Abstract
A growing body of research is demonstrating concordance between mother and child diurnal cortisol production. In the context of maternal history of depression, intergenerational concordance of cortisol production could contribute to hypercortisolemia in children of depressed mothers, which has been shown to increase risk for MDD. The current study is the first to examine concordance in diurnal cortisol production between mothers with a history of depression and their never-depressed, but high-risk, children. We collected salivary cortisol across two days from mothers with (remitted; RMD) and without (CTL) a history of recurrent episodes of depression and their never-depressed daughters. As expected, RMD mothers and their daughters both exhibited higher cortisol production than did their CTL counterparts. Moreover, both across and within groups, mothers’ and daughters’ cortisol production was directly coupled. These findings suggest that there is an intergenerational concordance in cortisol dysregulation that may contribute to hypercortisolemia in girls at familial risk for depression.
Keywords: depression, intergenerational transmission of risk, HPA axis, cortisol, concordance
In the United States alone, 10 to 15 million children under the age of 18 live with a depressed parent (England & Sim, 2009); approximately half of these children will develop depression by adulthood (Beardslee, Versage, & Gladstone, 1998; Goodman et al., 2011; Williamson, Birmaher, Axelson, Ryan, & Dahl, 2004). Indeed, parental depression is associated with a three- to five-fold increase in children’s risk for developing a depressive episode. Moreover, compared with children of well parents who develop depression, children of depressed parents tend to have an earlier age of depression onset, longer episode duration, and greater functional impairment (Hammen, Shih, Altman, & Brennan, 2003; Keller et al., 1986). Little is known, however, about mechanisms through which risk for Major Depressive Disorder (MDD) is transmitted from parent to child.
Dysregulation of the hypothalamus-pituitary-adrenal (HPA) axis has been found consistently to be related to elevated depressive symptoms and to familial risk for depression across a variety of ages, making it a potential mechanism underlying the transmission of risk for depression (Chrousos & Gold, 1992; Ehlert, Gaab, & Heinrichs, 2001; Goodman & Gotlib, 1999; Guerry & Hastings, 2011; Holsboer, 2000). Researchers have shown that anomalous diurnal cortisol production is a core neurobiological abnormality in depression. Compared to never-depressed controls, currently and formerly depressed adults exhibit a steeper cortisol awakening response (CAR), higher peak cortisol in the morning, and higher overall cortisol levels across the day (Bhagwagar, Hafizi, & Cowen, 2005; Dienes, Hazel, & Hammen, 2013; Vreeburg et al., 2009; although see Knorr, Vinberg, Kessing, & Wetterslev, 2010, for more equivocal results). Although altered functioning of the HPA axis may be a consequence of depression, a growing body of research is now beginning to document that dysregulation of this system precedes and even contributes to the onset of illness. Indeed, several investigations of children and adolescents at high risk for depression show that increased vulnerability for MDD is associated with heightened diurnal cortisol secretion (Chen, Joormann, Hallmayer, & Gotlib, 2009; Mannie, Harmer, & Cohen 2007). For example, Mannie et al. (2007) observed risk-related elevations in cortisol levels in the first 30 minutes after waking. These findings parallel data presented by Halligan, Herbert, Goodyer, and Murray (2004) showing that youth at risk for psychopathology exhibit higher morning cortisol secretion than do their low-risk peers. Importantly, in their sample of high-risk youth, Halligan and colleagues also found that hypercortisolemia predicted symptoms of depression three years later (Halligan, Herbert, Goodyer, & Murray, 2007; see also Goodyer, Tamplin, Herbert, & Altham, 2000; Harris et al., 2000). This finding was replicated in an unselected sample of older adolescents: those participants who showed a steeper CAR were more likely to develop MDD in the upcoming year (Adam, Doane, Zinbarg, Mineka, Craske & Griffith, 2010).
Investigators have attempted to understand and predict children’s diurnal HPA-axis activity by examining the concordance, or synchrony, between parent and child diurnal cortisol production. In samples of healthy dyads, children’s diurnal cortisol patterns have been closely related to their mothers’ cortisol production (e.g., Mörelius, Broström, Westrup, Sarman, & Örtenstrand, 2012; Stenius, Theorell, Lilja, Scheynius, Alm, & Lindblad, 2008). In fact, maternal cortisol level is among the strongest determinants of children’s cortisol production (Bright, Granger, & Frick, 2012). Mörelius et al. (2012), for example, examined diurnal cortisol secretion in mothers and their six-month-old infants and found strong correlations between mother and child cortisol levels in the morning, afternoon, and evening. Although studies of dyadic concordance have typically focused on mothers and their infants (e.g., Laurent, Ablow, & Measelle, 2011), high levels of concordance between mother and child diurnal cortisol production have been reported throughout childhood and adolescence (Young, Vazquez, Jiang, & Pfeffer, 2006; Papp, Pendry, & Adam, 2009).
Concordance between mother and child diurnal cortisol production becomes particularly important in the context of maternal psychopathology, especially in the context of disorders such as depression that are frequently associated with increased cortisol production. In the context of mothers’ depression-related hypercortisolemia, intergenerational concordance of cortisol production could potentiate hypercortisolemia in their children, which has been shown to increase individuals’ risk for depression (Halligan et al., 2007; Adam et al., 2010). Despite considerable evidence showing intergenerational concordance of cortisol production, it is not yet clear whether the concordance between mother and child cortisol production is equivalent in mothers with and without a history of depression. It is possible that mothers’ history of depression will decrease maternal sensitivity and responsiveness, which has been shown to attenuate dyadic concordance of cortisol production (Gunnar, Brodersen, Nachmias, Buss, & Rigatuso, 1996; Tu et al., 2007). Alternatively, higher levels of maternal negative affect have been reported to increase concordance (Papp et al., 2009). Elucidating the nature of concordance between depressed mothers’ and their children’s diurnal cortisol production is a critical next step toward understanding mechanisms underlying both hypercortisolemia in children of depressed parents and the intergenerational transmission of risk for depression. Importantly, no study has yet examined the concordance in diurnal cortisol production between formerly depressed mothers and their never-depressed adolescent offspring.
