Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic congener of the polyhalogenated aromatic hydrocarbons (PAH), which causes anatomical abnormalities and developmental defects, impairs ovulation and reduces fertility. TCDD’s endocrine-disrupting effects are, in part, caused by a direct action at the ovary.
Herein we investigated the in-vitro effects of environmentally relevant doses of TCDD on estradiol-17β (E2) production by human luteinizing granulosa cells (hLGC) obtained from women stimulated for in-vitro fertilization (IVF). TCDD at all concentrations tested (3.1 fM, 3.1 pM and 3.1 nM) significantly decreased E2 secretion when assayed for by radioimmunoassay (RIA). Herein we confirm that TCDD alters E2 secretion by hLGC in a time-, not dose-dependent fashion and are the first to show decreases in E2 secretion with fM concentrations of TCDD. Using real-time quantitative PCR (RT-qPCR), the decreased E2 secretion correlates with a decrease in the mRNA expression levels two enzymes in the estrogen biosynthesis pathway: CYP11A1 and CYP19A1.
Keywords: dioxin, estrogen, granulosa cells, low dose, ovary, RT-qPCR
1. INTRODUCTION
In recent years, numerous researchers have focused their studies on environmental contaminants capable of acting as endocrine disruptors [1–4]. The xenobiotic 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD; dioxin) is one such compound. Dioxins constitute one of the most ubiquitous and persistent environmental pollutants produced by man [5]. TCDD is prevalent in the environment as the result of the production and over-treatment with herbicides, as by-products of waste incineration and combustion processes, paper processing and the manufacture of plastics, primarily polyvinyl chloride [6–8]. Due to TCDD’s substantial biological activity and lipophilic nature, years of widespread production and improper and unregulated disposal of large quantities of this highly toxic chemical have resulted in global environmental contamination [5]. The Environmental Protection Agency suggests that the maximum contaminant level (MCL) for TCDD is 30 parts per quintillion (ppq) and TCDD is considered safe at levels below this MCL [9], but scientists have only recently discovered TCDD’s ability to perturb the normal endocrine-signaling pathways of an animal, potentially causing serious developmental and reproductive defects [10].
TCDD exerts its toxic effects and alters the hormonal profile of an organism by binding the aromatic hydrocarbon receptor (AHR). AHR belongs to the basic helix-loop-helix/ Per-Arnt-Sim (bHLH/PAS) family of proteins and is a ligand-inducible transcription factor [11]. Upon binding of TCDD, the TCDD/AHR complex undergoes a conformational change, is translocated to the nucleus and forms a heterodimer with the AHR nuclear translocator protein (ARNT; also called BMAL1) [12,13]. This heterodimeric complex (AHR-ARNT) is then able to bind cis-genomic dioxin response elements (DREs) or more correctly aromatic hydrocarbon response elements (AHREs), thereby altering gene expression in a wide variety of tissues [14–16] and interacting in multiple signaling pathways [17].
We have previously demonstrated the presence of a functional AHR capable of binding DNA in the rat ovary [18] and in primate ovarian tissue, including whole rhesus monkey ovarian tissue and human granulosa cells (GC) [19]. Recently we demonstrated that radiolabeled TCDD binds to AHR in rhesus monkey ovarian follicles at specific locations (GC>oocyte>theca); further supporting the notion that TCDD directly affects primate ovarian function through the AHR [20]. Similarly Wojtowicz et al. [21] detected a greater expression of AHR in porcine GCs versus theca cells and further demonstrated that only the AHRs in the GCs were activated following exposure to TCDD and dioxin-like PCBs. In addition we showed that weanling female Holtzman rats exposed to TCDD in utero and lactationally exhibited decreased serum E2 (estradiol-17β) concentrations and increased estrogen receptor (ER) mRNA in the ovary [22]. In human luteinized granulosa cells (hLGC) cultured with TCDD, Heimler et al., [23] illustrated that dioxin perturbed E2 secretion in a time-dependent manner, but did not alter progesterone (P) accumulation; and that it augmented inhibin A secretion [24]. Trewin et al., [25] demonstrated that in-vitro pulsatile gonadotropin- releasing hormone (GnRH) secretion from rat hypothalamic explants and basal or stimulable release of gonadotropins from hemi-pituitary cultures remained unaffected following TCDD exposure. Other studies in rat, nonhuman primates and human LGC in culture [23–30] have also corroborated the adverse effects of TCDD on steroid hormone production. Previous studies have also examined the enzymatic activity of enzymes in the estrogen biosynthesis pathway using TCDD-treated cells with inconsistent results [29,31,32], but never at fM concentrations and without the detailed time course we conduct here. Collectively, these data suggest that TCDD is capable of rendering its endocrine-disrupting effects directly at the level of the ovary by disrupting the steroidogenic process.
