Abstract
Background
From 1993 through 1995, the Pediatric Oncology Group (POG) enrolled patients with high-risk neuroblastoma on 3 sequential, conjoined studies: a phase II induction window (9340), followed by intensive multiagent induction chemotherapy (9341), and subsequent myeloablative therapy with autologous stem cell rescue (9342). We report here the outcomes of patients treated on these studies.
Patients and Methods
Patients were between one and 21 years old with high-risk neuroblastoma. Phase II window therapy consisted of 2 courses of either paclitaxel, topotecan, or cyclophosphamide wth topotecan for the phase II window. Induction therapy consisted of at least five cycles of intensive chemotherapy, followed by myeloablative therapy with purged autologous stem cell reinfusion. Patient responses, treatment toxicities, and overall and event-free survival rates were calculated.
Results
84% of patients responded to induction chemotherapy, with 39% achieving complete response. Toxicities were primarily hematologic. The 7-year EFS and OS rates for all eligible patients on POG 9341 were 23% +/− 4% and 28% +/− 4%, respectively. The 7-year EFS and OS rates for patients treated on POG 9342 were 27% +/− 6% and 29% +/− 6%, respectively.
Conclusions
These studies were the first attempt by POG to use autologous stem cell transplantation for neuroblastoma treatment in a cooperative group setting. Toxicities and outcomes were comparable to contemporary cooperative group studies. The phase II induction window had no detectable effect on outcomes. New strategies are needed to improve survival for this devastating disease.
Keywords: neuroblastoma, autologous stem cell transplantation
Introduction
Neuroblastoma is the most common extracranial tumor of childhood. Approximately 40% of neuroblastoma cases are considered high risk. Recent improvements in outcomes have been attributed to improved supportive care and more intensive chemotherapy regimens [1]. However, overall survival rates for children with high-risk neuroblastoma remain at approximately 30% with current treatment regimens combining intensive chemotherapy, surgery, radiation, and autologous stem cell transplantation [2–7].
In an attempt to further intensify treatment, myeloablative chemotherapy followed by autologous stem cell rescue (ASCR) has been used in small studies with mixed results [8–15]. A Pediatric Oncology Group (POG) pilot study using ASCR reported a 32% two-year event-free survival (EFS) for children with high-risk neuroblastoma treated after complete response to induction [16]. More recent cooperative group trials report 26–53% EFS and overall survival (OS) rates [2–4,7].
In 1993, POG opened a tandem treatment series of 3 studies for children with high-risk neuroblastoma: an upfront phase II induction window (POG 9340), followed by intensive induction chemotherapy (POG 9341) with surgery and local radiation therapy, and subsequent myeloablative therapy with ASCR (POG 9342). Response results from POG 9340 have been previously reported [17]. We report here long-term outcomes and toxicities for patients with high-risk neuroblastoma treated with intensive chemotherapy and ASCR on these studies.
Methods
Eligibility criteria for POG 9341/9342
Patients were enrolled between 1993 and 1995 at participating POG institutions. Eligible patients were between 365 days and 21 years of age with untreated metastatic neuroblastoma (INSS stage 4) or with stage 2B or 3 disease if the MYCN gene was amplified, and could have received one cycle of treatment following the POG 9244 protocol [18]. Histological verification of disease was required, with the exception of patients with tumor cells in the bone marrow and elevation of urine catecholamine levels.
Patients were enrolled initially onto POG 9340. However, patients could be entered directly onto POG 9341 in cases of life-threatening disease or parent/patient refusal of Phase II treatment. Direct enrollment onto POG 9341 also occurred between the second and third phase II windows. Patients enrolled on 9341 were eligible for 9342 if they had attained complete response (CR), partial response (PR), or minor response (MR) to induction therapy (Supplemental Appendix I), with negative bilateral bone marrow aspirates and biopsies by light microscopy and with <5% tumor cells detected by immunofluorescence.
Patient evaluation
Initial evaluation of patients included CT or MRI imaging of the primary site, with bone scan, skeletal survey, bone marrow aspirates and biopsies, urine catecholamine levels and renal and liver function studies. Pathology was confirmed by central review or by the combination of bone marrow samples containing tumor cells with elevated urine catecholamine levels. MYCN gene amplification was determined by Southern blot analysis [19] before or fluorescent in situ hybridization (FISH) analysis after July 1993 [20]. Written informed consent was obtained from patients or their guardians according to National Cancer Institute guidelines. Protocols were approved by participating institutional review boards prior to patient enrollment.
