Abstract
Objectives
It was recently demonstrated that adverse cardiovascular events (ACVE) complicate a high proportion of hospitalizations for patients with acute drug overdoses. The aim of this study was to derive independent clinical risk factors for ACVE in patients with acute drug overdoses.
Methods
This prospective cohort study was conducted over 3 years at two urban university hospitals. Patients were adults with acute drug overdoses enrolled from the ED. In-hospital ACVE was defined as any of myocardial injury, shock, ventricular dysrhythmia, or cardiac arrest.
Results
There were 1,562 patients meeting inclusion/exclusion criteria (mean age, 41.8 years; female, 46%; suicidal, 38%). ACVE occurred in 82 (5.7%) patients (myocardial injury, 61; shock, 37; dysrhythmia, 23; cardiac arrests, 22) and there were 18 (1.2%) deaths. On univariate analysis, ACVE risk increased with age, lower serum bicarbonate, prolonged QTc interval, prior cardiac disease, and altered mental status. In a multivariable model adjusting for these factors as well as patient sex and hospital site, independent predictors were: QTc > 500 msec (3.8% prevalence, odds ratio [OR] 27.6), bicarbonate < 20 mEql/L (5.4% prevalence, OR 4.4), and prior cardiac disease (7.1% prevalence, OR 9.5). The derived prediction rule had 51.6% sensitivity, 93.7% specificity, and 97.1% negative predictive value; while presence of two or more risk factors had 90.9% positive predictive value.
Conclusions
The authors derived independent clinical risk factors for ACVE in patients with acute drug overdose, which should be validated in future studies as a prediction rule in distinct patient populations and clinical settings.
Introduction
With nearly 100 deaths per day since 2007, the United States is currently experiencing its worst drug overdose epidemic of all time.1 The death rate of 11.8 per 100,000 persons in 2007 was roughly three times the rate in 1991.2 Over 2.5 million poisonings are reported to poison control centers in the United States each year.3 National estimates of drug-related visits to hospital emergency departments (EDs) in the Drug Abuse Warning Network (DAWN) demonstrate a marked increase in drug-related ED visits over the past decade, particularly those involving prescription drug abuse. DAWN estimates that there are approximately 1.5 million annual drug-related ED visits,4 of which 22% are admitted to hospital, with an associated 1% mortality rate according to the National Hospital Ambulatory Medical Care Survey (NHAMCS).5
Patients with suspected drug overdose represent many sub-populations of patients, such as suicidal drug overdose and acute substance or illicit drug abuse. Patient management is individualized by the clinician based on the exposure history, physical examination, and often includes consultation with a regional poison control center or an on-site medical toxicologist. In addition, routine ED evaluation typically includes an electrocardiogram (ECG)6 and basic metabolic laboratory serum profile. Using this information, clinical decisions must be made regarding medical clearance (for psychiatric evaluation or discharge home), or hospitalization for acute medical consequences or the risk of impending adverse events.
Adverse cardiovascular events (ACVE) comprise a large proportion of the morbidity and mortality in drug overdose emergencies reported to the National Poisoning Data System.3 A formal definition of drug-overdose related ACVE was initially informed by the International Liaison Committee on Resuscitation,7 subsequently explored with preliminary data,8 and then formally validated by a large prospective cohort study of acute drug overdose patients identified from the ED and followed to hospital discharge.9 In accordance with this extensive prior work, the following is the current definition of drug-related ACVE: myocardial injury (by biomarker or electrocardiogram [ECG] evidence); shock (hypotension or hypo-perfusion requiring vasopressors); ventricular dysrhythmias (ventricular tachycardia/fibrillation, torsade de pointes); and cardiac arrest (loss of pulse requiring cardiopulmonary resuscitation [CPR]). It is estimated that the incidence of ACVE from hospitalized drug overdose patients may be as high as 16.9%.9 Using the admission statistics previously cited from DAWN and NHAMCS,4,5 a clinical tool that could identify ACVE with 100% efficiency could theoretically prevent over 250,000 medical admissions per year in the United States.
Unfortunately, predicting which ED patients with suspected overdose will subsequently suffer ACVE remains difficult. Given the recent rise in drug overdose mortality as the leading cause of injury-related fatalities nationwide,1,2,10 clinicians must be equipped with valid clinical predictive tools to accurately identify those at risk for morbidity or mortality. Thus, we sought to derive independent risk factors for ACVE to guide management for ED patients with acute drug overdoses using commonly used and rapidly available tests. We hypothesized that elements of the routine ED workup would predict ACVE when combined into a simple clinical risk prediction tool, with high negative predictive value to safely identify low-risk patients without various high-risk clinical features.
Methods
Study Design
This prognostic indicator study was prospectively performed on consecutive adult ED patients with acute drug overdoses (prescription, over-the-counter, and illicit) over 36 months (2009 to 2012). The study protocol was approved by the institutional review board for the two participating institutions with a waiver of informed consent.
Study Setting and Population
Two urban, tertiary-care hospitals (Mount Sinai Hospital, Elmhurst Hospital Center, both in New York, NY) were used for enrollment with EDs that have a combined annual visit volume in excess of 150,000 and are staffed 24 hours per day with board-certified emergency physicians. Neither study hospital was a regional referral center for toxicology patients, but a medical toxicology consulting service was available if deemed necessary in addition to routine clinical care.