Several studies, including those that we have performed on rats, have elucidated some of the mechanisms by which TCDD alters steroid hormone production. However, the effects of TCDD on nonhuman ovarian steroidogenesis might differ from that which occurs in humans. In the present study, we set out to evaluate the effect of several concentrations of TCDD on E2 secretion by human LGC in culture. This will lend support to our hypothesis that TCDD acts directly on the ovary to disrupt steroid secretion by modulating the steroidogenic cascade at more than one locus.
2. MATERIALS AND METHODS
2.1 Chemicals
TCDD (>99% purity) was obtained from Cambridge Isotope Laboratories, Inc. (Andover, MA; USA). Treatment solutions containing TCDD in culture medium were prepared from a stock solution containing 1 mg TCDD/ml dissolved in 0.1% DMSO (Sigma Chemical Co., St. Louis, MO; USA).
2.2 Procurement and stimulation of ovarian tissue
Information on normal human ovarian tissue is invaluable, but it is extremely difficult to procure unstimulated ovaries; therefore, we obtained GCs from women whose ovaries were stimulated for in-vitro fertilization (IVF). GCs were obtained from Rush Presbyterian University Medical Center (RUMC) IVF Program (Chicago, IL; USA). In concordance with ethical guidelines, materials were collected with informed patient consent under Institutional Review Board (IRB) approval from RUMC. Pituitary desensitization and down-regulation was used to achieve ovarian stimulation. Specifically, a combination of leuprolide acetate (Lupron; TAP Pharmaceuticals, Abbott Park, IL; USA) and human menotropins (human menopausal gonadotropin and follicle-stimulating hormone: Pergonal; Serono Laboratories Inc., Randolph, MA; USA) were used. This method of ovarian stimulation enabled us to collect up to three million GCs from a single woman.
2.3 Human Granulosa Cell Isolation, Purification, and Culture
Ovarian follicular fluid (FF) was obtained using transvaginal aspiration approximately 36 h after a 10,000-IU human chorionic gonadotropin (hCG) injection to stimulate the midcycle luteinizing hormone (LH) surge. Oocytes were immediately harvested and we processed the remaining FF. Note that FF aspirates were pooled when samples from multiple women were obtained on the same day. Luteinizing granulosa cells (LGC) were then collected from the FF aspirates and centrifuged to remove red blood cells. Specifically, FF was collected in 15-ml centrifuge tubes and centrifuged at 300 x g for five minutes followed by five minutes at 600 x g to isolate the LGC. The firm white layer of LGCs over a red blood cell pellet was then transferred to a new 15-ml centrifuge tube (approximately 1 ml). LGC aggregates were mechanically dispersed through a 1-ml pipette tip. Up to 2 ml of culture medium was then added to the cell suspension. Stock culture medium contained 475 ml DMEM/F-12 (Sigma, St. Louis, MO; USA), 25 ml (5%) fetal bovine serum (Sigma, St. Louis, MO) and 2.5 ml gentamycin solution (Sigma, St. Louis, MO; USA) equivalent to a dose of 50 ug/ml. Approximately 2–3 ml of the cell suspension was then layered over 5 ml 45% Percoll (Sigma, St. Louis, MO; USA) and centrifuged at 300 x g for 30 minutes. The LGC layer was then aspirated off the top of the Percoll and the LGCs were pooled from all tubes into one fresh 15-ml centrifuge tube and mixed. If red blood cell contamination was still high, an additional Percoll extraction was performed. The LGC pool was then diluted with up to 10 ml of culture medium and washed twice by centrifugation (300 x g), discarding the supernatant each time. The final volume was brought up to 1 ml. The purified LGC were counted in a hemacytometer and the concentration was adjusted to 1 x 106 cells/ml. Cell viability was determined using 0.2% trypan blue exclusion dye. In all cases, cell viability was greater than 85%. The LGC were plated into 8-well Permanox-coated Lab-Tek slides (Nunc, Naperville, IL; USA) where each well contained 50,000 cells/well in 500 ml stock culture medium. Cells were incubated at 37°C, 5% CO2 and >98% humidified air overnight in culture medium. After allowing cells to plate so as to adhere to slide wells for at least 12 h, media were aspirated, discarded and replaced with 500 μl of treatment media. Treatment medium contained either stock medium + 0.1% DMSO (Control), 3.1 fM TCDD, 3.1 pM TCDD, or 3.1 nM TCDD. Fresh medium, with or without TCDD, was added to each of the wells at specific time intervals over a 48-h incubation period. We used Falcon 8-chamber Lab-Tek slides, each containing control, nM, pM, and fM in duplicate. After the initial overnight incubation, the medium was aspirated and collected for estrogen analysis by RIA. Specifically, medium was collected 4, 8, 12, 24, 36 and 48 h after TCDD administration. Each treatment group at each time point was analyzed in duplicate and comprised of pooled cells obtained from two or three women. These pooled cells were obtained from a total of ten different women on four separate occasions. Cells obtained from two individuals in the study produced no estrogen at 48 h of culture and were not found to be viable. As a result of this unfortunate occurrence, these pooled samples were removed, reducing the total sample size (N) for the study to eight. At each time point, culture wells were examined for bacterial or fungal contamination. In addition, cell counts were performed on random samples at the end of each experiment. Both in the control and three treatment groups, we observed a <22% reduction in cell number due to aspiration of non-adhering dead cells. Viable cells were lysed with 1 ml Tri Reagent (Sigma, St. Louis, MO; USA) at each time point and treatment concentration for use in the RNA studies.