Treatment
Patients initially enrolled on POG 9340 were treated with up to two courses of paclitaxel, topotecan, or cyclophosphamide/topotecan (Table I). Patients were then enrolled on POG 9341 for 5 cycles of induction therapy (Table I), followed by attempted resection and an additional cycle of CAV; patients with persistent tumor in the bone marrow could receive additional courses of IE, and repeated courses could be given for patients declining ASCR. Chemotherapy could be delayed up to 14 days for neutropenia or thrombocytopenia or for elevated serum creatinine or bilirubin. Doses were not reduced for hematologic toxicity.
Table I.
Chemotherapy Regimens on POG 9340, 9341, 9342
| study | drug | schedule |
|---|---|---|
| POG 9340 | paclitaxel | 350 mg/m2 IV over 24 hours every 14 days |
| topotecan | 2 mg/m2/day IV for 5 days every 21 days | |
| cyclophosphamide/topotecan | 250 mg/m2/day IV for 5 days every 21 days | |
| 0.75 mg/m2/day IV, for 5 days every 21 days | ||
| POG 9341 | ||
| courses 1,5 (HDP/VP) | cisplatin | 40mg/m2/day IV on days 1 through 5 |
| etoposide | 100mg/m2/dose IV twice daily on days 1 through 3 | |
| course 2 (CAV) | cyclophosphamide | 1000mg/m2/day IV on days 1 and 2 |
| doxorubicin | 60mg/m2 IV on day 1 | |
| vincristine | 1.5mg/m2/day IV on days 1, 8, and 15 | |
| course 3 (IE) | ifosfamide | 2g/m2/day IV on days 1 through 5 |
| etoposide | 75mg/m2/dose IV twice per day on days 1 through 3 | |
| course 4 (CB/VP) | carboplatin | 500mg/m2/day IV on days 1 and 2 |
| etoposide | 75mg/m2/dose IV twice per day on days 1 through 3 | |
| POG 9342 | ||
| days −6 to −4 | carboplatin | 667mg/m2/day IV |
| etoposide | 800mg/m2/day IV | |
| days −3 to −2 | cyclophosphamide | 60mg/kg/day IV |
At the completion of induction therapy, eligible patients were enrolled on POG 9342 for myeloablative chemotherapy and ASCR with purged bone marrow. Bone marrow stem cells were harvested if immunofluorescent testing demonstrated <5% marrow tumor cells with a peripheral platelet count of at least 100,000/mL and neutrophil count (ANC) of at least 1000/mL. For ASCR, patients required at least 4×108 nucleated marrow cells/kg. Bone marrow was purged using immunomagnetic bead purging with a mixture of antibodies UJ13A (anti-NCAM), M340 (anti-L1 CAM), UJ127.11 (anti-L1 CAM), Thy-1 (anti-CD 90), and 5.1.H11 (anti-NCAM), that reacts with >95% of neuroblastoma cells from >90% of patients [21].
Following bone marrow harvesting, patients were treated with radiation to the primary tumor site with a 2cm margin to 2400cGy in 20 fractions over 10 days (radiation at diagnosis to sites of symptomatic metastases was permitted). Myeloablative therapy began 7 to 10 days following radiation therapy (Table I). GM-CSF (10mcg/kg/day) was given after bone marrow infusion until the ANC reached 1000/mL.
Disease evaluations were performed after the completion of phase II therapy, after the second and fifth cycles of induction chemotherapy or within three weeks preceding ASCR, at the completion of therapy, and at any time that disease progression was suspected. Responses were measured and submitted by individual institutional investigators. Determination of the best disease response prior to transplant was made at approximately week 15 after the fifth course, or after subsequent cycles of continuation therapy given prior to transplantation. After the completion of therapy, clinical, laboratory, and radiologic disease evaluations were required per protocol every 3 months for the first year, and then every 6 months for the second year. Thereafter, clinical and laboratory evaluations (including urine catecholamines) were required every six months.