The current study was designed to examine the concordance in diurnal cortisol production between mothers with a history of depression and their never-depressed, but high-risk, children. Given the high rates of depression in daughters of depressed mothers (Gotlib & Colich, 2014; Hops, 1996), we recruited a sample of adolescent daughters at high or low risk for depression based on whether their mothers did or did not experience recurrent episodes of depression during the daughter’s lifetime. We first examined whether our sample of high-risk daughters and their mothers exhibited elevated diurnal cortisol production compared to their low-risk counterparts by assessing risk-group differences in CAR, peak morning cortisol, and daytime cortisol production. We then examined the concordance between mother and daughter diurnal cortisol production, and tested whether maternal history of depression moderated the strength of this association. We expected that mothers with a history of recurrent MDD would exhibit greater overall cortisol production than would mothers with no current or past Axis I disorder. Similarly, we expected that daughters who were at high risk for depression based on their mothers’ history of the disorder would exhibit greater overall cortisol production than would low-risk daughters. Finally, we expected to find significant intergenerational concordance in cortisol production in both the high- and low-risk groups, a pattern that could contribute to hypercortisolemia in high-risk daughters and, in turn, increase our understanding of the intergenerational transmission of risk for depression.
Materials and Methods
Participants
The current sample consisted of 112 mother-daughter dyads. All dyads included daughters who were between the ages of 9 and 15 years and who had no current or past Axis I disorder. Fifty-three daughters had mothers without a current or past Axis I disorder (low-risk daughters; CTL), and 59 daughters had mothers who had a history of recurrent episodes of MDD but whose depression was in full remission, defined as not meeting criteria for depression for the past two months (high-risk daughters; RSK). Mother-daughter dyads were recruited through advertisements posted throughout the community. A telephone screen was used to assess initial inclusion/exclusion criteria; these dyads were invited to the laboratory for a more extensive interview. No participant had any major neurological or metabolic conditions, significant head trauma, or clinically significant learning disorder, and all were fluent in English.
Assessment of Psychopathology
Interviews
All mothers and daughters were administered structured clinical interviews by trained interviewers. Mothers were administered the Structured Clinical Interview for DSM-IV (SCID: First, Gibbon, Spitzer, & Williams, 1997) to assess the presence of at least two distinct episodes of MDD since the birth of their daughter and the absence of a current depressive episode for past two months (remitted; RMD) or the absence of any current or past Axis I disorder (control; CTL). Daughters and mothers (about the daughters) were administered the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS; Kaufman et al., 2000) to assess the absence of any current or past Axis I disorder in the daughters. To assess interrater reliability, an independent rater who was blind to group membership evaluated 10% of SCID and K-SADS interviews. In all cases, diagnoses and group assignment matched those given by the original interviewer, Κ = 1.00.
Questionnaires
Daughters completed the 10-item version of the Children’s Depression Inventory (CDI; Kovacs, 1992), a self-report measure of depressive symptomatology for children between 8 and 17 years of age. Pubertal status was assessed via self-report Tanner Staging (Tanner & Whitehouse, 1976). Daughters also completed the Anxiety Sensitivity Index for Children (ASIC; Laurent et al., 1998), a 16-item self-report measure of anxiety sensitivity in children. Five daughters elected to not provide Tanner stage and one elected to not complete the ASIC. Mothers completed the Beck Depression Inventory-II (BDI; Beck, Steer, & Brown, 1996), a 21-item self-report measure of depressive symptom severity for adults. They also completed the Beck Anxiety Inventory (BAI; Beck & Steer, 1990), a 21-item measure of anxiety symptoms in adults. The ASIC and BAI were included to test as possible covariates.
Diurnal cortisol collection
Within 2 weeks of the diagnostic assessment, mother-daughter dyads were given Salivette kits (Sarstedt, Germany) for at-home cortisol collection. The CTL and RMD groups did not differ with respect to the time between diagnostic assessment and cortisol collection, t(110) = 1.06, p = .291. Both members of the dyad provided cortisol samples on 2 consecutive weekdays by providing a saliva sample at the following four time points: immediately upon waking, 30 minutes post-waking, mid-afternoon (3:30 pm), and 30 minutes before bedtime. Participants were instructed not to eat, drink, or brush their teeth for two hours before each sample. For a subset of participants (n = 58), smart caps were used to track the time when participants opened the bottle to retrieve the Salivette. Cortisol collection time obtained from the smart caps did not significantly differ from self-reported collection time at any time point for mothers, ts < 1.60, ps > .05, or daughters, ts < 1.80, ps > .05. After saliva collection, participants stored Salivettes in their home freezer until they could be transferred to the −20 °F freezer at Stanford University. Cortisol levels were assayed by luminescence immunoassay reagents using a commercial kit from Immuno-Biological Laboratories Inc. (Hamburg, Germany), and the assay sensitivity was set at 0.015 μg/dl. Samples were assayed together in large batches to control for interassay error, and control samples were included to evaluate variability. The intraassay variation on three saliva pools of the low, medium, and high controls were averaged 2.78%, 10.45%, and 4.79%, respectively. The mean values of the low, medium, and high controls were .054, .228, and .863 μg/dL, respectively. The interassay coefficients of the variations of the low, medium, and high controls were 10.9%, 10.5%, and 5.5%, respectively.