2.4 Estrogen Radioimmunoassay
Estradiol-17β concentrations were measured in the medium at 4, 8, 12, 24, 36 and 48 h using the E2 Coat-A-Count kit (Diagnostic Products Corp., Los Angeles, CA; USA). All samples were assayed in duplicate with the appropriate E2 standards. The intra-assay coefficient of variation derived from four replicate aliquots was 3.7 %.
2.5 mRNA Extraction and cDNA synthesis
Beta Mercaptoethanol (βME) was added to Tri Reagent-lysed cells samples and mRNA was extracted following the Aurum Total RNA mini kit spin protocol for adherent animal tissues (Bio-Rad, Hercules, CA; USA). The concentration and quality of extracted RNA was analyzed spectrophotometrically.
Total extracted RNA from each sample was reverse transcribed to cDNA using the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA; USA), with iScript reverse transcriptase, RNA template, and nuclease-free water (Fisher Scientific, Waltham, MA; USA). Resulting cDNA was analyzed spectrophotometrically and a portion was diluted to a working concentration of 25 ng/μL.
Sterile, RNase-free, DNase-free aerosol-barrier pipette tips (Fisher Scientific, Waltham, MA; USA) were used throughout all steps to limit the potential for contamination.
2.6 Measurement of mRNA levels via real-time quantitative PCR
Absolute quantification of mRNA expression was determined using real-time quantitative polymerase chain reaction (RT-qPCR) with a CFX Real-Time System with C1000 Touch Thermal Cycler (Bio-Rad, Hercules, CA; USA). The expression of CYP11A1 (cholesterol side-chain cleavage enzyme) and CYP19A1 (aromatase) was normalized to the expression of the housekeeping gene ribosomal 60S protein L13 (RPL13). Validated PrimePCR Assays (Bio-Rad, Hercules, CA; USA) RPL13 (Human) CYP11A1 (Human), and CYP19A1 (Human) were used for all qPCR assays with an iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA; USA). Three technical replicates of three biological replicates were performed at each time point. All components were mixed directly into the corresponding wells of a hard-shell 96-well clear PCR plate (Bio-Rad, Hercules, CA; USA) and covered with a Microseal ‘B’ Film (Bio-Rad, Hercules, CA; USA) prior to placement in the thermal cycler.
All Validated PrimePCR assays were verified to conform to MIQE [33] standards established for RT-qPCR. Melt curves for each run showed only one product, confirming specificity and the absence of both nonspecific annealing and primer dimerization.
2.7 Statistical Analysis
All E2 RIA data were spline-transformed prior to analysis. Due to the variability in E2 secretion by hLGC among patients, data were normalized and represented as a percent of control. Statistical analysis was performed using SPSS (Version 21.0; SPSS Inc., Chicago, IL). Data were analyzed statistically using a one-way analysis of variance (ANOVA). Conservative post-hoc tests were performed, when applicable, using Tukey’s method. Normality and homogeneity of variances (homoscedasticity) were also tested. P < 0.05 was considered to be statistically significant.
RT-qPCR data were analyzed using the Bio-Rad CFX Manager software with mRNA expression normalized to the housekeeping gene RPL13. Based on the MIQE guidelines, individual wells were excluded from analysis if the Cq standard deviation of sample replicates was greater than 0.20 [33].
3. RESULTS
3.1 E2 Secretion Diminishes Following TCDD Exposure
We observed statistically significant differences in E2 secretion by human LGC at 8, 12 and 24 h (Table 1) [3]. Specifically E2 secretion relative to control was significantly inhibited by the concentrations of TCDD considered to be environmentally relevant (3.1 fM, 3.1 pM and 3.1 nM) [34] (Table 1). Significant decreases in E2 secretion were not observed among any treatment groups andcontrols at 4 (Table 1), 36 or 48 h. Analysis of total E2 secretion in the medium throughout the 48-h culture period showed that environmentally relevant doses of TCDD inhibited E2 secretion by greater than 50% (data not shown).
Table 1.