Results
Patient characteristics
A total of 146 patients were enrolled on POG 9341, of whom 4 were ineligible (2 with incorrect diagnosis, one with progressive disease after the phase II window, and one without pathology specimen submission). 74 of these patients were enrolled on POG 9342, of whom 6 were ineligible (3 also ineligible for POG 9341, one with residual tumor in the marrow, one with progressive disease prior to transplant, and one registration error) (Figure 1).
Figure 1.
Distribution of patients enrolled on POG 9341 and 9342.
100 patients were initially treated on the phase II window trial POG 9340, of whom 92 were subsequently enrolled on POG 9341. 88 of these patients were eligible (see above), of whom 24 had received paclitaxel, 32 topotecan, and 31 cyclophosphamide plus topotecan. Two of these patients had a CR to phase II window therapy, 25 had a PR, 26 had MR, 20 had SD, and 14 had PD [17]. 54 patients enrolled on POG 9341 without prior phase II therapy, 32 during a 4-month interval between the second and third phase II window options, 13 who presented with stage 2B or 3 disease, and 9 due to patient/parent or physician choice.
The study patient characteristics are summarized in Table II. 80 of the patients on POG 9341were male and 62 were female. The median age at diagnosis was 3.3 years (range: 1.1 – 17.8 years). Of the 68 eligible patients treated on POG 9342, 39 were male and 29 were female. The median age at diagnosis was also 3.3 years (range: 1.1 – 14 years). 17 patients (12.0%) enrolled on POG 9341 and 10 patients (14.7%) enrolled on POG 9342 were between 12 and 18 months of age at diagnosis.
Table II.
Characteristics of Eligible Patients on POG 9341 and 9342
| Cohort | POG 9341 n (%) |
POG 9342 n (%) |
|---|---|---|
|
| ||
| Overall Eligible | 142 (100%) | 68 (100%) |
|
| ||
| Age | ||
| 12–18 months | 17 (12%) | 10 (14.7%) |
| >18 months | 125 (88%) | 58 (85.3%) |
|
| ||
| MYCN status | ||
| Non-amplified | 79 (61%) | 40 (63%) |
| Amplified | 51 (39%) | 24 (37%) |
| Unknown | 12 | 4 |
|
| ||
| Ploidy | ||
| Diploid | 52 (42%) | 27 (46%) |
| Hyperdiploid | 69 (56%) | 32 (54%) |
| Hypodiploid | 2 (2%) | |
| Unknown | 19 | 9 |
|
| ||
| INSS Stage | ||
| Stage 2b | 1 (0.7%) | 1 (1.5%) |
| Stage 3 | 12 (8.5%) | 5 (7.3%) |
| Stage 4 | 129 (90.8%) | 62 (91.1%) |
|
| ||
| Primary tumor site | ||
| Adrenal | 85 (59.9%) | |
| Retroperitoneal | 33 (23.2%) | |
| Thoracic | 11 (7.7%) | |
| Other abdominal | 4 (2.8%) | |
| Pelvic | 3 (2.1%) | |
| Insufficient data | 3 (2.1%) | |
| Adrenal & thoracic | 2 (1.4%) | |
| No known primary | 1 (0.7%) | |
|
| ||
| Toxicities (grade 4 only) | ||
| Neutropenia | 133 (93.6%) | 61 (92.4%) |
| Thrombocytopenia | 127 (89.4%) | 60 (90.9%) |
| Anemia | 25 (17.6%) | 6 (9.1%) |
| Sepsis/infection | 16 (11.3%) | 9 (13.6%) |
| Electrolyte abnormalities | 3 (2.1%) | 0 |
| Hearing loss | 3 (2.1%) | 0 |
| Hemorrhage | 2 (1.4%) | 0 |
| Diarrhea | 2 (1.4%) | 2 (3.0%) |
| Other | 2 (1.4%) | 8 (12.1%) |
POG 9341 Induction
Three patients received only a single cycle of induction therapy (one patient died after the first cycle, one developed progressive disease after the first cycle, and one patient was lost to follow-up after the first cycle). Five patients received two to four induction cycles (3 patients had disease progression, one patient refused further therapy, one patient transferred to a different institution and was lost to follow-up). The remaining 134 patients received at least five cycles of induction chemotherapy. 44 patients received an additional one to five courses of post-induction chemotherapy. The mean number of chemotherapy induction cycles per patient was 5.6 +/− 1.0. Toxicities are listed in Table II.