Statistical Analyses
Given the dependent nature of the primary outcome data (cortisol levels measured within a dyad over the course of the day), we used multilevel modeling as the analytic framework. Within-dyad variation in mother or daughter cortisol over time was modeled at Level 1. We evaluated linear, quadratic, cubic, piecewise, and exponential models, and we selected the model that best fit the data, based on the smallest value of Akaikie's Information Criterion (AIC), and that was consistent with our visual inspection of the data. For concordance analyses, mother cortisol was added as a time-varying, within-dyad characteristic at Level 1 (see also Laurent et al., 2011). At Level 2, between-dyad variability in Level 1 parameters was explained by characteristics that varied across mother-daughter dyads, in this case, mothers’ history of depression. In all analyses, time was allowed to vary across persons, thereby accounting for each individual’s unique cortisol sampling schedule. At Level 1, we assumed an independent and normally distributed error structure, eti, with mean of 0 and constant variance of σ2. At Level 2, we specified a random effect, rpi, with mean of 0 and a 3 × 3 covariance matrix, T.
We used hierarchical linear modeling (HLM; Raudenbush & Byrk, 2002; Raudenbush, Bryk, & Congdon, 2004) to test a series of models examining the impact of mothers’ history of depression on their own and their daughters’ diurnal cortisol patterns. This modeling technique allowed us to test the effects of mothers’ history of depression not only on the cortisol response trajectories themselves, but also on the concordance of these responses across mother-daughter dyads. Because salivary cortisol data continued to be positively skewed after logarithmic and other transformations, we winsorized cortisol values 2 standard deviations above the mean to the 2-standard deviation value (4% of samples). Dichotomous variables were effect coded and continuous variables were centered. Models were fit using full information maximum likelihood estimates for the purposes of calculating deviance, degrees of freedom, and AIC; and restricted maximum likelihood for estimating model parameters.
Results
Participant Characteristics
Demographic and clinical characteristics of the CTL and RMD mothers and the CTL and RSK daughters are presented in Table 1. There were no significant group differences in mothers’ age, t(110) = 1.61, p = .110, ethnicity, χ2(N=111) = 0.14, p = .713, education, χ2(N=111) = 2.49, p = .115, body mass index (BMI), t(100) = 0.98, p = .328, or percent taking oral contraceptives, χ2(N=111) = 1.41, p = .236. There were also no significant group differences in daughters’ age, t(110) = 0.07, p = .942, ethnicity, χ2(N=112) = 0.20, p = .654, Tanner stage, t(105) = 0.51, p = .611, menarche status, χ2(N=97) = 0.27, p = .606, or BMI, t(83) = 1.32, p = .191. The RMD mothers obtained significantly higher scores on the BAI than did the CTL mothers, t(110) = 5.97, p < .001; however, the RSK and CTL daughters did not differ in their scores on the ASIC, t(109) = 1.17, p = .245. The RMD mothers obtained significantly higher scores on the BDI than did the CTL mothers, t(110) = 6.94, p < .001, and the RSK daughters obtained slightly but significantly higher scores on the CDI than did the CTL daughters, t(110) = 3.50, p = .001. Importantly, sample collection times did not differ as a function of group for mothers or daughters at any time point, ts(110) < 1.98, ps > .05. Mothers’ first and second cortisol samples were taken an average of 30 minutes earlier than their daughters’ samples, ps < .001, the timing of mothers’ afternoon samples did not significantly differ from their daughters’ sampling times, and mothers’ evening samples were taken an average of 8 minutes after her daughters, p = .041.
Table 1.
Demographic Data for the Low-Risk and High-Risk Participants
|
| ||
| Mothers | ||
| CTL | RMD | |
|
| ||
| Age, M (SD) | 44.43 (4.30) | 42.76 (6.35) |
| % Caucasian | 75.00 | 77.97 |
| % College Education | 86.54 | 75.86 |
| % Psychotropic Meds, Current | 5.66 | 47.46 |
| % Psychotropic Meds, Lifetime | 5.66 | 76.27 |
| % Taking Oral Contraceptives | 11.32 | 5.08 |
| BAI, M (SD) | 3.61 (2.82) | 9.90 (7.18) |
| BDI, M (SD) | 3.08 (3.86) | 13.73 (10.55) |
| BMI, M (SD) | 24.85 (5.16) | 25.81 (4.70) |
|
| ||
| Daughters | ||
| CTL | RSK | |
|
| ||
| Age, M (SD) | 12.04 (1.53) | 12.02 (1.50) |
| % Caucasian | 71.70 | 67.80 |
| Tanner Stage, M (SD) | 6.08 (1.90) | 6.28 (2.14) |
| % Post-menarche | 46.67 | 51.92 |
| % Psychotropic Meds, Current | 0.00 | 0.00 |
| % Psychotropic Meds, Lifetime | 0.00 | 0.00 |
| % Taking Oral Contraceptives | 0.00 | 0.00 |
| ASIC, M (SD) | 8.08 (5.50) | 9.34 (5.83) |
| CDI, M (SD) | 1.08 (1.19) | 2.35 (2.40) |
| BMI, M (SD) | 19.46 (2.36) | 20.76 (3.30) |
|
| ||
Note. Mothers with (RMD) or without (CTL) a history of depression, and their daughters who are at high (RSK) or low (CTL) risk for depression based on mothers’ history of the disorder; Meds = Medications; CDI = Children’s Depression Inventory; BDI = Beck Depression Inventory; BMI = body mass index.