Estradiol accumulation by human luteinizing granulosa cells (hLGC) treated in vitro with 3.1 nM, 3.1 pM, or 3.1 fM TCDD at various time intervals. Values are given as a percentage relative to accumulation by untreated control cells (control = 100%).
| Time Points | Control | 3.1 nM TCDD | 3.1 pM TCDD | 3.1 fM TCDD |
|---|---|---|---|---|
| 4 hour | 100% | 153.2% (16.0) | 158.2% (11.2) | 137.5% (23.9) |
| 8 hour | 100% a | 50.1% (16.6) b | 51.6% (14.5) b | 43.8% (12.3) b |
| 12 hour | 100% a | 41.2% (10.6) b | 26.7% (9.2) b | 37.3% (7.8) b |
| 24 hour | 100% a | 46.9% (15.6) b | 44.7% (15.8) b | 44.9% (18.8) b |
| 36 hour | 100% a | 104.0% (9.3) | 83.5% (8.2) | 112.8% (11.7) |
| 48 hour | 100% a | 88.2% (16.3) | 93.0% (14.4) | 84.6% (13.9) |
Letter superscripts denote significance at each time period (within a given row), based on Tukey’s post-hoc test after one-way ANOVA (P < 0.05). Values within columns sharing the same superscripts do not differ significantly from one another. Parentheses denote ± (S.E.M.) reported as a percentage. N = 8 for each treatment group.
3.2 mRNA Levels Decrease in Response to fM TCDD
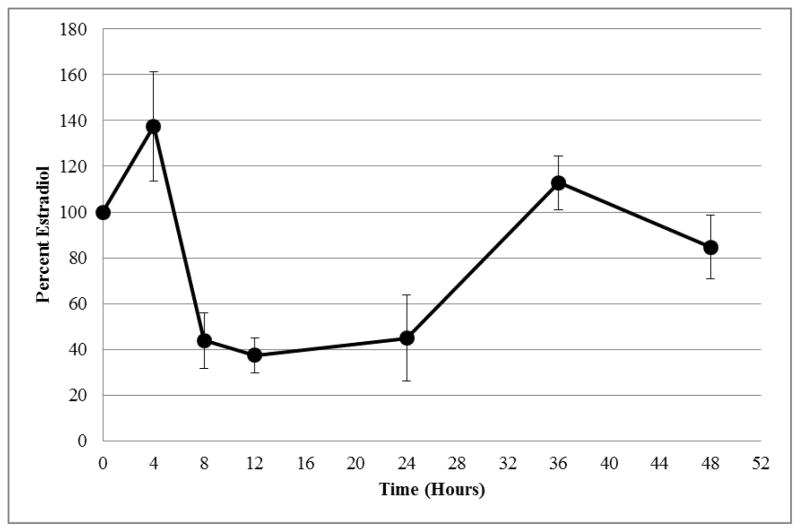
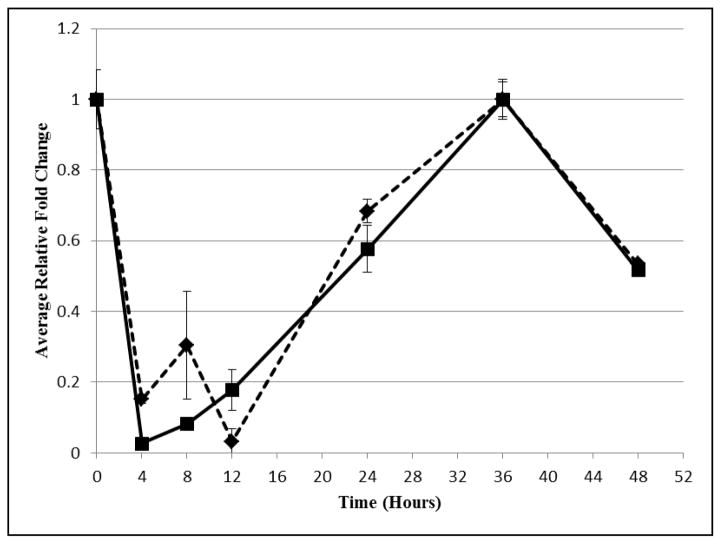
In addition, RT-qPCR experiments showed a drop in message for both CYP11A1 and CYP19A1 by 4 hours with the fM concentration, preceding the decrease in secreted E2 concentration (Figures 1 and 2). Following the recovery of message copy number by 24 hours, E2 concentration rose to the control by 36 hours. As expected, changes in the message for estrogen biosynthetic enzymes led to similar changes in E2 concentration.
Figure 1. Estrogen Accumulation by hLGC Treated with 3.1 fM TCDD.
Estradiol accumulation by human luteinizing granulosa cells (hLGC) treated in vitro with 3.1 fM TCDD at various time intervals. Values are given as a percentage relative to accumulation by untreated control cells (control = 100%).