Of the 44 patients who received additional cycles of chemotherapy, 15 eventually enrolled on POG 9342. 11 of the 44 patients received additional cycles due to organizational delays in the stem cell transplant process, of whom 7 subsequently were enrolled on POG 9342 and 3 received other stem cell transplants. 22 of the 44 patients received additional cycles in order to achieve a better response. 8 of these 22 patients were subsequently enrolled on POG 9342, all of whom had residual positive bone marrow analysis but cleared with additional treatment. 5 of the 44 patients received additional chemotherapy due to parental refusal of the transplant, and 6 received other stem cell transplants or other forms of therapy.
Out of the 134 patients treated on POG 9341 who received at least five cycles of induction chemotherapy, 48 (35.8%) patients achieved CR, 65 (48.5%) PR, 11 (8.2%) MR, 6 (4.5%) had SD, and 4 (3.0%) had PD. Of the 13 patients with INSS stage 2B or 3 disease, 12 attained CR and 1 attained PR.
60.2% (53/88) of the patients treated initially with phase II therapy on POG 9340 attained CR, PR, or MR prior to enrolling on POG 9341, with 46 of 63 patients (73.0%) responding to topotecan or to cyclophosphamide-topotecan. Response to the phase II window therapy did not correlate with later response to induction on POG 9341 therapy or to overall outcomes [17](and data not shown). Of the 54 patients who enrolled on POG 9341 without prior window therapy, 25 patients attained CR, 20 PR, 3 MR, 4 SD and 2 developed PD, and there was no statistical difference in response to 9341 therapy regardless of whether patients had received prior treatment with POG 9340 therapy (70/88 attained CR/PR/MR, 79.5%) or not (48/54, 88.9%).
POG 9342 Stem Cell Transplantation
68 eligible patients underwent ASCR on POG 9342. 28 patients were transplanted in CR, 37 were transplanted in PR, and 2 in MR. Of the 72 patients treated on POG 9341 who did not enroll on 9342, 19 were reported to have undergone stem cell transplantation off protocol. 4 patients received allogeneic stem cell transplants, 8 received stem cell transplants with peripheral blood stem cells, 3 patients received other stem cell transplants, and 4 patients transferred to other institutions for stem cell transplant therapy of unknown type. Other reasons for not enrolling on POG 9342 are listed in Table III.
Table III.
Off-Study Reasons for Patients Not Eligible for POG 9342
| Off-Study Reasons | n | % |
|---|---|---|
| wrong diagnosis | 1 | 1.4 |
| lost to follow up | 1 | 1.4 |
| protocol violation | 1 | 1.4 |
| refusal of further treatment | 2 | 2.9 |
| death | 2 | 2.9 |
| other/unknown | 14 | 19.4 |
| other bone marrow transplant | 19 | 27.1 |
| relapsed/progressive disease | 32 | 45.7 |
The time from patient diagnosis to ASCR varied from 171 to 377 days, with a mean of 234 days and a median of 228 days. The median time to engraftment post-transplantation was 26.5 days with a mean of 28.7 days (range 14–120). Toxicities are listed in Table II. There were no toxic deaths.
Outcomes
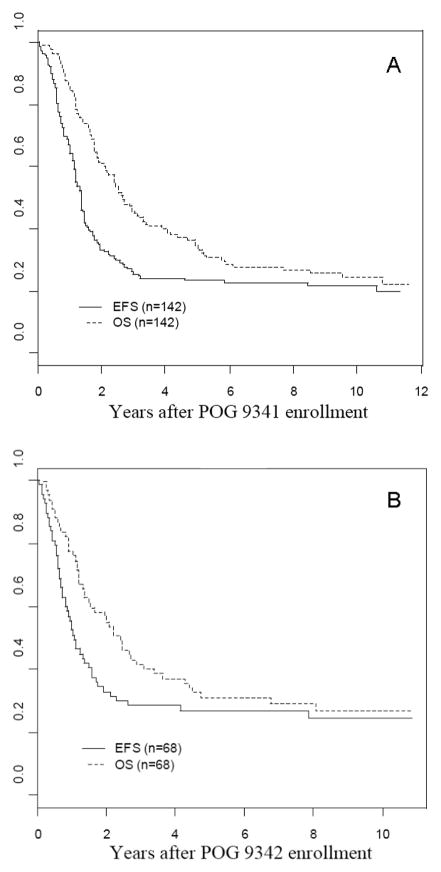
The 7-year EFS and OS rates for 142 patients on POG 9341 were 23% +/− 4% and 28% +/− 4%, respectively (Figure 2A). The 7-year EFS and OS rates for the 68 patients on POG 9342 were 27% +/− 6% and 29% +/− 6%, respectively (Figure 2B). Outcomes for the 100 patients initially enrolled on and eligible for POG 9340 have been previously reported, with no reported difference in outcome for patients not treated with phase II window therapy [17].