Patients with suspected acute drug overdoses were initially screened for inclusion by trained research assistants using one of two triggers: rounding with ED attending physicians several times daily during normal business hours, or by telephone referral of the case to the regional poison control center that was staffed 24 hours/day and 7 days/week by certified specialists in poisoning information. Reporting of suspected acute poisoning to the poison control center in New York City is mandated by public health law.11
Following screening, formal inclusion and exclusion criteria were applied to determine whether patients would be analyzed for prognostic indicators. Patients were included who met both of the following criteria: 1) acute presentation (within 24 hours of exposure), and 2) suspected overdose (i.e., illicit drug dose sufficient to cause symptoms or any prescription drug exposure greater than its therapeutic dose). Exclusion criteria were the following: alternative diagnosis (e.g., trauma or infection), chronic presentation (i.e., not meeting acute criteria above), non-drug overdose (e.g., plant), exposures limited to dermal or inhalational means only, age younger than 18 years, anaphylaxis, patients with incomplete data (i.e., left against medical advice, transferred to an outside institution, or otherwise eloped from the hospital), and patients with prior do-not-resuscitate orders (i.e., prior to hospital admission).
Study Protocol
Data collection from the medical chart occurred in accordance with accepted guidelines for valid medical chart abstraction, including training of abstractors and 95% agreement of a random sampling of ten test charts prior to mass data abstraction.12 While poison control center data were used to enhance initial screening and inclusion of subjects, actual medical record data was relied upon for all other data measurements, including study outcomes. This data included demographics (sex, age), exposure information (timing of exposure, number of exposures, intent, suicidality), toxin identification (detail from history and physical interview, serum drug concentrations if available), initial mental status (Glasgow Coma Scale [GCS] score, agitation, coma), prior cardiac disease (coronary artery disease or congestive heart failure), history of diabetes or hypertension, and toxicology screens (urine ELISA panel and serum concentration, if any). Blood and urine toxicology screen results sent as routine part of clinical care (i.e., no confirmatory gas chromatographic method coupled with mass spectrometry) were recorded in order to confirm exposure. Traditional cardiac biomarkers were measured using Bayer reagents (Bayer Healthcare, Cambridge, MA) on the Bayer Advia Centaur analyzer, and the standard cutoff concentration was used for cardiac troponin I (0 to 0.09 ng/ml, detection limit 0.01 ng/ml, 10% imprecision, 99th percentile cutoff > 0.1 ng/ml). Subjects without laboratory evaluation of troponin I were recorded as “missing” in the database to avoid misclassification bias, but for the purposes of ACVE coding, the missing value was assumed to be negative. Inpatient rhythm strips in the paper charts of inpatients receiving telemetry monitoring were reviewed to evaluate the alarmed segments. Data were abstracted to a de-identified electronic database with password protection.
Subjects were prospectively followed during the entirety of their hospital stays through to hospital discharge with data that included electronic medical records, paper medical records, consult records, poison control center records (for initial screening/inclusion only, not for data acquisition or outcome data), as well as inpatient telemetry monitoring (if any). Hospital medical record follow-up for all patients was performed by research assistants trained in medical abstraction and recorded using standardized data collection forms. Results (from electronic physician notes, laboratory records, radiology results, and discharge summaries) were prospectively available to the study investigators. Patients discharged from the hospital had no further follow-up. The primary outcome was in-hospital ACVE, defined as a composite outcome according to four criteria (see Outcome Measures below).
Cardiac disease was defined as the prior history of either coronary artery disease (diagnosed by cardiac catheterization or positive stress testing) or congestive heart failure (of any type), as denoted in the patient's medical chart. Altered mental status was defined according to charting of any of the following: GCS < 15, “coma,” “agitation,” or “altered mental status.” Suicidal ingestion was defined according to the treating physician's initial impression and confirmed by psychiatry consultation, with disagreements settled by psychiatry consultation note. Multi-drug exposures were defined as exposures to more than one drug of any kind, including two drugs of the same drug class (e.g., exposure to oxycodone and hydrocodone counted as two drug exposures, but only one drug class).
Outcome Measures
The criteria for in-hospital ACVE have been previously validated,9 and were therefore defined in this study as the occurrence of at least one of the following: 1) myocardial injury (troponin > 0.09 ng/mL at any time during hospitalization), 2) shock (hypotension requiring vasopressors), 3) ventricular dysrhythmia (ventricular tachycardia [VT], ventricular fibrillation [VF], or torsade de pointes), and 4) cardiac arrest (loss of pulses requiring chest compressions). ACVE was abstracted based on information from electronic medical records (laboratory results, discharge summary, discharge diagnosis), pharmacy records (dispensed and administered medications), alarmed rhythm strip segments (printed daily as part of routine clinical care if patient admitted to telemetry unit, interpreted by a blinded cardiologist), and electronic billing records (diagnosis codes, current procedural terminology codes). These records were reviewed daily for all enrolled patients and recorded using standardized data collection forms.