Baseline Models
We first ran baseline models of mothers’ and daughters’ diurnal cortisol containing no Level 2 predictors in order to describe the average diurnal cortisol patterns and between-dyad variability in these patterns. We then tested linear, quadratic, cubic, piecewise, and exponential models to determine the model that best captured the pattern of diurnal cortisol production across the day. Cubic and exponential models failed to converge. For both mothers and daughters, the smallest AIC was associated with the piecewise linear growth model (AIC = −191.9 and −191.74 for mothers and daughters, respectively), which estimated the cortisol awakening response (CAR; reflecting the slope from awakening to 30 minutes post-waking) and daytime slope (slope from 30 minutes post-waking through evening). Because time was centered at the second cortisol sample, the intercept term represents the level of cortisol 30 minutes after waking (see Randenbush & Bryk, 2002, for additional details).
The baseline cortisol model indicated that mothers’ average cortisol increased from awakening to 30 minutes after waking, β = 0.26, p < .001, reached a peak cortisol value that was significantly greater than zero, β = 0.65, p < .001, and then declined throughout the day, β = −0.04, p < .001. Daughters’ diurnal cortisol production followed the same pattern, increasing from awakening to 30 minutes after waking, β = 0.30, p < .001, reaching a peak cortisol value that was significantly greater than zero, β = 0.65, p < .001, and then declining throughout the day, β = −0.05, p < .001. Significant variability in these parameters, however, demonstrates that these patterns differed across dyads and could therefore be explained by Level 2 predictors.
Potential Covariates
A series of variables were tested as potential covariates in relation to mothers’ and daughters’ cortisol production. For mothers, we tested the following variables: current psychotropic medications, lifetime history of psychotropic medications, oral contraceptive use, treatment, body mass index (BMI), BAI scores, and BDI scores. For daughters, we tested the following variables: ASIC scores, preversus post-menarche, Tanner stage, BMI, and CDI scores; given the exclusion criteria for this study, daughters were not taking any psychotropic medication and were not in treatment. None of these variables predicted mothers’ or daughters’ cortisol response patterns, ps ≥ .05, and, therefore, were not included as covariates.
Group Differences in Mothers’ and Daughters’ Diurnal Cortisol Patterns
For each of the outcomes reported, we tested the effects of mothers’ history of depression on each of the Level 1 parameters: CAR, peak cortisol, and daytime slope. For mothers, history of depression significantly predicted the CAR, β = 0.09, p = .037, peak cortisol, β = 0.08, p = .005, and daytime slope, β = −0.01, p = .006, and significantly improved model fit, χ2(df=3) = 7.98, p = .046. Specifically, compared to CTL mothers, RMD had a steeper CAR, higher peak cortisol levels 30 minutes after waking, and a steeper daytime slope.1 In parallel for daughters, mothers’ history of depression significantly predicted the daughters’ CAR, β = 0.13, p = .008, peak cortisol, β = 0.09, p = .002, and daytime slope, β = −0.01, p = .001, and significantly improved model fit, χ2(df=3) = 11.31, p = .010. Specifically, compared to CTL daughters, RSK daughters had a steeper CAR, higher peak cortisol levels 30 minutes after waking, and a steeper daytime slope.2
Concordance in Mother and Daughter Cortisol
To directly test the concordance of mother and daughter cortisol production, daughter cortisol outcome was modeled as a function of an intercept and her mother’s cortisol production, both of which were allowed to vary across dyads. Overall, mother and daughter diurnal cortisol production was significantly concordant, β = 0.84, p < .001. Mothers’ history of depression was entered at Level 2 to explain the significant between-dyad variation in concordance. Importantly, mothers’ history of depression was not a significant predictor of the concordance between mothers’ and daughters’ diurnal cortisol production, β = −0.01, p = .765, and did not significantly improve model fit, χ2(df=2) = 0.86, p > .500. Moreover, when the association between mother and daughter cortisol production was examined separately based on mothers’ history of depression, there was evidence of strong intergenerational concordance in diurnal cortisol production for dyads in which mothers did have a history of depression, rs(59) > .332, ps < .010, and for dyads in which mothers did not, rs(53) > .393, ps < .010 (see Table 2).
Table 2.
Correlations between Mother-Daughter Diurnal Cortisol Across Groups and for Dyads in which the Mother does (RMD) and does not (CTL) have a History of Depression
| Across Groups |
RMD | CTL | |
|---|---|---|---|
| CAR | 0.410** | 0.332* | 0.426* |
| Peak Cortisol | 0.486** | 0.481** | 0.401* |
| Daytime Slope | 0.487** | 0.485** | 0.393* |
p < .01
p < .001
Discussion
This is the first study to examine the concordance in diurnal cortisol production between formerly depressed mothers and their never-depressed adolescents who are at risk for depression based on their mothers’ history of the disorder. Using multiple indicators of diurnal cortisol, we found that mothers’ history of depression predicted both their own and their daughters’ cortisol production. As expected, formerly depressed mothers exhibited a steeper CAR, higher peak cortisol 30 minutes after waking, and steeper afternoon slope than did healthy mothers. Importantly, this pattern of findings was paralleled in their daughters: daughters of formerly depressed mothers exhibited a steeper CAR, higher peak cortisol 30 minutes after waking, and steeper afternoon slope than did daughters of healthy mothers. We also found consistent and robust concordance between mothers’ and daughters’ diurnal cortisol production across both groups of participants: in both groups, mothers with higher-than-average diurnal cortisol production at a given time point had daughters with higher-than-average cortisol production at the same time point. Thus, concordance of mothers’ and daughters’ cortisol production may contribute to the hypercortisolemia observed in high-risk daughters’, which has been shown to increase risk for MDD (e.g., Adam et al., 2010; Halligan et al., 2007).