Figure 2.

A - Kaplan-Meier event-free and overall survival curves for 142 eligible patients on POG 9341, B - Kaplan-Meier event-free and overall survival curves for 68 eligible patients on POG 9342, C - Kaplan-Meier event-free and overall survival curves for 118 patients with CR, PR, or MR after induction on POG 9341.
For the 118 pts who had induction response of CR, PR, or MR on 9341, the 7-year EFS and OS rates were 25% +/− 4% and 30% +/− 5%, respectively (Figure 2C). Patients who received additional cycles of induction therapy prior to enrollment on POG 9342 had equivalent 7-year survival rates (EFS 30±9%, OS 33±10%) to those who achieved CR on scheduled therapy (EFS 24±9%, p=0.8536; OS 24±9%, p=0.6512).
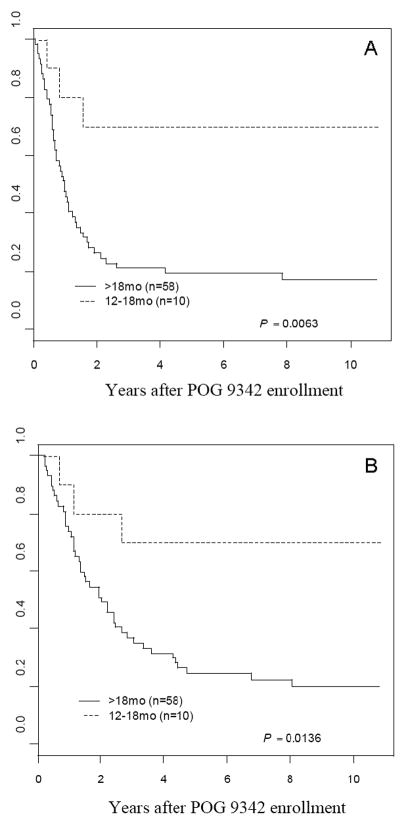
Patients aged 12–18 months at diagnosis (n=10) enrolled on POG 9342 had significantly higher EFS OS and OS rates than those over 18 months of age at diagnosis (n=58) (7-year EFS: 70±17% vs. 19±6%, p=0.0063; 7-year OS: 70±17% vs. 22±6%, p=0.0136) (Figure 3). Of the 68 patients on POG 9342, there were only 5 stage 3 patients. This sample size is too small for a logrank comparison of stage 3 to the rest of the cohort. However, only one of these patients had an event, as compared (descriptively) to the stage 4 patients who had 7-year EFS and OS rates of 25±7% and 27±7%, respectively.
Figure 3.
A - Kaplan-Meier event-free survival (EFS) curve for patients on POG 9342 between 12 and 18 months of age at diagnosis (dotted line) and over 18 months of age (solid line), B- Kaplan-Meier overall survival (OS) curve for patients on POG 9342 between 12 and 18 months of age at diagnosis (dotted line) and over 18 months of age (solid line).
Of the 142 patients treated on POG 9341, 111 were reported as having an event. 49 (44.1%) of these events occurred within one year of the onset of therapy, and 94 (84.7%) occurred within two years of therapy onset. For the 68 patients enrolled on POG 9342, 50 were reported as having an event. Of these 50, 31 (62%) occurred within one year of enrollment and 45 (90%) occurred within two years of enrollment.
Discussion
Overall survival rates for children with high-risk neuroblastoma are approximately 30% with current treatment [2–7]. Autologous stem cell transplantation has been shown in cooperative group trials to improve EFS, but not OS, rates in children with high-risk neuroblastoma [3,7]. We report here the results of the first cooperative group trial by the Pediatric Oncology Group (POG) to determine the outcomes and toxicities of intensive chemotherapy in combination with ASCR for children with high-risk neuroblastoma.