Data Analysis
Sample size was calculated a priori. Assuming a conservative 5% dichotomous ACVE incidence (based on prior literature),9 we calculated the need to enroll 1,500 patients to allow a 10:1 ratio of outcomes:covariates for the prediction model. We calculated 95% confidence intervals (CI) using the estimated standard error method. Normality testing of demographic data (e.g., age) was confirmed using the Shapiro-Wilk test. Chi-square (with two-tailed Fisher's exact test when appropriate) and t-tests were calculated for categorical and continuous variables, respectively, with 5% alpha (two-tailed).
The final multivariable model was built around four separate conceptual clusters (defined a priori on the basis of clinical experience and prior data,8 as demographic, clinical history, laboratory, and ECG variables) considered as separate groups to achieve a parsimonious and clinically meaningful model. Associations between clinical factors and the primary outcome were calculated first using univariate analysis. To avoid over-fitting of the model, the single most clinically significant variable from each conceptual cluster was then agreed upon by all senior study investigators and added to the multivariable regression, which included covariates that also achieved univariate significance (SPSS version 21.0). Regression model fit was assessed by the Nagelkerke method with R2 statistics. Missing data was handled by listwise deletion. Multicollinearity was assessed using the variance inflation factor. No interaction terms were introduced. The model was additionally tested for diagnostic performance using recursive partitioning analysis (JMP, SAS, Inc.). Diagnostic test characteristics (sensitivity, specificity, etc.) of the final model's prediction of the primary outcome were calculated with 95% CI.
Subgroup Analysis
As a pre-planned subgroup analysis in order to account for patient population heterogeneity and thus possible differential performance of the risk factors based on overdose intent, we calculated subgroup test characteristics after subdividing patients into each of the following groups: suicidal, recreational, therapeutic error, unknown/other intentions. In addition, we assessed performance of the risk factors in patients with single drug overdoses as well as multidrug overdoses. No adjustment was necessary for multiple comparisons, as there were fewer than five subgroups.
Results
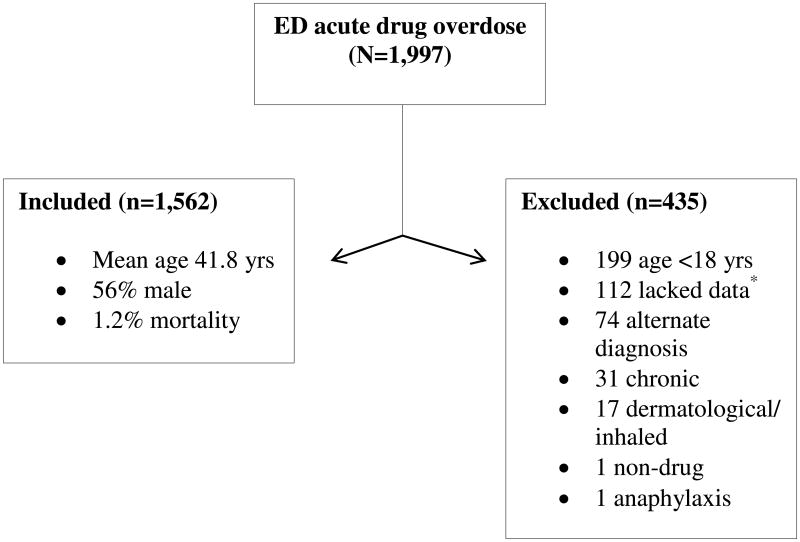
Over the course of the study period there were 1,997 suspected acute drug overdoses, of which 435 were excluded, yielding 1,562 individual subjects for analysis. Details of study enrollment are outlined in Figure 1. Baseline clinical characteristics of subjects who were included are summarized in Table 1.
Figure 1. Study Enrollment.
* Those lacking data left against medical advice, were transferred to an outside institution, or otherwise eloped from the hospital.
Table 1.
Clinical Characteristics of Patients in the Study.
| Clinical Characteristics | ACVE n=82 (5.3%) | NO ACVE n=1,475 (94.7%) |
|---|---|---|
|
| ||
| Median (IQR) or n (%) | ||
| Median age (yrs)† | 54.0 (43-67) | 40.0 (28-52) |
| Women | 39 (45) | 723 (49) |
| Past cardiovascular history | ||
| Hypertension* | 47 (57) | 391 (27) |
| Diabetes | 22 (27) | 247 (17) |
| Coronary disease* | 22 (27) | 88 (6) |
| Heart failure* | 15 (18) | 22 (1.5) |
| Cardiac disease† | 28 (34) | 96 (6.5) |
| Altered mental status* | 68 (83) | 917 (62) |
| ECG findings | ||
| Median heart rate | 96 (71.3-110) | 85 (74-103) |
| Median QRS | 102 (83-122) | 88 (75-100) |
| Median QTc† | 454 (428-485) | 432 (410-453) |
| Cutoff ≥ 500 ms† | 29 (35) | 152 (10) |
| Basic metabolic panel | ||
| Median bicarb (mEq/L)* | 24 (20.8-27.3) | 26 (24-29) |
| Cutoff < 20 mEq/L† | 14 (17) | 52 (3.5) |
| Number of exposures | ||
| 1 | 22 (27) | 491 (33) |
| 2 | 24 (29) | 459 (31) |
| 3 | 15 (18) | 256 (17) |
| 4+ | 15 (18) | 226 (15) |
| Unknown | 6 (7.3) | 48 (3.3) |
Univariate p<0.05
Univariate p<0.001
ACVE = adverse cardiovascular events; cardiac disease = coronary disease or heart failure; ECG = electrocardiogram; mEq/L = milliequivalents per liter; QTc = computer generated Bazett QT correction as printed on presentation ECG.