Our finding of hypercortisolemia in formerly depressed mothers adds to previous studies documenting cortisol dysregulation in individuals with a history of depression (e.g., Appelhof et al., 2006; Tressider et al., 1991; Zobel, Nickel, Sonntag, Uhr, Holsboer, & Ising, 2001). Although these findings are consistent with the formulation that HPA-axis dysregulation is a consequence of the depressive episode, it is noteworthy that we also found evidence of cortisol dysregulation in healthy daughters of formerly depressed mothers. Indeed, the high-risk daughters, who themselves had no current or past Axis I disorder, showed evidence of cortisol dysregulation on every metric of diurnal cortisol activity. Our findings add to a handful of studies demonstrating cortisol dysregulation in children and adolescents at risk for depression (Goodyer et al., 2000; Halligan et al., 2004; Mannie et al., 2007; Young et al., 2006). Thus, hypercortisolemia may be a marker of risk for depression that signals the need for early intervention. This possibility is consistent with several longitudinal studies that found adolescents who exhibit greater diurnal cortisol production to be more susceptible to developing a mood disorder (Adam et al., 2010; Ellenbogen, Hodgins, Linnen, & Ostiguy, 2011). Moreover, researchers have shown that chronic cortisol elevations can increase atrophy of such brain regions as the prefrontal cortex and hippocampus, which in turn can impair individuals’ ability to regulate emotional responses to negative events (Gold, Drevets, & Charney, 2002; McEwen, 1998).
It is noteworthy that we documented concordance of HPA-axis functioning between formerly depressed mothers and their at-risk, yet never-depressed, daughters. This finding increases our understanding of hypercortisolemia in children at familial risk for depression (e.g., Mannie et al., 2007; Halligan et al., 2004). Our results are consistent with the broader literature showing concordance between parent and child biological functioning in both unselected and healthy samples (e.g., Bornstein & Suess, 2000; Mörelius et al., 2012; Mörelius, Theodorsson, & Nelson, 2009; Stenius et al., 2008). Moreover, our results support and extend Young et al.’s (2006) finding of concordance between parent and child cortisol in the context of parental depression. It is important to note, however, that approximately half of the children in Young et al.’s study met criteria for an Axis I disorder; moreover, in one-third of the depressed dyads, it was the nondepressed parent, rather than the depressed parent, who provided the cortisol sample. Our study extends Young et al.’s results by documenting significant concordance in diurnal cortisol production between formerly depressed mothers and their at-risk children before any of the children had developed a psychiatric disorder. Our results are also consistent with findings reported by Laurent et al. (2011), who showed that neither current nor past maternal depressive symptoms influenced the degree of concordance between mother and infant cortisol production during a psychosocial stressor. Laurent and colleagues did find, however, that increasing levels of depressive symptoms over time intensified the concordance between mother and infant cortisol production. It will be important in future research to examine whether changes in depressive symptoms also affect concordance in diurnal cortisol production between depressed mothers and their adolescent children.
Theorists have suggested that both genetic and environmental factors contribute to concordance between parent and child cortisol production. Increasing evidence indicates that cortisol production and regulation is heritable. For example, individuals who are homozygous for the short allele in the promoter region of the serotonin transporter gene have been found to produce higher levels of cortisol, both throughout the day (Chen et al., 2009) and in response to a psychosocial stressor (Gotlib, Joormann, Minor & Hallmayer, 2008). Moreover, data from twin studies suggest heritability estimates of .40 for the CAR (Wüst, Federenko, Hellhammer, & Kirschbaum, 2000). Evidence from twin studies also demonstrates the importance of environmental factors on HPA-axis activity. For example, concordant cortisol production has been found between twins living in the same environment (Custodio et al., 2007; Schreiber et al., 2006) but not between twins living in different environments (Franz et al., 2010). Moreover, instability and stress in individuals’ home environments can directly affect cortisol production (de Weerth, Buitelaar, & Beijers, 2013; Repetti, Taylor, & Seeman, 2002). Similarly, evidence from both human and animal models suggests that early environmental stress can have lasting effects on cortisol dysregulation and neural structures that support HPA-axis activity (Spinelli et al., 2009; Taylor, 2010). It will be important that future studies quantify the relative contributions of genetic and environmental factors in order to gain a better understanding of mechanisms underlying intergenerational concordance in cortisol production.
We should note three limitations of the present study. First, despite the fact that daughters in the high- and low-risk groups did not meet diagnostic criteria for a current or past Axis I disorder, there were nevertheless group differences in CDI scores. It is important to note, however, that CDI scores for both groups of girls were still far below the recommended cutoff score of 8 (Kovacs, 1992). Moreover, we examined CDI scores as a covariate and found no evidence that, within this narrow and low range of CDI scores, girls’ depressive symptomatology affected cortisol levels. Second, although this study provides important first evidence of the concordance in patterns of diurnal cortisol between mothers and daughters, the cross-sectional nature of our data limits our ability to make statements about causation. In this context, it is possible that daughters’ cortisol level influenced their mothers’ cortisol production, or that there is a bidirectional and interactive relation between mother and daughter cortisol production (Saxbe et al., 2013). Third, we did not assess a number of variables known to influence salivary cortisol functioning, including sleep disturbances, history of trauma, and the phase of participants’ menstrual cycle. Future research should examine the influence of these variables on the concordance of cortisol production. Similarly, the use of self-report Tanner stage as a proxy for pubertal development is a limitation of this study. Although self-reported measures of physical maturation have been found to be significantly associated with levels of sex hormones, both testosterone and DHEA levels have been shown to influence diurnal cortisol production. Thus, future research should consider directly assessing gonadal hormones. Finally, although hypercortisolemia has been shown consistently to increase susceptibility to depression in both high-risk and unselected samples (Adam et al., 2010; Halligan et al., 2007; Goodyer et al., 2000; Harris et al., 2000), it will be important to follow this sample of girls over time to examine whether high levels of cortisol production predict the subsequent onset of depression.