Our patient cohort appears similar to other contemporary cooperative group protocols for high-risk neuroblastoma treatment, with 89% of patients older than 18 months at diagnosis, 91% having INSS stage 4 disease, and 38% of tumors having N-myc gene amplification. By comparison, Matthay et al. also included patients with Evans stage III disease with concurrent elevated serum ferritin levels or unfavorable histology [3,24], while Berthold et al. included patients with stage 1 and 2 disease with N-myc gene amplification [7]. Other cooperative group trials excluded patients with stage 2 or 3 disease and N-myc gene amplification [2,4]. This early national cooperative group study encountered difficulties with successive protocol patient registration, data collection, and prompt access to stem cell transplantation facilities. Some patients and physicians also refused treatment with the upfront phase II window therapy. Despite these challenges, this study represents one of the earliest cooperative group trials completed successfully incorporating stem cell transplantation for neuroblastoma.
No toxic deaths occurred during either induction treatment or stem cell transplantation. Reported toxicities were primarily hematologic and were expected with the intensity of therapy administered. The Children’s Cancer Group [3] reported a similar 71% incidence for all grade 3 or 4 toxicities during induction, and a recent German study [7] reported a 95% incidence of grade 3 and 4 bone marrow toxicity during induction.
83.1% of patients achieved CR, PR, or MR on POG 9341, with no statistical difference in response whether patients had received phase II window treatment (70/88, 79.5%) or not (48/54, 88.9%). 8 of 22 patients (36.3%) who received additional cycles of chemotherapy were able to attain CR or PR status and undergo ASCR on 9342, suggesting additional induction chemotherapy may be beneficial in select cases.
Our response rates and long-term outcomes are comparable to other contemporary stem cell transplant protocols. A 3-year progression-free survival rate of approximately 40% was reported [3] for patients surviving induction and autologous stem cell transplantation, without maintenance therapy with retinoic acid. The 5-year progression free survival rate was 24% for 436 patients in the European Bone Marrow Transplantation Registry treated with single bone marrow transplantation [2]. Berthold et al. reported a 47% 3 year EFS rate for patients treated with stem cell transplantation [7], but these patients received additional immunotherapy with ch14.18 or retinoic acid, and some patients received additional I-MIBG therapy and radiation therapy.
The outcomes for patients aged 12–18 months treated on POG 9342 were clearly improved compared to older patients. More recent trials have also concluded that this population of patients has a better prognosis [25], and recent COG trials have been modified to exclude these patients from high-risk therapy. Unfortunately, due to the small number of stage 3 patients enrolled on POG 9342, we are unable to determine whether have significantly improved outcomes compared to the remainder of the cohort. Other cooperative group studies have shown that stage 3 patients treated using high-risk neuroblastoma protocols have improved outcomes [7,26], suggesting a small population that may benefit from reduced therapy intensity.
Cooperative group studies that have compared autologous stem cell transplantation with continued chemotherapy in randomized fashion reported improved EFS/PFS rates with transplantation, but with no difference in OS rates [3,7]. The survival rate for patients treated on POG 9342 was not significantly better than the entire POG 9341 population. A sizeable fraction (19/72, 26%) of patients not enrolled on POG 9342 underwent myeloablative therapies off study, and patient selection may have affected the outcomes of patients on POG 9342. The absence of post-transplant therapy with retinoic acid or other immunotherapy also likely resulted in lower EFS rates for this patient cohort compared to more recent trials employing ASCR.
In summary, the sequential POG 9340/9341/9342 studies represent the first attempt by POG to utilize ASCR in a cooperative group setting for the treatment of children with high-risk neuroblastoma. We report similar response rates, toxicities, and EFS and OS rates when compared to contemporary cooperative group neuroblastoma trials utilizing stem cell transplantation. Initial phase II therapy had no effect on response rates or overall outcomes. Novel treatment strategies are needed to improve the overall outcomes in children with high-risk neuroblastoma.
Supplementary Material
Acknowledgments
We would like to acknowledge the contributions of Vijay Joshi, MD, in the design and execution of this study.
