Abstract
Osteoarthritis (OA) is associated with articular cartilage abnormalities and affects people of older age: preventative or therapeutic treatment measures for OA and related articular cartilage disorders remain challenging. In this perspective review, we have integrated multiple biological, morphological, developmental, stem cell and homeostasis concepts of articular cartilage to develop a paradigm for cartilage regeneration. OA is conceptually defined as an injury of cartilage that initiates chondrocyte activation, expression of proteases and growth factor release from the matrix. This regenerative process results in the local activation of inflammatory response genes in cartilage without migration of inflammatory cells or angiogenesis. The end results are catabolic and anabolic responses, and it is the balance between these two outcomes that controls remodelling of the matrix and regeneration. A tantalizing clinical clue for cartilage regrowth in OA joints has been observed in surgically created joint distraction. We hypothesize that cartilage growth in these distracted joints may have a biological connection with the size of organs and regeneration. Therefore we propose a novel, practical and nonsurgical intervention to validate the role of distraction in cartilage regeneration in OA. The approach permits normal wake-up activity while during sleep; the index knee is subjected to distraction with a pull traction device. Comparison of follow-up magnetic resonance imaging (MRI) at 3 and 6 months of therapy to those taken before therapy will provide much-needed objective evidence for the use of this mode of therapy for OA. We suggest that the paradigm presented here merits investigation for treatment of OA in knee joints.
Keywords: articular cartilage, osteoarthritis, cartilage injury, cartilage repair, cartilage regeneration, cartilage organ size, osteoarthritis treatment, joint distraction, nonsurgical intervention
Introduction
It is estimated that about 10% of the world’s population aged 60 years or older have significant clinical problems that could be attributed to osteoarthritis (OA) [Cooper et al. 2013]. The healthcare burden of OA on the society is enormous because this disease progressively affects the ageing population. Moreover, the ever changing population demographics further increase the cost of healthcare to society, and add pain and disability to a large segment of the population. Trauma, degenerative joint diseases, metabolic factors such as obesity and mechanical factors such as joint instability are among the common aetiological factors of OA [Felson, 2009]. Presently, there are no pharmacological agents for the prevention or treatment of OA; the only medical option for OA involves pain management. In this context, cartilage tissue engineering has not held the high promises of therapy in OA [Huey et al. 2012] and the potential for cartilage regeneration through stem cell therapy has not yet fully materialized [Djouad et al. 2009; Johanson et al. 2012]. Accordingly, total joint replacement, though expensive, is considered a final option for relieving pain and regaining function in patients with OA [Gossec et al. 2011].
Articular cartilage is thought to have limited regeneration potential [Huey et al. 2012]. However, it has been observed that symptomatic pain relief and cartilage regeneration are possible in OA joints that have been surgically pulled apart or distracted for a prolonged periods of time [Wiegant et al. 2013]. This might have been a clue that cartilage regeneration is possible in OA joints. However, the mechanism(s) by which cartilage growth might occur in the distracted joint space are unknown. In this perspective, we analyse the biological aspects of cartilage development, including injury and growth, and present a conceptual context for cartilage regeneration in the distracted OA joints. Accordingly, we propose a novel paradigm for nonsurgical joint distraction treatment of OA.
Cartilage structure
Articular cartilage is a unique tissue between the ends of bones in the joints that helps in load bearing and lubrication. It is rich in extracellular matrix with scattered chondrocytes [Poole, 2005]. Adult cartilage has zonal architecture consisting of superficial, middle, and deep zones [Grogan et al. 2013]. These zones vary in matrix biochemical composition, cell density and morphology, cell metabolism and the pericellular matrix (PCM) [Wilusz et al. 2014]. The superficial zone extends ~10–20% of the full thickness of the articular cartilage. Chondrocytes in this zone are elongated, flattened, oriented parallel to the cartilage surface and are surrounded by densely packed collagen fibrils with low levels of aggrecan but higher concentrations of decorin and biglycan [Poole et al. 1996]. Superficial zone proteins homologous to proteoglycan 4 are encoded by the Prg4 gene, which provides lubrication and a load-bearing surface for the articular cartilage [Ikegawa et al. 2000]. The middle or transitional zone comprises ~40–60% of the cartilage thickness. Chondrocytes in this middle zone are surrounded by randomly organized collagen type II fibrils with high concentrations of aggrecan, hyaluronic acid and dermatan sulfate (Figure 1). However, chondrocytes in the deep zone are ellipsoid, and these cells are stacked in cell columns surrounded by a specialized PCM and interspersed by a radially oriented collagen matrix [Poole, 2005; Wilusz et al. 2014]. Between the deep zone of cartilage and calcific cartilage in adults is a specialized amorphous material resembling a basement membrane called the tidemark (Figure 1).
Figure 1.
