behavior. J Neurophysiol. 2001;86:1555–1572. doi: 10.1152/jn.2001.86.4.1555. [DOI] [PubMed] [Google Scholar]
- Tan AYY, Atencio CA, Polley DB, Merzenich MM, Schreiner CE. Unbalanced synaptic inhibition can create intensity-tuned auditory cortex neurons. Neuroscience. 2007;146:449–462. doi: 10.1016/j.neuroscience.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Tan AYY, Wehr M. Balanced tone-evoked synaptic excitation and inhibition in mouse auditory cortex. Neuroscience. 2009;163:1302–1315. doi: 10.1016/j.neuroscience.2009.07.032. [DOI] [PubMed] [Google Scholar]
- Trehub SE, Bull D, Schneider BA. Infants' detection of speech in noise. J Speech Hear Res. 1981;24:202–206. doi: 10.1044/jshr.2402.202. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kraus N. Learning to encode timing: mechanisms of plasticity in the auditory brainstem. Neuron. 2009;62:463–469. doi: 10.1016/j.neuron.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky N, Las L, Farkas D, Nelken I. Multiple time scales of adaptation in auditory cortex neurons. J Neurosci. 2004;24:10440–10453. doi: 10.1523/JNEUROSCI.1905-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovsky N, Las L, Nelken I. Processing of low-probability sounds by cortical neurons. Nat Neurosci. 2003;6:391–398. doi: 10.1038/nn1032. [DOI] [PubMed] [Google Scholar]
- Volkov IO, Galazjuk AV. Formation of spike response to sound tones in cat auditory cortex neurons: interaction of excitatory and inhibitory effects. Neuroscience. 1991;43:307–321. doi: 10.1016/0306-4522(91)90295-y. [DOI] [PubMed] [Google Scholar]
- Ward WD, Duvall AJ. Behavioral and ultrastructural correlates of acoustic trauma. Ann Otol Rhinol Laryngol. 1971;80:881–896. doi: 10.1177/000348947108000615. [DOI] [PubMed] [Google Scholar]
- Watson CS. Masking of Tones by Noise for the Cat. J Acoust Soc Am. 1963;35:167–172. [Google Scholar]
- Weber DL. Growth of masking and the auditory filter. J Acoust Soc Am. 1977;62:424–429. doi: 10.1121/1.381542. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron. 2005;47:437–445. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Wersäll J, Björkroth B, Flock A, Lundquist PG. Experiments on ototoxic effects of antibiotics. Adv Otorhinolaryngol. 1973;20:14–41. doi: 10.1159/000393087. [DOI] [PubMed] [Google Scholar]
- West CD. The relationship of the spiral turns of the cochlea and the length of the basilar membrane to the range of audible frequencies in ground dwelling mammals. J Acoust Soc Am. 1985;77:1091–1101. doi: 10.1121/1.392227. [DOI] [PubMed] [Google Scholar]
- Willard FH, Ryugo DK. Anatomy of the central auditory system. In: Willott JF, editor. The Auditory Psychobiology of the Mouse. Charles C Thomas Publisher; Springfield: 1983. pp. 201–304. [Google Scholar]
- Willott JF. Factors affecting hearing in mice, rats, and other laboratory animals. J Am Assoc Lab Anim Sci. 2007;46:23–27. [PubMed] [Google Scholar]
- Winer JA, Schreiner CE. The Inferior Colliculus. Springer New York; New York: 2005. The Central Auditory System: A Functional Analysis. pp. 1–68. [Google Scholar]
- Wong PCM, Skoe E, Russo NM, Dees T, Kraus N. Musical experience shapes human brainstem encoding of linguistic pitch patterns. Nat Neurosci. 2007;10:420–422. doi: 10.1038/nn1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost WA, Shofner WP. Critical bands and critical ratios in animal psychoacoustics: an example using chinchilla data. J Acoust Soc Am. 2009;125:315–323. doi: 10.1121/1.3037232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerlin S. Electrophysiological evidence for the critical band in humans. J Acoust Soc Am. 1986;79:1612–1616. doi: 10.1121/1.393297. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Bao S, Merzenich MM. Disruption of primary auditory cortex by synchronous auditory inputs during a critical period. Proc Natl Acad Sci USA. 2002;99:2309–2314. doi: 10.1073/pnas.261707398. [DOI] [PMC free article] [PubMed] [Google Scholar]