Figure 2. Average Relative Fold Change of Aromatase and Side Chain Cleavage Treated with 3.1 fM TCDD.
Relative fold changes as determined by RT-qPCR of mRNA expression of side chain cleavage (diamonds) and aromatase (squares) in cells treated with 3.1 fM concentration of TCDD. Fold changes are relative to untreated hGLC normalized to RPL13.
4. DISCUSSION
Previous studies have shown that μM and nM doses of TCDD can induce apoptosis in hLGC in a dose-dependent manor [23]. In the present study, we evaluated concentrations considered to be sub-environmental and showed that in-vitro administration of fM TCDD disrupts normal E2 secretion/accumulation by human LGC. Specifically we noted a significant reduction in E2 secretion into culture media at 8, 12 and 24 hours, which correlated with a diminution in mRNAs for CYP11A1 and CYP19A1 at 4 and 12 hours. We did not observe this decrease at later time points (36 and 48 h), suggesting that the reduction in E2 secretion observed herein was time-dependent and is related to the recovery of CYP11A1 and CYP19A1 expression. Our data indicated no statistically significant differences among environmentally relevant (3.1 fM-ppq, 3.1 pM-ppt, and 3.1 nM-ppb) doses of TCDD, indicating that any dose of TCDD within the limits of detection might be capable of modulating ovarian steroid secretion. Future studies using RT-qPCR to evaluate expression levels of steroidogenic enzymes versus concentrations of TCDD may explain this lack of a dose response. However it is important to note that for the first time, we have demonstrated that even a 3.1 fM dose of TCDD, which is approximately 1000-fold lower than our laboratory has previously shown to have an effect [3,22,23], is capable of altering steroid hormone secretion by human LGC in culture; this suggests that a no-observable-adverse-effects level (NOAEL) might not exist for TCDD, at least within the sensitivity of our assays. Collectively, these data showed that TCDD was able to disrupt E2 secretion by hLGC in a time- but not dose-dependent manner. In addition these aforementioned results of reduced E2 secretion confirm previous results reported by our laboratory and others, using a number of in-vitro and in-vivo model systems [22,23,29,31,32].
Our group, as well as others, has illustrated perturbations in steroid hormone secretion with TCDD over the past 20 years. Researchers have demonstrated that female Holtzman rats exposed to TCDD in utero and lactationally showed marked decreases in serum E2 concentrations [22,35] and that peripubertal female rats exposed during the same period exhibited reduced pituitary follicle-stimulating hormone (FSH) mRNA content with no effect on circulating progesterone or androstenedione concentrations [18]. In hLGC cultured with TCDD, Heimler et al., [23] illustrated that dioxin perturbs E2 secretion in a time- and dose-dependent manner, but does not alter progesterone (P) accumulation; however, E2 secretion returns to normal when exogenous androgen is included in the culture medium. Conversely, Enan et al., [36] noted a decrease in P production after a 24-h exposure of hLGC to TCDD. Moran et al. [29] corroborated Heimler’s findings and additionally showed a marked decrease in 17alpha-hydroxylase/17,20-lyase cytochrome P450 (P450c17; CYP17A1) protein expression and 17,20-lyase activity proportional to the decrease in E2 secretion following 10 days in culture with 10 nM TCDD. We suspect the difference between our findings and Moran et al. may be caused by the sensitivity in method or the length of TCDD incubation. Regardless of steroidogenic endpoint affected (and controversy still remains), these data certainly suggest that much lower concentrations of TCDD are capable of rendering endocrine-disrupting effects directly at the level of the human ovary by disrupting the steroid biosynthetic pathway.
The mechanism(s) by which TCDD alters human ovarian steroidogenesis remains elusive. Studies conducted previously in our laboratory have depicted mechanisms by which TCDD reduced mRNA levels for cytochrome P450 side-chain cleavage (CYP11A1, SCC), and mRNA copy number and activity of cytochrome P450 aromatase (CYP19A1) in rat luteinized granulosa cells [31,37]. Specifically our laboratories have found that in rat granulosa cell cultures that TCDD reduced aromatase and P450 SCC mRNAs but exerted no effect on 3β-hydroxysteroid dehydrogenase mRNA concentrations [31]. The study we present here confirms this in hLGC. Following in-vivo exposure of zebrafish to TCDD, Heiden et al. [38] evaluated the expression of genes important in estrogen synthesis and signaling. TCDD exposure significantly reduced mRNA expression for steroid acute regulatory (STAR) protein, CYP19A1, activins, and FSH and LH receptor, and resulted in decreased serum E2 concentrations, fewer large vitellogenic follicles, increased atretic follicles, a 50% reduction in egg production and a 96% decrease in spawning success in zebrafish [32]. Interestingly Vidal et al. [39] found in hLGC exposed to TCDD significantly increased gene expression of microsomal catechol-O-methytransferase (COMT) and CYP1B1, which are involved in hydroxylating estrogens to catechol forms. These data suggest that TCDD is capable of altering E2 synthesis and metabolism by modulating the steroidogenic cascade at more than one locus. In addition other researchers are now designing experiments to elucidate other possible locus/loci of TCDD action on the steroidogenic process. Miyamoto [40], e.g., has shown that more than 100 genes in rat ovary, GC, and placenta are induced or suppressed following dioxin exposure. It is obvious, therefore, that much remains unknown with regard to dioxin effects on steroidogenesis specifically and reproduction generally.