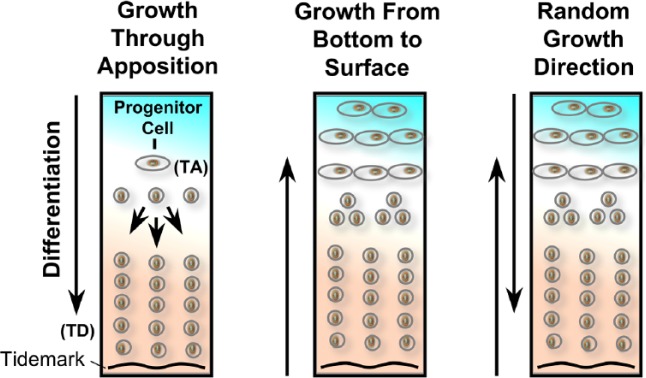
Hypothetical models of development of articular cartilage. Left panel shows growth through apposition. Middle panel suggests growth from bottom of cartilage attached to the bone towards cartilage surface in the joint cavity. Right hand panel suggests cartilage growth could be random from bottom and top of the cartilage.
Left side of figure is modified from Hayes et al. [2001]
TA, transit-amplifying; TD, terminal differentiation.
Cartilage development
Development of articular cartilage and synovial joint structures is tightly synchronized [Koyama et al. 2008; Sandell, 2012; Iwamoto et al. 2013]. Initially at the prospective joint sites, expression of Sox9/Col2/doublecortin (DCX) in anlagen occurs in response to undefined upstream morphogenetic and determination mechanisms, and results in the expression of Gdf5 in the interzone mesenchymal population [Decker et al. 2014]. This specific population of Gdf5-expressing joint progenitor interzone cells maintain DCX expression [Zhang et al. 2011]. In developing (digits) joints, Tgfbr2 positive cells reach the interzone sites in spatiotemporal distribution [Li et al. 2013]. First, Tgfbr2 positive cells are limited to the dorsal and ventral regions but undetected in the central region of the interzone [Spagnoli et al. 2007; Li et al. 2013]. Later on, anlagen-bound chondrocytes turn on the expression of matrillin-1. Gdf5 cells that subsequently express Sox9/Col2, and are positive for the expression of matrillin-1, differentiate into all other chondrocytes, while cells negative for mitrillin-1 expression evolve into articular chondrocytes [Hyde et al. 2007]. This process involves specific action of regulatory genes including Erg and PTHrP [Iwamoto et al. 2007; Chen et al. 2008; Li et al. 2013]. Postnatally, Gdf5 and Tgfbr2-positive and -negative interzone cells and their progeny give rise to multiple joint tissues including articular cartilage, intrajoint ligaments, synovial lining cells and other joint-specific structures such as the meniscus in the knee [Decker et al. 2014]. Moreover, Tgfbr2 expressing cells constitute the slow cycling stem/progenitor cell population that are distributed with specific cell niches of joint such as in the groove of Ranvier [Li and Xie, 2005; Li et al. 2013].
Observations made through examining cartilage development in mice that are mutant for Noggin, Wnt4/Wnt9a, Tgfbr2 or β-catenin revealed the presence of joint ablations and cartilage fusions. Similarly, mouse embryos deficient in the Tgfβ-activated kinase 1, heparin sulfate synthase Ext1 or Indian hedgehog (Ihh) also demonstrated joint defects [Gunnell et al. 2010; Mundy et al. 2011]. Furthermore, mouse embryo mutants for the master chondrogenic genes, Sox5 and Sox6, also display defects of limb skeletogenesis and joint development [Dy et al. 2010]. Similarly, the absence of the zinc finger transcription factors Osr1 and Osr2 limits the sustained expression of Gdf5, Wnt4 and Wnt9a in interzone cells leading to fusion of limb joints [Gao et al. 2011]. Also, ablation of C-Jun, which is an upstream regulator of Wnt9a, results in deranged Wnt signalling and lack of progression of joint formation [Kan and Tabin, 2013]. Collectively, these molecular regulators act at both local and distant levels and participate in the regulation of gene expression in interzone cells, and therefore control their cell fate and function. Adding to the ever expanding list of factors regulating cartilage development that were delineated above, low expression of chemokines and in particular MCP-5, compared with growth plate chondrocytes, is also required for interzone cells and joint formation [Longobardi et al. 2012].
Developing joints next undergo cavitation to create the synovial cavity that is filled with lubricants containing the synovial fluid. It has been known for a long time that immobilization by experimental nerve damage inhibits cavity formation in the developing joints [Fell and Canti, 1934]. Joint movement stimulates the establishment of joint superficial cell phenotype and the production of essential lubricants such as hyaluronate and lubricin [Dowthwaite et al. 2003]. Recent studies using muscleless mouse embryo mutants have revealed that joint progenitor cell fate is not maintained in these embryos; neither Wnt/β-catenin signalling is activated nor the joints have formed properly [Pazin et al. 2012]. Further studies revealed that there might be differential requirements for muscle-derived movements at joints such as the elbow joint compared with a more essential requirement for Tgfbr2 signalling for knee joint formation [Pazin et al. 2012]. Nevertheless, data from ongoing research studies that are examining joint development indicate that, developmentally, articular chondrocytes are different from growth plate hypertrophic chondrocytes [Iwamoto et al. 2013]. Perhaps these studies may provide a key towards our understanding of cartilage regeneration and finding clues for treatment of cartilage injury.