Overall, the current findings contribute to our understanding of HPA-axis functioning in children of depressed mothers. Although there is considerable evidence demonstrating that children of depressed mothers are at increased risk for depression, the mechanisms underlying this risk are not well understood. Data from this study indicate that daughters of formerly depressed mothers exhibit hypercortisolemia throughout the day. Moreover, there was an intergenerational concordance in cortisol dysregulation that may contribute to hypercortisolemia in girls whose mothers’ have a history of depression. Given the consequences of hypercortisolemia for susceptibility to develop depressive episodes (e.g., Halligan et al., 2007) and for neural development and functioning (Gold et al., 2002; McEwen, 1998), elucidating intergenerational concordance in cortisol dysregulation is likely to have important implications for understanding the onset of MDD in girls at familial risk for depression.
Highlights.
- Diurnal cortisol was collected from mothers with (RMD) and without (CTL) a history of depression and their never-depressed daughters
- RMD mothers and their daughters exhibited higher cortisol production than did their CTL counterparts
- Both across groups and within each group, daughters’ cortisol production was directly coupled with their mothers’ cortisol production
- Mothers with higher cortisol production at a given point in time had daughters with higher cortisol production at the same time point
- Findings suggest that there is an intergenerational concordance in cortisol dysregulation that could contribute to hypercortisolemia in daughters of depressed mothers
Figure 1.
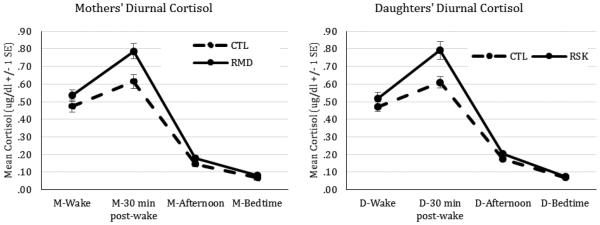
Diurnal cortisol at waking, 30 minutes post-waking, mid-afternoon (approximately 3:30pm), and 30 minutes before bedtime for mothers with (RMD) and without (CTL) a history of depression and their high-risk (RSK) and low-risk (CTL) daughters.
Acknowledgements
We thank Maria Lemus and Kirsten Gilbert for their assistance in scheduling and running the participants. This research was supported by NIMH Grants MH74849 to IHG and F32-MH102013 to JL. Please address correspondence to Joelle LeMoult, Ph.D., Department of Psychology, Bldg. 420, Jordan Hall, Stanford University, Stanford, CA 94305; jlemoult@stanford.edu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
One mother in the RMD group was taking a corticosteroid; findings did not change when this participant was removed from the analyses.
Given that BMI has been associated with diurnal cortisol production in other studies (e.g., Daniel et al., 2006), we tested BMI as a covariate in the model predicting mothers’ and daughters’ diurnal cortisol production. BMI did not significantly predict the CAR, peak cortisol, or the daytime slope for mothers or daughters, ps > .05; moreover, the main effect of mothers’ history of depression on each index of diurnal cortisol remained significant, ps < .02, and the addition of BMI did not significantly improve model fit, χ2(3) < 1.80, ps > .05.
References
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35(6):921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhof BC, Huyser J, Verweij M, Brouwer JP, van Dyck R, Fliers E, Schene AH. Glucocorticoids and relapse of major depression (dexamethasone/corticotropin-releasing hormone test in relation to relapse of major depression) Biological Psychiatry. 2006;59(8):696–701. doi: 10.1016/j.biopsych.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Atkins DC. Using multilevel models to analyze couple and family treatment data: basic and advanced issues. Journal of Family Psychology. 2005;19(1):98–110. doi: 10.1037/0893-3200.19.1.98. [DOI] [PubMed] [Google Scholar]
- Beardslee WR, Versage EM, Gladstone TR. Children of affectively ill parents: A major depressive disorder: A review. American Journal of Psychiatry. 1998;54:1254–1268. doi: 10.1097/00004583-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Beck Anxiety Inventory. Psychological Corporation; San Antonio, TX: 1990. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Bhagwagar Z, Hafizi S, Cowen PJ. Increased salivary cortisol after waking in depression. Psychopharmacology. 2005;182(1):54–57. doi: 10.1007/s00213-005-0062-z. [DOI] [PubMed] [Google Scholar]
- Bolger N, Laurenceau JP. Intensive longitudinal methods: An introduction to diary and experience sampling research. Guilford Press; 2013. [Google Scholar]
- Bornstein MH, Suess PE. Child and mother cardiac vagal tone: continuity, stability, and concordance across the first 5 years. Developmental Psychology. 2000;36(1):54. [PubMed] [Google Scholar]
- Bright MA, Granger DA, Frick JE. Do infants show a cortisol awakening response? Developmental Psychobiology. 2012;54(7):736–743. doi: 10.1002/dev.20617. [DOI] [PubMed] [Google Scholar]
- Chen MC, Joormann J, Hallmayer J, Gotlib IH. Serotonin transporter polymorphism predicts waking cortisol in young girls. Psychoneuroendocrinology. 2009;34(5):681–686. doi: 10.1016/j.psyneuen.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- Custodio RJ, Junior CEM, Milani SLS, Simões AL, De Castro M, Moreira AC. The emergence of the cortisol circadian rhythm in monozygotic and dizygotic twin infants: the twinopair synchrony. Clinical Endocrinology. 2007;66(2):192–197. doi: 10.1111/j.1365-2265.2006.02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M, Moore DS, Decker S, Belton L, DeVellis B, Doolen A, Campbell MK. Associations among education, cortisol rhythm, and BMI in blue-collar women. Obesity. 2006;14(2):327–335. doi: 10.1038/oby.2006.42. [DOI] [PubMed] [Google Scholar]