Abbreviations
- POG
Pediatric Oncology Group
- CCG
Children’s Cancer Group
- ASCR
autologous stem cell rescue
- CR
complete response
- PR
partial response
- MR
minimal response
- SD
stable disease
- PD
progressive disease
- CT
computed tomography
- MRI
magnetic resonance imaging
- FISH
fluorescent in situ hybridization
- ANC
absolute neutrophil count
- INSS
International Neuroblastoma Staging System
- EFS
event-free survival
- PFS
progression-free survival
- OS
overall survival
- MIBG
meta-iodobenzylguanidine
References
- 1.Cheung NV, Heller G. Chemotherapy Dose Intensity Correlates Strongly with Response, Median Survival, and Median Progression-Free Survival in Metastatic Neuroblastoma. J Clin Oncol. 1991;9:1050–8. doi: 10.1200/JCO.1991.9.6.1050. [DOI] [PubMed] [Google Scholar]
- 2.Ladenstein R, Philip T, Lasset C, et al. Multivariate Analysis of Risk Factors in Stage 4 Neuroblastoma Patients Over the Age of One Year Treated with Megatherapy and Stem Cell Transplantation: A Report from the European Bone Marrow Transplantation Solid Tumor Registry. J Clin Oncol. 1998;16:953–65. doi: 10.1200/JCO.1998.16.3.953. [DOI] [PubMed] [Google Scholar]
- 3.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of High-Risk Neuroblastoma with Intensive Chemotherapy, Radiotherapy, Autologous Bone Marrow Transplantation, and 13-cis-Retinoic acid. New Engl J Med. 1999;341:1165–73. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 4.Frappaz D, Michon J, Coze C, et al. LMCE3 Treatment Strategy: Results in 99 Consecutively Diagnosed Stage 4 Neuroblastomas in Children Older than 1 Year at Diagnosis. J Clin Oncol. 2000;18:468–76. doi: 10.1200/JCO.2000.18.3.468. [DOI] [PubMed] [Google Scholar]
- 5.Kaneko M, Tsuchida Y, Mugishima H, et al. Intensified Chemotherapy Increases the Survival Rates in Patients with Stage 4 Neuroblastoma with MYCN Amplification. J Pediatr Hematol Oncol. 2002;24:613–21. doi: 10.1097/00043426-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 6.de Bernardi B, Nicolas B, Boni L, et al. Disseminated Neuroblastoma in Children Older than One Year at Diagnosis: Comparable Results with Three Consecutive High-Dose Protocols Adopted by the Italian Co-Operative Group for Neuroblastoma. J Clin Oncol. 2003;21:1592–601. doi: 10.1200/JCO.2003.05.191. [DOI] [PubMed] [Google Scholar]
- 7.Berthold F, Boos J, Burdach S, et al. Myeloablative Megatherapy with Autologous Stem-Cell Rescue Versus Oral Maintenance Chemotherapy as Consolidation Treatment in Patients with High-Risk Neuroblastoma: A Randomized Controlled Trial. Lancet Oncol. 2005;6:649–58. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 8.Pritchard J, McElwain TJ, Graham Pole J. High-Dose Melphalan with Autologous Marrow for Treatment of Advanced Neuroblastoma. Br J Cancer. 1982;45:86–94. doi: 10.1038/bjc.1982.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.August CS, Serota FT, Koch PA, et al. Treatment of Advanced neuroblastoma with Supralethal Chemotherapy, Radiation, and allogeneic or autologous bone marrow reconstitution. J Clin Oncol. 1984;2:609–16. doi: 10.1200/JCO.1984.2.6.609. [DOI] [PubMed] [Google Scholar]
- 10.Hartmann O, Lumbroso JD, Lemerle M, et al. Repeated High-Dose Chemotherapy Followed by Purged Autologous Bone Marrow Transplantation as Consolidation Therapy in Metastatic Neuroblastoma. J Clin Oncol. 1987;5:1205–11. doi: 10.1200/JCO.1987.5.8.1205. [DOI] [PubMed] [Google Scholar]
- 11.Berthold F, Bender-Gotze C, Dopfer R, et al. Myeloablative Chemo- and Radiotherapy with Autologous and Allogenic Bone Marrow Reconstitution in Children with Metastatic Neuroblastoma. Klin Padiatr. 1988;200:221–5. doi: 10.1055/s-2008-1033712. [DOI] [PubMed] [Google Scholar]