Cartilage stem cells
After skeletal maturity, articular chondrocytes stop proliferating and are slow to replace. These cellular characteristics are also exhibited by stem cells [Blanpain et al. 2007]. When cultured, isolated articular chondrocytes form cobblestone sheets of overlapping cells and cartilage-like tissue. Chondrocytes exhibit characteristics of transformed cells, such as anchorage-independent growth, preference of a rounded cell shape and growth in nude mice [Benya and Shaffer, 1982; Dell’accio, 2001]. These cell characteristics have been mostly associated with the cell biology of chondrocytes and need further reconsideration in the context of the features of cartilage stem cells.
Several independent research groups have demonstrated that cells isolated from the superficial zone of adult articular cartilage from various sources including human tissues have progenitor cell characteristics such as high colony formation and expression of mesenchymal stem cell markers [Dowthwaite et al. 2004; Hattori et al. 2007]. Furthermore, after multiple passages, these cells express a chondrogenic phenotype and, in response to Tgfb, cells respond with enhanced synthesis of lubricin and cartilage specific matrix components [Dowthwaite et al. 2004; Hattori et al. 2007]. Furthermore, Tgfb and Wnt/β-catenin signalling regulate proliferation and differentiation of these cells [Dowthwaite et al. 2004; Hattori et al. 2007].
Immunohistochemistry of normal cartilage sections shows large numbers of chondrocytes with staining for stem cell markers such as Notch-1, Stro-1 and VCAM-I throughout various zones [Grogan et al. 2009]. Stem cell surface markers such as CD44, CD151, CD105 and CD166 have also been demonstrated in chondrocytes [Alsalameh et al. 2004]. Interestingly, Hoechst 33342 dye exclusion, a feature of stem cell side population, is equally distributed in normal and OA chondrocytes [Grogan et al. 2009]. Furthermore, normal and osteoarthritic articular cartilage contains high number of CD105 and CD166 cells. In particular, the cell population containing CD166-positive cells have strong chondrogenic potential and were exclusively located in the superficial and middle zone [Pretzel et al. 2011].
Studies have also demonstrated that the synovium and infrapatellar fat pad contain mesenchymal stem cells, and it has been suggested that these progenitor cells could be utilized for cell-based tissue engineering and treatment of cartilage regeneration [De Bari et al. 2001; Dragoo et al. 2003; Futami et al. 2012]. Mesenchymal stem cells have also been found in the synovial fluid of normal knee joints and their number increases in synovial fluid of diseased joints [Jones et al. 2008; Morito et al. 2008]. Karlsson and colleagues studied BrdU labelling of adult rabbit knee joints and showed that cell labelling in the perichondrial groove of Ranvier is indicative for progenitor cells [Karlsson et al. 2009]. Moreover, these progenitor cells also stained for stem cell markers including Stro-1, Jagged1 and BMPr1a [Karlsson et al. 2009]. As described above, Tgfbr2 expressing cells contribute to joint development and populations of these slow cycling stem/progenitor cells are present in the perichondrial groove of Ranvier, synovium, articular cartilage superficial layer and tendon sites of joints. These authors suggested that this might be a specific site for a stem cell niche in the joint that could have significant roles in repair and regeneration [Li et al. 2013]. However, given these observations, the progenitor cells in perichondrial groove of Ranvier will have an anatomical and physical barrier to traverse to the site of injury in the cartilage that usually occurs where the joint is subjected to repeated traumas – away from groove of Ranvier. However, it is still possible that progenitor cells of groove of Ranvier could be involved in increasing the circumference of the developing adult articular cartilage.
Bone marrow is rich source of hematopoietic and mesenchymal stem cells [Li and Xie, 2005]. Based on this concept, procedures for marrow stimulation including transcortical Pridie drilling, abrasion arthroplasty and microfracture rely on mesenchymal stem cells and growth factors coming from the bone marrow and have been advocated for cartilage regeneration [Schindler, 2011]. Koelling and colleagues observed that chondrogenic progenitor cells migrate to the damaged articular cartilage from the bone marrow through the subchondral bone [Koelling et al. 2009].
Collectively, it is unclear if there is a special niche within the confines of the articular cartilage that might support stem cell regeneration. Differentiated chondrocytes showing stem cell markers indicate the transit-amplifying nature of these cells but their significance in cartilage regeneration is still unclear [Blanpain et al. 2007; Doupe et al. 2012; Tata et al. 2013].