- de Weerth C, Buitelaar JK, Beijers R. Infant cortisol and behavioral habituation to weekly maternal separations: Links with maternal prenatal cortisol and psychosocial stress. Psychoneuroendocrinology. 2013;38(12):2863–2874. doi: 10.1016/j.psyneuen.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Dienes KA, Hazel NA, Hammen CL. Cortisol secretion in depressed, and at-risk adults. Psychoneuroendocrinology. 2013;38(6):927–940. doi: 10.1016/j.psyneuen.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus–pituitary–adrenal axis. Biological psychology. 2001;57(1):141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- England MJ, Sim LJ. Depression in parents, parenting, and children: Opportunities to improve identification, treatment, and prevention. National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN) Biometrics Research, New York State Psychiatric Institute; New York: Nov, 2002. [Google Scholar]
- Franz CE, York TP, Eaves LJ, Mendoza SP, Hauger RL, Hellhammer DH, Kremen WS. Genetic and environmental influences on cortisol regulation across days and contexts in middle-aged men. Behavior Genetics. 2010;40(4):467–479. doi: 10.1007/s10519-010-9352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PW, Drevets WC, Charney DS, Drevets WC. New insights into the role of cortisol and the glucocorticoid receptor in severe depression. Biological Psychiatry. 2002;52(5):381–385. doi: 10.1016/s0006-3223(02)01480-4. [DOI] [PubMed] [Google Scholar]
- Goodman SH. Depression in mothers. Annual Review in Clinical Psychology. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychological Review. 1999;106(3):458. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: A meta-analytic review. Clinical Child and Family Psychology Review. 2011;14(1):1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Tamplin A, Herbert J, Altham PME. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. The British Journal of Psychiatry. 2000;177(6):499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Colich NL. Children of depressed parents. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. Third Guilford Press; New York: 2014. pp. 240–258. [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63(9):847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry JD, Hastings PD. In search of HPA axis dysregulation in child and adolescent depression. Clinical Child and Family Psychology Review. 2011;14(2):135–160. doi: 10.1007/s10567-011-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Nachmias M, Buss K, Rigatuso J. Stress reactivity and attachment security. Developmental Psychobiology. 1996;29(3):191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer IM, Murray L. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biological Psychiatry. 2004;55(4):376–381. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer I, Murray L. Disturbances in morning cortisol secretion in association with maternal postnatal depression predict subsequent depressive symptomatology in adolescents. Biological Psychiatry. 2007;62(1):40–46. doi: 10.1016/j.biopsych.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Hammen C, Shih J, Altman T, Brennan PA. Interpersonal impairment and the prediction of depressive symptoms in adolescent children of depressed and nondepressed mothers. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42(5):571. doi: 10.1097/01.CHI.0000046829.95464.E5. [DOI] [PubMed] [Google Scholar]
- Harris TO, Borsanyi S, Messari S, Stanford K, Brown GW, Cleary SE, Herbert J. Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. The British Journal of Psychiatry. 2000;177(6):505–510. doi: 10.1192/bjp.177.6.505. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Hops H. Intergenerational transmission of depressive symptoms: Gender and developmental considerations. In: Mundt C, Goldstein MJ, Hahlweg K, Fiedler P, editors. Interpersonal Factors in the Origin and Course of Affective Disorders. Royal College of Psychiatrists; London: 1996. pp. 113–129. [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. Historical development and present status of the schedule for affective disorders and schizophrenia for school-age children (K-SADS) Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(1):49–58. doi: 10.1097/00004583-200001000-00016. [DOI] [PubMed] [Google Scholar]
- Keller MB, Beardslee WR, Dorer DJ, Lavori PW, Samuelson H, Klerman GR. Impact of severity and chronicity of parental affective illness on adaptive functioning and psychopathology in children. Archives of General Psychiatry. 1986;43(10):930. doi: 10.1001/archpsyc.1986.01800100020004. [DOI] [PubMed] [Google Scholar]
- Knorr U, Vinberg M, Kessing LV, Wetterslev J. Salivary cortisol in depressed patients versus control persons: A systematic review and meta-analysis. Psychoneuroendocrinology. 2010;35(9):1275–1286. doi: 10.1016/j.psyneuen.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children's depression inventory: manual. Multi-Health Systems. 1992 [Google Scholar]
- Lange C, Zschucke E, Ising M, Uhr M, Bermpohl F, Adli M. Evidence for a normal HPA axis response to psychosocial stress in patients remitted from depression. Psychoneuroendocrinology. 2013;38(11):2729–2736. doi: 10.1016/j.psyneuen.2013.06.033. [DOI] [PubMed] [Google Scholar]
- Laurenceau JP, Bolger N. Using diary methods to study marital and family processes. Journal of Family Psychology. 2005;19(1):86. doi: 10.1037/0893-3200.19.1.86. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC, Measelle J. Risky shifts: How the timing and course of mothers' depressive symptoms across the perinatal period shape their own and infant's stress response profiles. Development and Psychopathology. 2011;23(02):521–538. doi: 10.1017/S0954579411000083. [DOI] [PubMed] [Google Scholar]