5. CONCLUSIONS
Collectively these data provide evidence for direct effects of TCDD at the ovary, which could ultimately result in disrupted fertility. Our present study unequivocally shows that TCDD is able to reduce E2 secretion by hLGC in vitro in a time-dependent fashion at an extremely low exposure dose and that it is preceded by a decrease in the copy number of mRNAs for cytochrome P450 (CYP11A1) side-chain cleavage (SCC) and cytochrome P450 aromatase (CYP19A1). However, conflicting results among researchers show that a controversy exists as to the exact locus/loci of TCDD’s actions. In order to further clarify the effects of TCDD on the expression of enzymes in the estrogen synthetic pathway, we plan to continue RT-qPCR studies on the remaining enzymes in the pathway as well as explore the dose-dependent effects of TCDD on cell death by both apoptosis and autophagy. We expect that the information gathered from this and future studies will further enhance our understanding of the potential effects of environmental toxicants (especially dioxins) on female reproductive health.
Supplementary Material
Research Highlights.
TCDD decreases E2 secretion in human luteinizing granulosa cells.
TCDD alters E2 secretion by hLGC in a time-, not dose-dependent fashion.
E2 secretion is decreased with concentrations of TCDD as low as fM.
TCDD decreases mRNA expression levels of CYP11A1 and CYP19A1.
Acknowledgments
Supported in part by Grants NIH-ES-011569 and P30 ES004184 from the National Institutes of Health and the Office on Research for Women’s Health, National Institutes of Health, Research Triangle Park, North Carolina.
The authors wish to thank Dr. Rebecca Stahl for her assistance with the RIA. We would also like to thank Elizabeth Ebensperger and Mikayla Callen for their assistance completing the RT-qPCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baldridge MG, Stahl RL, Gerstenberger SL, Tripoli V, Hutz RJ. Modulation of Ovarian Follicle Maturation in Long-Evans Rats Exposed to Polychlorinated Biphenyls (PCBs) in Utero and Lactationally. Reprod Toxicol. 2003;17(5):567–73. doi: 10.1016/s0890-6238(03)00095-9. [DOI] [PubMed] [Google Scholar]
- 2.Baldridge MG, Stahl RL, Gerstenberger SL, Tripoli V, Hutz RJ. In Utero and Lactational Exposure of Long-Evans Rats to Ammonium Perchlorate (AP) Disrupts Ovarian Follicle Maturation. Reprod Toxicol. 2004;19:155–61. doi: 10.1016/j.reprotox.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Hutz RJ, Carvan MJ, III, Baldridge MG, Conley LK, King Heiden T. Environmental Toxicants and Effects on Female Reproductive Function. Review Trends in Reprod Biol. 2006;2:1–11. doi: 10.1901/jaba.2006.2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig ZR, Leslie TC, Hatfield KP, Gupta RK, Flaws JA. Mono-hydroxy methoxychlor alters levels of key sex steroids and steroidogenic enzymes in cultured mouse antral follicles. Toxicol Appl Pharm. 2010;249:107–13. doi: 10.1016/j.taap.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safe SH. Modulation of gene expression and endocrine response pathways by 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related compounds. Pharmac Ther. 1995;67(2):247–81. doi: 10.1016/0163-7258(95)00017-b. [DOI] [PubMed] [Google Scholar]
- 6.Poland A, Knutson JC. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–54. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- 7.McLachlan JA, Korach KS. Proceedings of the Meeting: Estrogens in the Environment III: Global health implications. Suppl 7. Washington D.C: Environ Health Prospect; 1994. pp. 1–178. [Google Scholar]
- 8.Hutz RJ. Reproductive endocrine disruption by environmental xenobiotics that modulate the estrogen-signaling pathway, particularly by tetrachlorodibenzo-p-dioxin (TCDD) J Reprod Dev. 1999;45:1–12. [Google Scholar]
- 9.Basic Information about Dioxin (2,3,7,8-TCDD) in Drinking Water. http://water.epa.gov/drink/contaminants/basicinformation/dioxin-2-3-7-8-tcdd.cfm.