Cartilage maintenance
Homeostasis of the articular cartilage is maintained by the right balance between aggrecan and collagen contents to provide a physiochemical structure for compressive and tensile strength for joint load and mobility [Poole, 2005]. Aggrecan molecules are noncovalently associated with collagen fibrils and minor conditions such as immobility can cause its loss from the cartilage [Vertel, 1995]. However, chondrocytes rapidly replenish aggrecan. On the other hand, human collagen type II in cartilage matrix is a long-lived protein, with an estimated halflife of >117 years [Verzijl et al. 2000]. Thus, its breakdown, synthesis and maturation are complex and prolonged processes, and may be key limiting factors for regeneration of cartilage. Attrition of superficial chondrocytes by mechanical forces in the joint is likely replaced through its progenitor activity in a homeostatic process. However, the middle and deep zones of cartilage show slow turnover of cells or matrix.
Cartilage injury and mechanisms of repair
Ideally, a cartilage injury should heal without a scar formation. Scar tissues within the joint would interfere with the smooth surface and would have deleterious mechanical properties. However, embryonic skin wounds heal without scar formation and are achieved by rapid re-epithelialization of wounds with little involvement from basal epidermal cells [Degen and Gourdie, 2012]. Embryonic wound edges are pulled together in a purse-string manner and small wounds in the gut epithelium also heal in this fashion [Degen and Gourdie, 2012; Polk and Frey, 2012]. In an in vitro wound model of bovine and human cartilage, it was observed that superficial zone chondrocytes rapidly proliferated, migrated and spread over the injured cartilage surface in a manner resembling re-epithelialization of wounds [Seol et al. 2012]. A cartilage injury does not involve fibrin clot formation, recruitment of inflammatory cells or angiogenesis [Martin, 1997]. However, cartilage tissue actively resists vascularization by producing anti-angiogenic components such as thrombospondin-1, chondromodulin-1, SPARC (secreted protein acidic and rich in cysteine), collagen type II derived N-terminal propeptide (PIIBNP), and the type XVIII derived endostatin [Patra and Sandell, 2012]. Furthermore, calcific cartilage and tidemarks are two anatomical barriers for the vascularization of articular cartilage. In OA, there are multiple depositions of tidemarks, as if to ward off vascularization from the subchondral bone. In support of this notion, injection of vascular growth factors in the joint cavity induces OA demonstrating its adverse effect in the joint [Ludin et al. 2013].
Murine and other animal models of OA usually involve cutting the medial meniscotibial ligament of knee joints. Microarray analysis of genes expressed in whole joints or microdissected tissues of joints, including articular cartilage, meniscus and epiphysis, which are obtained from these OA models, have advanced the molecular understanding of cartilage wound healing. A cartilage injury sets in motion a series of molecular events, which has parallels in other wounds, such as the expression of proteases and the release of growth factors [Martin, 1997; Wong et al. 2013]. In response to injury, there is a specific activation of inflammatory response genes in the injured cartilage. The inflammatory gene responses that follow injured cartilage are not simply the same as those induced by agents such as interleukin 1α (IL-1α), and they are also independent of the effects of either gene activation or products from noncartilaginous tissues of the joints. In the injured cartilage, there is activation of the three MAP kinases JNK, p38 and extracellular-signal-regulated kinase (ERK), as well as Src kinases, phosphatidylinositol 3-kinase (PI3K) and NF-kB [Chong et al. 2013; Watt et al. 2013]. In addition, hosts of other genes are also activated in injured cartilage, including those coding for cytokines and chemokines such as IL-1α, IL-6 and CCL2 [Burleigh et al. 2012].
The injury response in cartilage can be categorized into catabolic and anabolic. Catabolic activity that is most pertinent to cartilage is the release of the aggrecan-degrading enzyme disintegrin, metalloproteinase with thrombospondin motifs 5 (ADAMTS-5) and the collagenase matrix metalloproteinase 13 (MMP-13), which specifically degrade type II collagen. ADAMTS-5 is released early in response to injury and MMP-13 expression shows a delayed response [Burleigh et al. 2012]. In contrast, the anabolic activity includes the induction of chondroprotective genes such as tumor necrosis factor-inducible gene 6 protein (TSG-6), hyaluronan-binding anti-inflammatory molecule, tissue inhibitor of metalloproteinases-1 (TIMP-1) and activin A – a transforming growth factor beta (TGF-β) family member [Burleigh et al. 2012].