- 12.Kushner BH, Gulati SC, Kwon JH, et al. High-Dose Melphalan with 6-Hydroxydopamine Purged Autologous Bone Marrow Transplantation for Poor-Risk Neuroblastoma. Cancer. 1991;68:242–7. doi: 10.1002/1097-0142(19910715)68:2<242::aid-cncr2820680204>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Dini G, Lanino E, Garaventa A, et al. Myeloablative Therapy and Unpurged Autologous Bone Marrow Transplantation for Poor-Prognosis Neuroblastoma: Report of 34 Cases. J Clin Oncol. 1991;9:962–9. doi: 10.1200/JCO.1991.9.6.962. [DOI] [PubMed] [Google Scholar]
- 14.Corbett R, Pinkerton R, Pritchard J, et al. Pilot Study of High-Dose Vincristine, Etoposide, Carboplatin, and Melphalan with Autologous Bone Marrow Rescue in Advanced Neuroblastoma. Eur J Cancer. 1992;28A:1324–8. doi: 10.1016/0959-8049(92)90509-z. [DOI] [PubMed] [Google Scholar]
- 15.Kletzel M, Becton DL, Berry DH. Single Institution Experience with High-Dose Cyclophosphamide, Continuous Infusion Vincristine, Escalating Doses of VP-16-213, and Total Body Irradiation with Unpurged Bone Marrow Rescue in Children with Neuroblastoma. Med Pediatr Oncol. 1992;20:64–7. doi: 10.1002/mpo.2950200114. [DOI] [PubMed] [Google Scholar]
- 16.Pole JG, Casper J, Elfenbein G, et al. High-Dose Chemoradiotherapy Supported by Marrow Infusions for Advanced Neuroblastoma: A Pediatric Oncology Group Study. J Clin Oncol. 1991;9:152–8. doi: 10.1200/JCO.1991.9.1.152. [DOI] [PubMed] [Google Scholar]
- 17.Kretschmar CS, Kletzel M, Murray K, et al. Response to Paclitaxel, Topotecan, and Topotecan-Cyclophosphamide in Children with Untreated Disseminated Neuroblastoma Treated in an Upfront Phase II Investigational Window: A Pediatric Oncology Group Study. J Clin Oncol. 2004;22:4119–26. doi: 10.1200/JCO.2004.08.174. [DOI] [PubMed] [Google Scholar]
- 18.Strother D, van Hoff J, Rao PV, Smith EJ, Shamberger RC, Halperin EC, Murray KJ, Castleberry RC. Event-Free Survival of Children’ with Biologically Favourable Neuroblastoma Based on the Degree of Initial Tumour Resection: Results from the Pediatric Oncology Group. Eur J Cancer. 1997;33:2121–5. doi: 10.1016/s0959-8049(97)00293-1. [DOI] [PubMed] [Google Scholar]
- 19.Brodeur GM, Seeger RC, Schwab M, et al. Amplification of N-myc in Untreated Human Neuroblastomas Correlates with Disease Stage. Science. 1984;224:1121–4. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro DN, Valentine MB, Rowe ST, et al. Detection of N-myc Gene Amplification by Fluorescence In Situ Hybridization. Diagnostic Utility for Neuroblastoma. Am J Pathol. 1993;142:1339–46. [PMC free article] [PubMed] [Google Scholar]
- 21.Bowman L, Grossmann M, Rill D, et al. IL-2 Adenovector-Transduced Autologous Tumor Cells Induce Antitumor Immune Responses in Patients with Neuroblastoma. Blood. 1998;6:1941–9. [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 23.Peto R, Pike MC, Armitage P, et al. Design and Analysis of Randomized Clinical Trials Requiring Prolonged Observation of Each Patient. II. Analysis and Examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans AE. Staging and Treatment of Neuroblastoma. Cancer. 1980;45:1799–802. [PubMed] [Google Scholar]
- 25.London WB, Boni L, Simon T, et al. The Role of Age in Neuroblastoma Risk Stratification: The German, Italian, and Children’s Oncology Group Perspectives. Canc Lett. 2005;228:257–66. doi: 10.1016/j.canlet.2004.12.054. [DOI] [PubMed] [Google Scholar]
- 26.Kaneko M, Tsuchida Y, Uchino J, et al. Treatment Results of Advanced Neuroblastoma With the First Japanese Study Group Protocol. J Pediatr Hematol Oncol. 1999;21:190–7. doi: 10.1097/00043426-199905000-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.