- Laurent J, Schmidt NB, Catanzaro SJ, Joiner TE, Jr, Kelley AM. Factor structure of a measure of anxiety sensitivity in children. Journal of anxiety Disorders. 1998;12(4):307–331. doi: 10.1016/s0887-6185(98)00017-6. [DOI] [PubMed] [Google Scholar]
- Mannie Z, Harmer C, Cowen P. Increased waking salivary cortisol levels in young people at familial risk of depression. American Journal of Psychiatry. 2007;164(4):617–621. doi: 10.1176/ajp.2007.164.4.617. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840(1):33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Mörelius E, Broström EB, Westrup B, Sarman I, Örtenstrand A. The stockholm neonatal family-centered care study: effects on salivary cortisol in infants and their mothers. Early Human Development. 2012;88(7):575–581. doi: 10.1016/j.earlhumdev.2011.12.033. [DOI] [PubMed] [Google Scholar]
- Mörelius E, Theodorsson E, Nelson N. Stress at threeomonth immunization: Parents’ and infants’ salivary cortisol response in relation to the use of pacifier and oral glucose. European Journal of Pain. 2009;13(2):202–208. doi: 10.1016/j.ejpain.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Weissman MM, Kidd KK. Children and depression: The children of depressed parents; the childhood of depressed patients; depression in children. Journal of Affective Disorders. 1980;2(1):1–16. doi: 10.1016/0165-0327(80)90017-8. [DOI] [PubMed] [Google Scholar]
- Papp LM, Pendry P, Adam EK. Mother-adolescent physiological synchrony in naturalistic settings: within-family cortisol associations and moderators. Journal of Family Psychology. 2009;23(6):882. doi: 10.1037/a0017147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Vol. 1. Sage; 2002. [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R. HLM 6 for Windows [Computer software] Scientific Software International, Inc; Skokie, IL: 2004. [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychological bulletin. 2002;128(2):330–366. [PubMed] [Google Scholar]
- Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression:A primer on neuron death. Biological Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Saxbe DE, Margolin G, Spies Shapiro L, Ramos M, Rodriguez A, Iturralde E. Relative influences: Patterns of HPA Axis concordance during triadic family interaction. Health Psychology. 2013;33:273–281. doi: 10.1037/a0033509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe D, Repetti RL. For better or worse? Coregulation of couples’ cortisol levels and mood states. Journal of Personality and Social Psychology. 2010;98(1):92. doi: 10.1037/a0016959. [DOI] [PubMed] [Google Scholar]
- Schreiber JE, Shirtcliff E, Hulle CV, Lemery-Chalfant K, Klein MH, Kalin NH, Goldsmith HH. Environmental influences on family similarity in afternoon cortisol levels: twin and parent–offspring designs. Psychoneuroendocrinology. 2006;31(9):1131–1137. doi: 10.1016/j.psyneuen.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, Stein E. Early-life stress induces long-term morphologic changes in primate brain. Archives of General Psychiatry. 2009;66(6):658–665. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenius F, Theorell T, Lilja G, Scheynius A, Alm J, Lindblad F. Comparisons between salivary cortisol levels in six-months-olds and their parents. Psychoneuroendocrinology. 2008;33(3):352–359. doi: 10.1016/j.psyneuen.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Steward MM, Sridhar A, Meyer JS. In New Perspectives in Regeneration. Springer Berlin Heidelberg; 2013. Neural regeneration; pp. 163–191. [DOI] [PubMed] [Google Scholar]
- Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Archives of Disease in Childhood. 1976;51(3):170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proceedings of the National Academy of Sciences. 2010;107(19):8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trestman RL, Coccaro EF, Bernstein D, Lawrence T, Gabriel SM, Horvath TB, Siever LJ. Cortisol responses to mental arithmetic in acute and remitted depression. Biological Psychiatry. 1991;29(10):1051–1054. doi: 10.1016/0006-3223(91)90361-o. [DOI] [PubMed] [Google Scholar]
- Tu MT, Grunau RE, PetrieoThomas J, Haley DW, Weinberg J, Whitfield MF. Maternal stress and behavior modulate relationships between neonatal stress, attention, and basal cortisol at 8 months in preterm infants. Developmental Psychobiology. 2007;49(2):150–164. doi: 10.1002/dev.20204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, van Pelt J, DeRijk RH, Verhagen JC, van Dyck R, Penninx BW. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from a large cohort study. Archives of General Psychiatry. 2009;66(6):617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- Weissman M, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H. Offspring of depressed parents: 20 years later. American Journal of Psychiatry. 2006;163(6):1001–1008. doi: 10.1176/ajp.2006.163.6.1001. [DOI] [PubMed] [Google Scholar]
- Williamson DE, Birmaher B, Axelson DA, Ryan ND, Dahl RE. First episode of depression in children at low and high familial risk for depression. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43(3):291–297. doi: 10.1097/00004583-200403000-00010. [DOI] [PubMed] [Google Scholar]
- Wüst S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25(7):707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Young EA, Vazquez D, Jiang H, Pfeffer CR. Saliva cortisol and response to dexamethasone in children of depressed parents. Biological Psychiatry. 2006;60(8):831–836. doi: 10.1016/j.biopsych.2006.03.077. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Sonntag A, Uhr M, Holsboer F, Ising M. Cortisol response in the combined dexamethasone/CRH test as predictor of relapse in patients with remitted depression: a prospective study. Journal of psychiatric research. 2001;35(2):83–94. doi: 10.1016/s0022-3956(01)00013-9. [DOI] [PubMed] [Google Scholar]