Several mechanisms control the signalling of inflammatory gene responses in a cartilage injury. Cartilage is a mechanosensitive tissue and perturbation of the matrix could signal the activation of chondrocyte surface receptors, such as that of integrins, calcium ion channels, primary cilium and discoidin domain receptors [Leong et al. 2011; Burleigh et al. 2012; Vincent, 2013]. When the integrity of the PCM is breached and subjected to cyclic compression, this results in the release of stored growth factors [Vincent, 2013]. The PCM is a specialized matrix that immediately surrounds chondrocytes and is rich in the nonfibrillar type VI collagen and the heparan sulfate proteoglycan perlecan, within which are sequestered various growth factors such as fibroblast growth factors (FGFs), connective tissue growth factor, insulin-like growth factor (IGF) and members of the TGF-β superfamily such as activin, bone morhogenetic protein (BMP) and their inhibitors [Vincent, 2013]. Of these, FGFs have become significant, and FGF-2 and FGF-18 are the most studied. FGF-2, through its interaction with FGF receptor (FGFR)-1, promotes cartilage degradation and the engagement with FGFR-3 drives joint protection [Ellman et al. 2013]. In mouse models, FGF-2 delays cartilage degradation. Mice in which the gene for FGF-2 has been deleted develop accelerated OA spontaneously and following joint destabilization [Chong et al. 2013]. In the human cartilage, engagement of FGF-2/FGFR-1 acts as an antagonist by enhancing ADAMTS-5 and MMP-13 induction [Ellman et al. 2013]. However, engagement of FGF-18/FGFR-3 results in an anabolic effect in human articular chondrocytes, as reflected by increased chondrocyte viability and cell-associated matrix formation [Ellman et al. 2013]. In addition to the TGF family of proteins and growth factors, other factors such as Wnt signalling molecules are also liberated following cartilage injury [Dell’accio et al. 2008]. All of these growth factors are involved in the normal cartilage organogenesis and would therefore also play a role in OA cartilage.
Taken together, articular chondrocytes and the extracellular matrix participate in cartilage injury. The first task for the repair and regeneration of cartilage is chondrocyte activation and the release of cells from their locations in the matrix. This is achieved mainly by the release of ADAMTS-5 and MMP-13. Reactive oxygen radical production by activated chondrocytes may additionally facilitate the breakdown of the matrix [Tiku et al. 2000]. Once the damaged tissue is degraded, chondrocytes with progenitor and migratory abilities populate the injured site and begin the regenerative process [Morales, 2007]. In the superficial zone, the repair is rapidly achieved by superficial zone chondrocytes. However, the breakdown and regeneration of the middle zone to deeper layers of cartilage could be a slow process. Until recently, it was generally believed that cartilage regeneration is almost impossible in mammals. However, it has been observed in murine models that articular cartilage regeneration is heritable in that some murine strains are robust healers while other strains are nonhealers [Rai et al. 2013; Rai and Sandell, 2014].
There is a body of evidence that shows attempts of cartilage regeneration occurring in the middle and deep zones. For example, studies in OA have revealed that there is an initial increase in matrix synthesis and cell proliferation. Chondrocyte clusters are formed in the middle and deep zones of cartilage [Brandt et al. 2008; Lotz et al. 2010]. However, in spite of these reparative processes, cartilage regrowth is generally not materialized in OA. The reasons for the failure of cartilage regrowth are unclear. Lessons from cartilage engineering have provided additional clues; for example, the lateral integration of neo-cartilage to adjacent cartilage is rarely observed [Huey et al. 2012]. Also, vertical fissures in OA cartilage have been known to fail to heal, indicting a failure of lateral integration of diseased OA cartilage tissue [Brandt et al. 2008].
Directional growth of articular cartilage
Superficial zone chondrocytes are thought to derive the appositional growth of cartilage (Figure 1) [Hayes et al. 2001]. This has enforced the notion that articular cartilage grows from the inside of a joint cavity towards the bone. This concept is at variance with the dynamics of development of other tissues, such as skin and mucosal linings of the gastrointestinal tract, which develop outwards from the corresponding underlying tissues. An embryonic stem cell population treated with Gdf5 and cyclopamine, a specific Hedgehog inhibitor, derived in vitro cartilage tissue, and showed zonal organization with columnar chondrocytes at the basal layer, a collagen type II-rich tidemark, and a distinct superficial layer [Craft et al. 2013]. This evidence indicates that cartilage can develop from the bottom up (Figure 1), suggesting that the mechanisms and dynamics of articular cartilage growth are still unresolved; in that regrowth may occur from top or bottom or may be random. Implications of these concepts also depend on the different levels of severity OA joints (Figure 2) and suggest that there might be a need of a layer of chondrocyte-rich matrix in ulcerated lesions that can be coaxed for regrowth.
Figure 2.
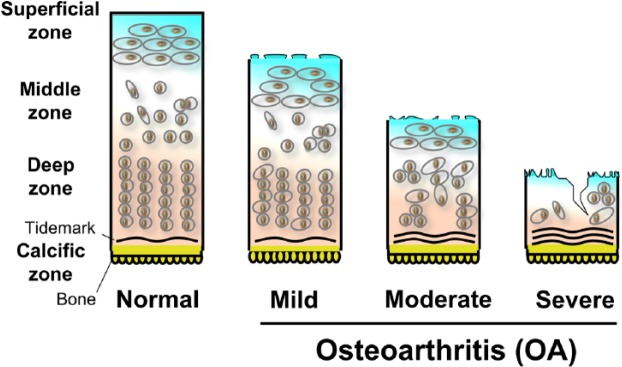
Diagrams representing normal and osteoarthritic (OA) cartilage. OA cartilage is depicted as mild, moderate and severe.