Primary Outcomes
All-cause mortality occurred in 18 patients representing 1.2% (95% CI = 0.6 to 1.7) of the entire cohort. Out of 1,562 included patients, there were 82 with the primary outcome of in-hospital ACVE (5.25% incidence, 95% CI = 4.1% to 6.4%). Of these, there were 61 (3.9%) patients with myocardial injury, 37 (2.3%) with shock, 23 (1.4%) with dysrhythmia, and 22 (1.4%) with cardiac arrest. Of the included patients, 1134 (72.6%) patients were missing troponin data, of whom 16 met criteria for ACVE via another outcome (four shock, five cardiac arrest, seven dysrhythmia), and 1,118 did not meet other outcome criteria and were thus coded as “no ACVE.”
Exposure Information
The median number of drug exposures in the cohort was two, and there no correlation between each additional number of drug exposures and ACVE incidence (see Table 1). The most common drug exposure class was opioids (n = 418), while the most common single drug was cocaine (n = 330). Opioid overdoses were most frequently oxycodone (n = 113, 7.1% ACVE), heroin (n = 88, 5.7% ACVE), and hydrocodone (n = 23, 4.35% ACVE). The most common antihypertensive overdoses were beta blockers (n = 28, 10.7% ACVE), calcium channel blockers (n = 22, 9.1% ACVE), and angiotensin converting enzyme (ACE) inhibitors (n = 13, 15.4% ACVE). The most frequent sympathomimetic exposure was cocaine (n = 330, 4.9% ACVE), while the most frequent over-the-counter exposures were ethanol (co-ingested in 273 patients) and acetaminophen (n = 178). The drug exposure with the highest ACVE incidence and mortality was digoxin (72.7% and 18.2%, respectively). Table 2 displays the top five exposures in each category.
Table 2.
Top Exposures According to Frequency, ACVE, and Mortality.
| Ranking | Most Common Drug Classes | ACVE Incidence | In-Hospital Mortality |
|---|---|---|---|
| 1 | Opioid (n=418) | Digoxin (n=8/11, 72.7%) | Digoxin (n=2/11, 18.2%) |
| 2 | Sympathomimetic (392) | ACE inhibitors (2/13, 15.4) | ACE inhibitors (2/13, 15.4) |
| 3 | Benzodiazepines (340) | Beta blockers (3/28, 10.7) | CCB (1/22, 4.6) |
| 4 | Antipsychotics (165) | Diuretics (1/10, 10.0) | Oxycodone (3/113, 2.7) |
| 5 | Antidepressants (163) | CCB (2/22, 9.1) | Heroin (2/88, 2.3) |
ACE = angiotensin converting enzyme; ACVE = adverse cardiovascular events; CCB = calcium channel blockers.
Univariate Analysis
Demographically, age was associated with ACVE, while sex had no association. Mean age in the ACVE group was 53.4 years, versus 38.2 years in the no event group (p < 0.001). The age cutpoint associated with the highest odds of ACVE was 50 years, which conferred over three-fold increased odds of ACVE (OR 3.05, 95% CI = 2.0 to 4.6). Males had 34% increased odds of ACVE, but this finding was not statistically significant (p = 0.58).
Main Analysis
In the main analysis to identify independent predictors of ACVE, we controlled for confounders by performing multivariable logistic regression (as described above in Methods). Covariates added to the regression model included the most clinically significant demographics (age, sex), clinical comorbidity (prior cardiac disease), laboratory result (serum bicarbonate), and ECG finding (QTc prolongation), as well as the remaining most highly statistically significant univariate predictors (ED site, mental status on presentation). Subsequent multivariable analysis with these variables yielded the following ACVE independent risk factors: QTc prolongation (3.8% prevalence > 500 ms, OR 27.6, p < 0.001), prior cardiac disease (7.1% prevalence, OR 9.5, p < 0.001), and low serum bicarbonate (5.4% prevalence under 20 mEq/L, OR 4.4, p < 0.01). Model fit was excellent, with R2 = 0.802 with negligible multi-collinearity (variance inflation factor < 5) of each variable. The final derived model is summarized in Table 3.
Table 3. Independent Predictors of ACVE.
| Risk Factor | OR (95% CI) | Adjusted OR* (95% CI) |
|---|---|---|
| QTc, (> 500 ms) | 25.7 (12.2-54.1) | 27.6 (11.1-69.1) |
| Bicarbonate, (<20 mEq/L) | 10.7 (5.9-19.4) | 4.4 (2.1-9.1) |
| Prior cardiac disease, (CAD or CHF) | 9.5 (5.6-16.4) | 9.5 (4.9-18.4) |
Multivariable model adjusted for: altered mental status, age, sex, and ED site.