- 10.Birnbaum LS. Developmental effects of dioxins and related endocrine disrupting chemicals. Toxicol Lett. 1995;82/83:743–50. doi: 10.1016/0378-4274(95)03592-3. [DOI] [PubMed] [Google Scholar]
- 11.Beischlag TV, Wang S, Rose D, Torchia J, Reisz-Porszasz S, Muhammed K, et al. Recruitment of the NCoA/SRC-1/p160 family of transcriptional coactivators by the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator complex. Mol Cell Biol. 2002;22(12):4319–33. doi: 10.1128/MCB.22.12.4319-4333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Probst MR, Reisz-Porszasz S, Agbunag RV, Ong MS, Hankinson O. Role of the aryl hydrocarbon receptor nuclear translocator protein in aryl hydrocarbon (dioxin) receptor action. Mol Pharmacol. 1993;87:306–14. [PubMed] [Google Scholar]
- 13.Xu CX, Krager SL, Liao DF, Tischkau SA. Disruption of CLOCK-BMAL1 transcriptional activity is responsible for aryl hydrocarbon receptor-mediated regulation of PERIOD1 gene. Toxicol Sci. 2010;115(1):98–108. doi: 10.1093/toxsci/kfq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolwick KM, Schmidt JV, Carver LA, Swanson HI, Bradfield CA. Cloning and expression of the human Ah receptor cDNA. Mol Pharmacol. 1993;44:911–17. [PubMed] [Google Scholar]
- 15.Whitlock JP., Jr Mechanistic aspects of dioxin action. Chem Res Toxicol. 1993;6:754–63. doi: 10.1021/tx00036a003. [DOI] [PubMed] [Google Scholar]
- 16.Janosek J, Hilscherova K, Blaha L, Holoubek I. Enviornmental xenobiotics and nuclear receptors-interactions, effects and in vitro assessments. Toxicol In Vitro. 2006;20:18–37. doi: 10.1016/j.tiv.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Ma C, Marlowe JL, Puga A. The aryl hydrocarbon receptor at the crossroads of multiple signaling pathways. EXS. 2009;99:231–57. doi: 10.1007/978-3-7643-8336-7_9. [DOI] [PubMed] [Google Scholar]
- 18.Chaffin CL, Hutz RJ. Regulation of the aromatic hydrocarbon receptor (AHR) by in utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) J Reprod Dev. 1997;43:47–51. [Google Scholar]
- 19.Chaffin CL, Heimler I, Rawlins RG, Wimpee BA, Sommer C, Hutz RJ. Estrogen receptor and aromatic hydrocarbon receptor in the primate ovary. Endocrine. 1996a;5:315–21. doi: 10.1007/BF02739065. [DOI] [PubMed] [Google Scholar]
- 20.Baldridge MG, Hutz RJ. Autoradiographic Localization of Aromatic Hydrocarbon Receptor (AHR) in Rhesus Monkey Ovary. Am J of Primatology. 2007;69:681–91. doi: 10.1002/ajp.20381. [DOI] [PubMed] [Google Scholar]
- 21.Wojtowicz A, Tomanek M, Augustowska K, Gregoraszczuk EL. Aromatic hydrocarbon receptor (AhR) in the porcine theca and granulosa cells: effect of TCDD, PCB 126 and PCB 153 on the expression of AhR. Endocr Regul. 2005;39(4):109–18. [PubMed] [Google Scholar]
- 22.Chaffin CL, Peterson RE, Hutz RJ. In utero and lactational exposure of female Holtzman rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin: modulation of the estrogen signal. Biol Reprod. 1996b;55:62–7. doi: 10.1095/biolreprod55.1.62. [DOI] [PubMed] [Google Scholar]
- 23.Heimler I, Rawlins RG, Owen H, Hutz RJ. Dioxin Perturbs, in a dose- and time-dependent fashion, steroid secretion, and induces apoptosis of human luteinized granulosa cells. Endocrinol. 1998;139(10):4373–9. doi: 10.1210/endo.139.10.6264. [DOI] [PubMed] [Google Scholar]
- 24.Ho HM, Ohshima K, Watanabe G, Taya K, Strawn E, Hutz RJ. TCDD Increases Inhibin A Production by Human Luteinized Granulosa Cells In Vitro. J Reprod Dev. 2006;52:523–8. doi: 10.1262/jrd.18006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trewin AL, Woller MJ, Wimpee BAB, Conley LK, Baldridge MG, Hutz RJ. Short-Term Hormone Release from Female Rat Hypothalamic and Pituitary Explants is not Altered by 2,3,7,8-Tetrachlorodibenzo-p-dioxin. J Reprod Dev. 2007;53(4):765–75. doi: 10.1262/jrd.18101. [DOI] [PubMed] [Google Scholar]