Distraction of OA joints helps cartilage regeneration
Recent investigations of the role of joint distraction in OA therapy provide a clue for cartilage regeneration [Marijnissen et al. 2002; Wiegant et al. 2013]. The rationale for distracting joints is based on the leg-lengthening surgical procedure of Ilizarov. In this procedure, the broken ends of the bone are continually distracted. The bone is then filled with cartilaginous tissue, which is later converted into bone through the bone’s healing process. The newly formed bone in the distracted space provides solid bone tissue for lengthening the leg. In joint distraction, it is presumed that new cartilage tissue formed in a distracted space is pliable like normal cartilage and able to withstand compression and exhibit tensile properties. There is some corroborative evidence indicating that new cartilage tissue formed in distracted joints exhibits cartilage-associated properties [Wiegant et al. 2013]. The mechanism of cartilage regrowth in distracted joint space is not known. Moreover, factors that control tissue or organ dimensions in the body in general are poorly understood, except to some extent in certain pathological conditions [Zhao et al. 2011; Tumaneng et al. 2012]. Transcription co-activators such as Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) are known to play a role in organ size control and regeneration, and it appears that these two processes are closely related. Also, these transcription factors have been identified as sensors and mediators of mechanotransduction, including in chondrocytes, suggesting that they play roles in cartilage regeneration [Dupont et al. 2011; Zhao et al. 2011; Zhong et al. 2013]. Another possibility is that negative pressure created in a distracted joint may accelerate cartilage wound healing. This concept is supported by the observations of the effects of negative pressure on the skin wound healing process [Orgill and Bayer, 2013; Schluck et al. 2013].
Although joint distraction by surgery provides a therapeutic opportunity for OA, it may not be an optimum or practical undertaking for a number of reasons. The surgical procedure for distraction is a serious undertaking that involves a prolonged period of convalescence, with associated adverse effects such as pin tract infection. This procedure usually involves joint immobilization, which can lead to unfavourable effects and cause atrophy of cartilage. In addition, the lack of cyclic compression on the cartilage can eliminate any beneficial anti-inflammatory and anabolic effects of mechanotransduction on chondrocytes [Leong et al. 2011].
Monitoring cartilage degeneration and regeneration
The lack of consistently reliable tools to monitor cartilage degeneration and regeneration has hindered clinical studies in OA. Recent studies have indicated an advantage of magnetic resonance imaging (MRI) studies of joints [Guermazi et al. 2013]. An MRI examination can detect early abnormalities of cartilage, ligaments, menisci, synovial joints, bone and bone marrow. An MRI examination shows a three-dimensional visualization of cartilage and can provide quantitative, semi-quantitative and biochemical assessments of cartilage. Abnormal features in an MRI can predict rapid cartilage loss [Roemer et al. 2009, 2012]. These observations indicate that it is possible to monitor cartilage pathology by MRI in OA [Hunter et al. 2011]. Moreover, molecular assays, gene expression profiling and biomarkers of OA severity such as microRNAs [Beyer et al. 2015] could be used as secondary outcome measures to group responders and nonresponders based on MRI studies for more qualitative measures and better understanding of the mechanisms of repair.
Paradigm of nonsurgical distraction therapy of OA
Considering the aforementioned observations, we suggest a study paradigm of cartilage regeneration in OA. Patients with OA of the knee would have MRI confirmation of their disease. According to MRI-defined qualitative, quantitative and biochemical assessment, cartilage degeneration may be graded as mild, moderate or severe (Figure 2). Mild disease is defined as being confined to the upper third of the cartilage; moderate disease extends up to two-thirds of the cartilage, while severe disease extends beyond these anatomical limits (Figure 2). Patients with mild or moderate cartilage degradation, with or without osteophytes or subchondral bone lesions with compartmental disease, will enter the clinical study protocol. Exclusion criteria would include a history of trauma, ligament or meniscal injury, obesity, joint instability or factors that could predispose to rapid cartilage degradation. Patients in the proposed pilot study will be allowed a normal wake-up activity of the index-knee joint using knee unloader braces to reduce stress in the diseased compartment and keep the opposing cartilage from causing mechanical friction [Lafeber et al. 2006]. During sleep, the index-knee joint will be subjected to a pull traction device to separate the bone ends of the knee joint and enhance negative intra-articular pressure. It should be noted that sleep-time traction would sufficiently distract the joint. An MRI study will be repeated at 3 and 6 months of therapy or later. In a blinded manner, the baseline MRI values and values obtained at the midpoint and endpoint of the study will be compared. The MRI technique will provide sophistication and precision in measuring cartilage and bone changes, bone marrow lesions, as well as the biochemical changes of cartilage. An advantage of the proposed study is that it is a noninvasive medical intervention and allows reasonable daily activity. In addition, it allows joint movements and mechanotransduction of chondrocytes without adverse effects of distraction on cartilage growth. Recently, patellofemoral braces have been shown to effectively reduce symptoms and bone marrow lesions in patients with patellofemoral OA [Callaghan et al. 2015]. Furthermore, studies of combination of subchondral drilling, joint motion and distraction promoted repair of osteochondral defect in the weight bearing area in a rabbit model of cartilage injury, therefore suggesting that joint distraction may be advantageous for cartilage regeneration [Kajiwara et al. 2005]. Distraction of osteoarthritic ankles also helps in the remodelling of subchondral bone [Wiegant et al. 2013]. We believe that the paradigm presented here merits full investigation for the treatment of OA.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Rutgers-Robert Wood Johnson Medical School, Rutgers Cancer Institute of New Jersey, NIH, and Department of Defence generously support the authors of this work. H.E.S. is founder and stockholder of Celvive, Inc., a company with profiles focused on stem cell therapy technologies. M.T. declares no conflicts of interest in preparing this article.