CAD = coronary artery disease; CHF = congestive heart failure; OR = odds ratio; mEq/L = milliequivalents per liter; ms = milliseconds
ACVE = adverse cardiovascular events; QTc = Bazett's corrected QT interval
Recursive Partitioning Analysis
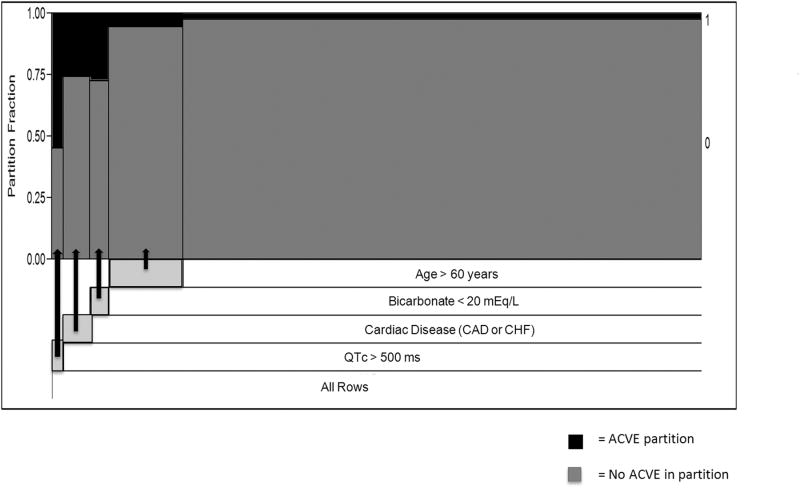
To maximize negative predictive value (NPV) of the main analysis, recursive partitioning was performed with four nodes using the above variables. The partitions displayed in Figure 2 represent the results of binary splits of the data that resulted in the top four successive partitions with the highest sensitivity at each step. The recursive partitioning model did not significantly improve on the NPV of the three-predictor model derived from logistic regression (98.10% vs. 97.51%, respectively).
Figure 2. Recursive Partitioning of the Risk Factors.
Recursive partitioning model using four binary nodes in order of highest to lowest sensitivity. Partition fraction (y-axis) denotes the ACVE percentage at each successive step, and x-axis spacing denotes numbers of individuals in each partition. The most efficient (i.e., higher diagnostic sensitivity) partitions start from the left (bottom) and move in decreasing order to the right (top).
ACVE = adverse cardiovascular events; QTc = Bazett's corrected QT interval; ms = milliseconds; mEq/L= milliequivalents per liter.
Prognostic Test Characteristics
Using the three independent predictors derived with logistic regression above (QTc > 500 ms, cardiac history, bicarbonate < 20 mEq/L), we analyzed the diagnostic test characteristics using these risk factors applied to the entire cohort. The decision rule (“positive” if ≥1 predictor, negative if 0 predictors) was highly specific (93.7%) and had excellent negative predictive value (97.1%). For those with zero risk factors, ACVE rates were exceptionally low (2.9%, 95% CI = 2.0% to 3.7%). In addition, the ACVE rate increased exponentially in the presence of multiple predictors, peaking at 90.9% (95% CI = 62.3% to 98.4%) ACVE rate for patients with two or more predictors. Table 4 displays a full overview of the test characteristics of the ACVE prediction rule for acute drug overdose patients.
Table 4. Test Characteristics of ACVE Prediction Rule in Acute Drug Overdose.
| # of Risk Factors* | ACVE Rate (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR- (95% CI) |
|---|---|---|---|---|---|---|
| Rule (≥1 factor) | 51.6 (41.1-62) | 93.7 (92.4-94.8) | 97.1 (96.2-97.9) | 8.2 (6.3-10.7) | 0.5 (0.3-0.6) | |
| 0 factors | 2.9 (2.0-3.7) | |||||
| 1 factor | 27.2 (20.0-34.6) | |||||
| 2+ factors | 90.9 (62.3-98.4) | |||||
ACVE = acute cardiovascular event; LR+ = likelihood ratio positive; NPV= negative predictive value; OR= odds ratio; QTc = Bazett's corrected QT interval.
Risk factors: 1) QTc prolongation, 2) cardiac disease, 3) low bicarbonate
Subgroup Analysis
Full results of the subgroup analysis are displayed in Table 5. The subgroup with the highest ACVE incidence was “unknown/other” intentions (10.87%, 95% CI = 3.67% to 23.58%). The rule had the highest sensitivity in patients with therapeutic errors (83.9%, 95% CI = 66.3% to 94.5%).