- 26.Enan E, Moran F, Vandervoot CA, Stewart DR, Overstreet JW, Lasley BL. Mechanism of toxic action of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in cultured human luteinized granulosa cells. Reprod Toxicol. 1996a;10:497–508. doi: 10.1016/s0890-6238(96)00137-2. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Johnson DC, Rozman KK. Reproductive effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in female rats: ovulation, hormonal regulation, and possible mechanisms. Toxicol Appl Pharmacol. 1995;133:321–7. doi: 10.1006/taap.1995.1157. [DOI] [PubMed] [Google Scholar]
- 28.Moran FM, Tarara R, Chen J, Santos S, Cheney A, Overstreet JW, et al. Effect of dioxin on ovarian function in the cynomolgus macaque (M. fascicularis) Reprod Toxicol. 2001;15(4):377–83. doi: 10.1016/s0890-6238(01)00138-1. [DOI] [PubMed] [Google Scholar]
- 29.Moran FM, VandeVoort CA, Overstreet JW, Lasley BL, Conley AJ. Molecular target of endocrine disruption in human luteinizing granulosa cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin: inhibition of estradiol secretion due to decreased 17alpha-hydroxylase/17,20-lyase cytochrome P450 expression. Endocrinol. 2003;144(2):467–73. doi: 10.1210/en.2002-220813. [DOI] [PubMed] [Google Scholar]
- 30.Rier SE, Martin DC, Bowman RE, Dmoski WP, Becker JL. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fund Appl Toxicol. 1993;21:433–41. doi: 10.1006/faat.1993.1119. [DOI] [PubMed] [Google Scholar]
- 31.Dasmahapatra AK, Wimpee BA, Trewin AL, Wimpee CF, Ghorai JK, Hutz RJ. Demonstration of 2,3,7,8-tetrachlorodibenzo-p-dioxin attenuation of P450 steroidogenic enzyme mRNA’s in rat granulosa cell in vitro by competitive reverse transcriptase-polymerase chain reaction assay. Mol Cell Endocrinol. 2000;164:5–18. doi: 10.1016/s0303-7207(00)00245-8. [DOI] [PubMed] [Google Scholar]
- 32.King Heiden T, Carvan MJ, III, Hutz RJ. Inhibition of follicular development, vitellogenesis, and serum 17{beta}-estradiol concentrations in zebrafish following chronic, sublethal dietary exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2006;90:490–9. doi: 10.1093/toxsci/kfj085. [DOI] [PubMed] [Google Scholar]
- 33.Bustin SA, Benes V, Garson JA, Hellemans J, Huggart J, Kubista M, et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin Chem. 2009;55(4):611–22. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 34.Birnbaum LS, DeVito MJ. Use of toxic equivalency factors for risk assessment for dioxins and related compounds. Toxicology. 1995;105(2–3):391–401. doi: 10.1016/0300-483x(95)03237-a. [DOI] [PubMed] [Google Scholar]
- 35.Salisbury TB, Marcinkiewicz JL. In utero and lactational exposure to 2,3,7,8-Tetrachlorodibenzo-p-dioxin and 2,3,4,7,8-Pentachlorodibenzofuran reduces growth and disrupts reproductive parameters in female rats. Biol Reprod. 2002;66:1621–6. doi: 10.1095/biolreprod66.6.1621. [DOI] [PubMed] [Google Scholar]
- 36.Enan E, Lasley B, Stewart D, Overstreet J, Vandervoot CA. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) modulates function of human luteinizing granulosa cells via cAMP signaling and early reduction of glucose transporting activity. Reprod Toxicol. 1996b;10:191–8. doi: 10.1016/0890-6238(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 37.Dasmahapatra AK, Wimpee BA, Trewin AL, Hutz RJ. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases steady-state estrogen receptor-β mRNA levels after CYP1A1 and CYP1B1 induction in rat granulosa cells in vitro. Mol Cell Endocrinol. 2001;182:39–48. doi: 10.1016/s0303-7207(01)00545-7. [DOI] [PubMed] [Google Scholar]
- 38.Heiden TC, Struble CA, Rise ML, Hessner MJ, Hutz RJ, Carvan MJ., III Molecular targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) within the zebrafish ovary: insights into TCDD-induced endocrine disruption and reproductive toxicity. Reprod Toxicol. 2008;25(1):47–57. doi: 10.1016/j.reprotox.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vidal JD, Vandevoort CA, Marcus CB, Lararewicz NR, Conley AJ. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induces CYP1B1 expression in human luteinized granulosa cells. Arch Biochem Biophys. 2005;439(1):53–60. doi: 10.1016/j.abb.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 40.Miyamoto K. Effects of dioxin on gene expression in female reproductive systems in the rat. Environ Sci. 2004;11(1):47–55. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.