Contributor Information
Moti L. Tiku, Department of Medicine, Robert Wood Johnson Medical School, New Brunswick, NJ 08903-2681, USA
Hatem E. Sabaawy, Rutgers Cancer Institute of New Jersey, Robert Wood Johnson Medical School, New Brunswick, NJ, USA
References
- Alsalameh S., Amin R., Gemba T., Lotz M. (2004) Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum 50: 1522–1532. [DOI] [PubMed] [Google Scholar]
- Benya P., Shaffer J. (1982) Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30: 215–224. [DOI] [PubMed] [Google Scholar]
- Beyer C., Zampetaki A., Lin N., Kleyer A., Perricone C., Iagnocco A., et al. (2015) Signature of circulating micrornas in osteoarthritis. Ann Rheum Dis 74: e18. [DOI] [PubMed] [Google Scholar]
- Blanpain C., Horsley V., Fuchs E. (2007) Epithelial stem cells: turning over new leaves. Cell 128: 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K., Dieppe P., Radin E. (2008) Etiopathogenesis of osteoarthritis. Rheum Dis Clin North Am 34: 531–559. [DOI] [PubMed] [Google Scholar]
- Burleigh A., Chanalaris A., Gardiner M., Driscoll C., Boruc O., Saklatvala J., et al. (2012) Joint immobilization prevents murine osteoarthritis and reveals the highly mechanosensitive nature of protease expression in vivo. Arthritis Rheum 64: 2278–2288. [DOI] [PubMed] [Google Scholar]
- Chen X., Macica C., Nasiri A., Broadus A. (2008) Regulation of articular chondrocyte proliferation and differentiation by indian hedgehog and parathyroid hormone-related protein in mice. Arthritis Rheum 58: 3788–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong K., Chanalaris A., Burleigh A., Jin H., Watt F., Saklatvala J., et al. (2013) Fibroblast growth factor 2 drives changes in gene expression following injury to murine cartilage in vitro and in vivo. Arthritis Rheum 65: 2346–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C., Dennison E., Edward M., Litwic A. (2013) Epidemology of osteoarthitis. Medicographia 35: 145–151. [Google Scholar]
- Craft A., Ahmed N., Rockel J., Baht G., Alman B., Kandel R., et al. (2013) Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development 140: 2597–2610. [DOI] [PubMed] [Google Scholar]
- De Bari C., Dell’accio F., Tylzanowski P., Luyten F. (2001) Multipotent mesenchyml stem cells from adult human synovial membrane. Arthritis Rheum 44: 1928–1942. [DOI] [PubMed] [Google Scholar]
- Decker R., Koyama E., Pacifici M. (2014) Genesis and morphogenesis of limb synovial joints and articular cartilage. Matrix Biol 39C: 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen K., Gourdie R. (2012) Embryonic wound healing: a primer for engineering novel therapies for tissue repair. Birth Defects Res C Embryo Today 96: 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’accio F., De Bari C., Eltawil N., Vanhummelen P., Pitzalis C. (2008) Identification of the molecular response of articular cartilage to injury, by microarray screening: WNT-16 expression and signaling after injury and in osteoarthritis. Arthritis Rheum 58: 1410–1421. [DOI] [PubMed] [Google Scholar]
- Dell’accio F., De Bari C., Luyten F. (2001) Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum 44: 1608–1619. [DOI] [PubMed] [Google Scholar]
- Djouad F., Bouffi C., Ghannam S., Noel D., Jorgensen C. (2009) Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol 5: 392–399. [DOI] [PubMed] [Google Scholar]
- Doupe D., Alcolea M., Roshan A., Zhang G., Klein A., Simons B., et al. (2012) A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science 337: 1091–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowthwaite G., Bishop J., Redman S., Khan I., Rooney P., Evans D., et al. (2004) The surface of articular cartilage contains a progenitor cell population. J Cell Sci 117: 889–897. [DOI] [PubMed] [Google Scholar]
- Dowthwaite G., Flannery C., Flannelly J., Lewthwaite J., Archer C., Pitsillides A. (2003) A mechanism underlying the movement requirement for synovial joint cavitation. Matrix Biol 22: 311–322. [DOI] [PubMed] [Google Scholar]
- Dragoo J., Samimi B., Zhu M., Hame S., Thomas B., Lieberman J., et al. (2003) Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pad. J Bone Joint Surg 85: 740–747. [PubMed] [Google Scholar]
- Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., et al. (2011) Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183. [DOI] [PubMed] [Google Scholar]
- Dy P., Smits P., Silvester A., Penzo-Mendez A., Dumitriu B., Han Y., et al. (2010) Synovial joint morphogenesis requires the chondrogenic action of SOX5 and SOX6 in growth plate and articular cartilage. Dev Biol 341: 346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman M., Yan D., Ahmadinia K., Chen D., An H., Im H. (2013) Fibroblast growth factor control of cartilage homeostasis. J Cell Biochem 114: 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell. H., Canti R. (1934) Experiments on the development in vitro of the avian knee joint. Proc R Soc Lon 116: 316–351. [Google Scholar]