Table 5. Subgroup Analysis of ACVE Risk Factor Test Characteristics.
| Subgroup | ACVE n (%) (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|
| Intent | |||||
| Suicidal | 638 (3.6) (2.3-5.4) | 47.8 (26.9-69.4) | 82.9 (79.7-85.8) | 9.5 (4.8-16.3) | 97.7 (96.0-98.8) |
| Recreational | 494 (4.7) (2.9-6.9) | 60.9 (38.6-80.3) | 83.2 (79.5-86.5) | 15.1 (8.5-23.9) | 97.8 (95.8-98.9) |
| Therapeutic error | 359 (8.6) (5.9-12.0) | 83.9* (66.3-94.5) | 77.4 (72.5-81.9) | 26.0 (17.7-35.7) | 98.1 (95.6-99.4) |
| Other/Unknown | 46 (10.87) (3.7-23.6) | 40.00 (6.5-84.6) | 90.3* (76.9-97.2) | 33.3* (5.3-77.3) | 92.5 (79.6-98.3) |
| Exposures | |||||
| Single drug | 508 (4.5) (2.9-6.7) | 78.3 (56.3-92.5) | 83.5 (79.9-86.7) | 18.4 (11.3-27.5) | 98.8* (97.2-99.6) |
| Multi-drug | 1,031 (5.3) (4.0-6.9) | 60.0 (45.9-72.9) | 81.3 (78.7-83.7) | 15.3 (10.8-20.8) | 97.3 (95.9-98.3) |
| Total cohort | 1,562 (5.3) (4.1-6.4) | 51.6 (41.1-62) | 93.7 (92.4-94.8) | 16.4 (12.5-20.9) | 97.1 (96.2-97.9) |
Risk factors: 1) QTc prolongation, 2) cardiac disease, 3) low bicarbonate
The subgroup with the highest test characteristic in each column.
ACVE = adverse cardiovascular events; NPV = negative predictive value; PPV = positive predictive value.
Discussion
This study has derived independent predictors of ACVE that may, if validated in future studies, provide the basis of new clinical tools to risk-stratify drug overdose patients for the occurrence of in-hospital adverse events. The strongest predictors of ACVE were QTc prolongation present on the presentation ECG, prior history of either coronary artery disease or congestive heart failure, and metabolic acidosis present on the initial basic metabolic panel. The aggregate derived risk factors were able to identify populations at exceptionally low risk (2.9%, 95% CI = 2.0% to 3.7%) and high risk (90.9%, 95% CI = 62.3% to 98.4%) for in-hospital ACVE, as identified on ED presentation. Overall, the pre-test likelihood of ACVE was 5.4% (1 in 18.5), which could be reduced to 2.9% (1 in 35) in patients with no risk factors. Use of these risk factors, if validated in the future as a clinical decision rule, may allow for safer disposition of lower risk patients as well as intensive care unit (ICU) triage for those at highest risk.
We intentionally analyzed a heterogeneous study population in an attempt to enhance generalizability for ED clinicians taking care of acute drug overdoses. However, we realize that a drug overdose population in fact represents very distinct patient sub-populations – suicide attempts, unintentional exposures, recreational overdoses, etc. Each sub-population represents different age groups, exposures, and doses with resulting differences in outcomes. For example, older patients were more likely to overdose on cardiovascular medications and more likely to suffer ACVE. To further address the issue of heterogeneity, we performed a pre-planned subgroup analysis of the ACVE risk factors stratified by intent of exposure (recreational, therapeutic error, etc.), the results of which are demonstrated in Table 5. In this subgroup analysis, we demonstrate that the rule performs with excellent specificity and negative predictive value for various subgroups, even for those with unknown intent and therapeutic errors. Therefore, these results suggest the general application of these risk factors is appropriate across all overdose intents, as well as for both single- and multi-drug exposures. Furthermore, higher relative mortality in our study, compared to National Poison Data Systems data, can be explained by both enhanced data ascertainment and higher acuity in the present study, as well as missing follow-up information in data provided by the poison control center.
Poisoning is an infrequent cause of cardiac arrest in elderly patients, but is the leading cause of cardiac arrest in patients younger than 40 years of age.3,5,13 Urgent consultation with a medical toxicologist or regional poison control center is recommended in cases of cardiotoxicity, because standard guidelines for non-drug related emergency cardiovascular care may not apply to the management of acute overdose.14,15 Rather, administering a life-saving antidote or performing continuous telemetry monitoring may be the deciding factors for whether or not a patient survives a severe overdose. Currently, many recommendations for emergency care of drug-related cardiovascular events16 lack scientific foundations; evidence-based tools for decision-making in the ED management of drug overdose are urgently needed. While the clinical implications of serum cardiac troponin I elevations in drug overdose patients are of unclear significance, they are probably of different clinical significance than for ACS patients, due to differences in pathophysiology and etiology. Therefore, further research on the clinical implications of drug-induced myocardial injury are urgently needed. The results of the present study address this need; however, future studies to validate these findings are warranted in external sites with different patient populations and at-risk subgroups.
Drug toxicity may cause myocardial injury through a variety of mechanisms. Myocardial injury is the most common adverse cardiovascular event that occurs due to drug overdose.9 Serum cardiac troponin I is a specific enzyme that is released into the bloodstream following myocardial cell necrosis or injury from drug cardiotoxicity.17 According to published guidelines from the American Heart Association, the approach to patients with symptoms of drug-induced myocardial injury should differ in both diagnostic and therapeutic management.18 However, such guidelines relied on expert consensus due to lack of evidence-based tools to risk-stratify and guide management. The present study thus verifies and improves on these guidelines to optimize adverse event prevention.
Shock is the second most common ACVE that occurs due to drug overdose.9 In the ED and ICU, drug-induced shock must be treated with vasopressor drugs (e.g., norepinephrine) in order to maintain adequate blood pressure to prevent end-organ injury.19 The most specific biomarker for end-organ injury due to shock is lactic acid, also referred to as lactate.20 This study paves the way for future work to combine clinical risk scores (i.e., those derived here) with biomarkers (e.g., lactate) for improvement of overdose risk stratification.
Sudden cardiac death in a young healthy population is statistically most likely to be drug-related.21,22 It was recently demonstrated in a large clinical cohort that ventricular dysrhythmia is the third most common ACVE that occurs due to drug overdose.9 VF is probably the final common pathway of most sudden cardiac deaths, but rhythm disturbances may begin with monomorphic or polymorphic VT as well as torsade de pointes,23 a form of polymorphic VT that is identified characteristically on the ECG. The dichotomization of QTc around 500 ms in this study, in lieu of the “QT nomogram,”24 is justified by prior drug overdose data using this cutoff to predict ACVE,8 as well as data from Chan and colleagues that demonstrated that the QT nomogram performed essentially the same as the 500 ms cutoff at predicting poisoning-related torsade de pointes (98.7% vs. 97.2% specificity, respectively).24 This study demonstrates that VT/VF is much more common than torsade de pointes in this population, and that dysrhythmia occurrence may be predictable. Future studies should therefore evaluate treatment modalities (e.g., magnesium sulfate, antidysrhythmics) for those identified by this study to be high-risk.
The majority of drugs found to be most commonly associated with ACVE and in-hospital mortality in this study were, perhaps not surprisingly, digoxin, anti-hypertensives, and opioids. This refutes a common notion that opioid overdose deaths are primarily respiratory in nature, given that in-hospital respiratory arrest is exceedingly uncommon due to routine monitoring and advanced airway techniques. Furthermore, our finding that ACE inhibitors were commonly associated with ACVE and death, more so than even calcium channel blockers/beta blockers or opioids, is novel and deserves further study. One possible explanation is that patients who had access to ACE inhibitors also had poor cardiovascular substrate (e.g., congestive heart failure), which interfered with the ability to cope with any substantial cardiotoxic insult.
Limitations
Because specific causes for admission were not recorded during enrollment, it is difficult to distinguish between ACVE being the underlying cause for admission versus ACVE occurring unexpectedly after admission; however, both of these are important causes of morbidity, and future studies should continue to evaluate both. The urban affiliate hospitals in this study represent only one medical school, and thus may not be generalizable to all settings globally; therefore, external validation studies are warranted. Exposure confirmation through analytical testing was lacking in a subset of patients; however, suspicion of overdose based on history, clinical findings, or ancillary testing was documented by treating clinicians and the regional poison control center. Given the heterogeneity of the patient population, our models did not account for dose of poisoning of specific agents; thus, dose response curves were not included. The protocol did not involve kappa statistics for data abstraction because there was only one abstracter per chart, due to study constraints. Finally, while missing troponin data may have led to a slight under-estimation of ACVE incidence, it is likely that clinicians did not order the test because it was in fact not indicated (i.e., would be negative if measured).
Conclusions
We derived independent clinical predictors of adverse cardiovascular events for patients with acute drug overdoses. The absence of all risk factors had excellent negative predictive value to potentially aid cardiovascular medical clearance. Presence of two or more risk factors conferred extremely high risk of in-hospital adverse cardiovascular events, which may justify intensive care unit admission decisions. Future validation of these risk factors with application to specific at-risk subgroups is warranted.
Acknowledgments
We would like to thank our research assistants Amy Chen, Janice Lam, and Elizabeth McInness for their hard work on this project.
This study was funded by grant DA026476 (PI: AFM) from the National Institutes of Health. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
Presented at the XXXIII International Congress, European Association of Poisons Centres and Clinical Toxicologists, Copenhagen, Denmark, May 2013, and the Society for Academic Emergency Medicine, Annual Meeting, Atlanta, GA, May 2013.
Conflicts of Interest: None.
References
- 1.Centers for Disease Control and Prevention. [Accessed Feb 21, 2015];Wide-ranging Online Data for Epidemiologic Research (WONDER database) Available at: wonder.cdc.gov.
- 2.Warner M, Chen L, Makuc D. Increase in fatal poisonings involving opioid analgesics in the USA, 1999--2006. [Accessed Feb 21, 2015];NCHS Data Brief. Available at: http://www.cdc.gov/nchs/data/databriefs/db22.htm. [PubMed]
- 3.Bronstein AC, Spyker DA, Cantilena LR, Jr, Rumack BH, Dart RC. 2011 Annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 29th Annual Report. Clin Toxicol (Phila) 2012;50:911–1164. doi: 10.3109/15563650.2012.746424. [DOI] [PubMed] [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. [Accessed Feb 21, 2015];The DAWN Report: Highlights of the 2009 Drug Abuse Warning Network (DAWN) findings on drug-related emergency department visits. Available at: http://www.samhsa.gov/data/sites/default/files/DAWN127/DAWN127/sr127-DAWN-highlights.pdf. [PubMed]
- 5.McCaig LF, Burt CW. Poisoning-related visits to emergency departments in the United States, 1993-1996. J Toxicol Clin Toxicol. 1999;37:817–26. doi: 10.1081/clt-100102460. [DOI] [PubMed] [Google Scholar]
- 6.Manini AF, Yates C. Utility of the electrocardiogram in drug overdose and poisoning: theoretical considerations and clinical implications. Clin Cardiol Rev. 2012;8:137–51. doi: 10.2174/157340312801784961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albertson TE, Dawson A, de Latorre F, et al. American Heart Association; International Liaison Committee on Resuscitation. TOX-ACLS: toxicologic-oriented advanced cardiac life support. Ann Emerg Med. 2001;37(4 Suppl):S78–90. doi: 10.1067/mem.2001.114174. [DOI] [PubMed] [Google Scholar]
- 8.Manini AF, Nelson LS, Skolnick AH, Slater W, Hoffman RS. Electrocardiographic predictors of adverse cardiovascular events in suspected poisoning. J Med Toxicol. 2010;6:106–15. doi: 10.1007/s13181-010-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manini AF, Nelson LS, Stimmel B, Vlahov D, Hoffman RS. Incidence of adverse cardiovascular events in adults following drug overdose. Acad Emerg Med. 2012;19:843–9. doi: 10.1111/j.1553-2712.2012.01397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warner M, Chen LH, Makuc DM, Anderson RN, Miniño AM. NCHS data brief, no 81. Hyattsville, MD: National Center for Health Statistics; 2011. Drug poisoning deaths in the USA, 1980–2008. [PubMed] [Google Scholar]
- 11.New York State. Public Health Law § 2500-d. [Accessed Feb 21, 2015]; Available at: http://www.health.ny.gov/regulations/recently_adopted/docs/2012-01-18_distributions_health_care_initiatives_pool_poison_control_center_operations.pdf.
- 12.Gilbert EH, Lowenstein SR, Koziol J, Barta DC, Steiner J. Chart reviews in emergency medicine research: where are the methods? Ann Emerg Med. 1996;27:305–8. doi: 10.1016/s0196-0644(96)70264-0. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Increases in age-group-specific injury mortality --United States, 1999-2004. MMWR Morbid Moral Wkly Rep. 2007;56(49):1281–4. [PubMed] [Google Scholar]
- 14.Facility assessment guidelines for regional toxicology treatment centers. American Academy of Clinical Toxicology. J Toxicol Clin Toxicol. 1993;31:211–7. doi: 10.3109/15563659309000387. No authors listed. [DOI] [PubMed] [Google Scholar]
- 15.American College of Emergency Physicians. Poison information and treatment systems. American College of Emergency Physicians. Ann Emerg Med. 1996;28:384. [PubMed] [Google Scholar]
- 16.Sayre MR, Koster RW, Botha M on behalf of the Adult Basic Life Support Chapter Collaborators. Part 5: adult basic life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122(suppl 2):S298–324. doi: 10.1161/CIRCULATIONAHA.110.970996. [DOI] [PubMed] [Google Scholar]
- 17.Gaze DC, Collinson PO. Cardiac troponins as biomarkers of drug- and toxin-induced cardiac toxicity and cardiac protection. Expert Opin Drug Metab Toxicol. 2005;1:715–25. doi: 10.1517/17425255.1.4.715. [DOI] [PubMed] [Google Scholar]
- 18.Vanden Hoek TL, Morrison LJ, Shuster M, et al. Part 12: cardiac arrest in special situations: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(suppl 3):S829–61. doi: 10.1161/CIRCULATIONAHA.110.971069. [DOI] [PubMed] [Google Scholar]
- 19.Levine M, Curry SC, Padilla-Jones A, Ruha AM. Critical care management of Verapamil and Diltiazem overdose with a focus on vasopressors: a 25-year experience at a single center. Ann Emerg Med. 2013;62(3):252–8. doi: 10.1016/j.annemergmed.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Manini AF, Kumar A, Olsen D, Vlahov D, Hoffman RS. Utility of serum lactate to predict drug-overdose fatality. Clin Toxicol (Phila) 2010;48(7):730–6. doi: 10.3109/15563650.2010.504187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–82. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 22.Roden D, Woosley R, Primm R. Incidence and clinical features of the quinidine-associated long QT syndrome: implications for patient care. Am Heart J. 1986;111:1088–93. doi: 10.1016/0002-8703(86)90010-4. [DOI] [PubMed] [Google Scholar]
- 23.Viskin S. Long QT syndromes and torsades de pointes. Lancet. 1999;354:1625–33. doi: 10.1016/S0140-6736(99)02107-8. [DOI] [PubMed] [Google Scholar]
- 24.Chan A, Isbister GK, Kirkpatrick CM, Dufful SB. Drug-induced QT prolongation and torsades de pointes: evaluation of a QT nomogram. QJM. 2007;100(10):609–15. doi: 10.1093/qjmed/hcm072. [DOI] [PubMed] [Google Scholar]