- Felson D. (2009) Developments in the clinical under-standing of osteoarthritis. Arthritis Res Ther 11: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan M., Parkes M., Hutchinson C., Gait A., Forsythe L., Marjanovic E., et al. (2015) A randomised trial of a brace for patellofemoral osteoarthritis targeting knee pain and bone marrow lesions. Ann Rheum Dis. Epub ahead of print 16 January 2015. 10.1136/annrheumdis-2014-206376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futami I., Ishijima M., Kaneko H., Tsuji K., Ichikawa-Tomikawa N., Sadatsuki R., et al. (2012) Isolation and characterization of multipotential mesenchymal cells from the mouse synovium. PLoS One 7: e45517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Lan Y., Liu H., Jiang R. (2011) The zinc finger transcription factors OSR1 and OSR2 control synovial joint formation. Dev Biol 352: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossec L., Paternotte S., Maillefert J., Combescure C., Conaghan P., Davis A., et al. (2011) The role of pain and functional impairment in the decision to recommend total joint replacement in hip and knee osteoarthritis: an international cross-sectional study of 1909 patients. Report of the Oarsi-Omeract Task Force on Total Joint Replacement. Osteoarthritis Cartilage 19: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan S., Duffy S., Pauli C., Koziol J., Su A., D’lima D., et al. (2013) Zone-specific gene expression patterns in articular cartilage. Arthritis Rheum 65: 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan S., Miyaki S., Asahara H., D’lima D., Lotz M. (2009) Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther 11: R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guermazi A., Roemer F., Felson D., Brandt K. (2013) Motion for debate: osteoarthritis clinical trials have not identified efficacious therapies because traditional imaging outcome measures are inadequate. Arthritis Rheum 65: 2748–2758. [DOI] [PubMed] [Google Scholar]
- Gunnell L., Jonason J., Loiselle A., Kohn A., Schwarz E., Hilton M., et al. (2010) TAK1 regulates cartilage and joint development via the MAPK and BMP signaling pathways. J Bone Miner Res 25: 1784–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori S., Oxford C., Reddi A. (2007) Identification of superficial zone articular chondrocyte stem/progenitor cells. Biochem Biophys Res Commun 358: 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A., Macpherson S., Morrison H., Dowthwaite G., Archer C. (2001) The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol 203: 469–479. [DOI] [PubMed] [Google Scholar]
- Huey D., Hu J., Athanasiou K. (2012) Unlike bone, cartilage regeneration remains elusive. Science 338: 917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D., Zhang W., Conaghan P., Hirko K., Menashe L., Reichmann W., et al. (2011) Responsiveness and reliability of MRI in knee osteoarthritis: a meta-analysis of published evidence. Osteoarthritis Cartilage 19: 589–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde G., Dover S., Aszodi A., Wallis G., Boot-Handford R. (2007) Lineage tracing using matrilin-1 gene expression reveals that articular chondrocytes exist as the joint interzone forms. Dev Biol 304: 825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegawa S., Sano M., Koshizuka Y., Nakamura Y. (2000) Isolation, characterization and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet Cell Genet 90: 291–297. [DOI] [PubMed] [Google Scholar]
- Iwamoto M., Ohta Y., Larmour C., Enomoto-Iwamoto M. (2013) Toward regeneration of articular cartilage. Birth Defects Res C Embryo Today 99: 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto M., Tamamura Y., Koyama E., Komori T., Takeshita N., Williams J., et al. (2007) Transcription factor erg and joint and articular cartilage formation during mouse limb and spine skeletogenesis. Dev Biol 305: 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson K., Zhu S., Tremblay M., Payette J., Wang J., Bouchez L., et al. (2012) A stem cell-based approach to cartilage repair. Science 336: 717–721. [DOI] [PubMed] [Google Scholar]
- Jones E., Crawford A., English A., Henshaw K., Mundy J., Corscadden D., et al. (2008) Synovial fluid mesenchymal stem cells in health and early osteoarthritis: detection and functional evaluation at the single-cell level. Arthritis Rheum 58: 1731–1740. [DOI] [PubMed] [Google Scholar]
- Kajiwara R., Ishida O., Kawasaki K., Adachi N., Yasunaga Y., Ochi M. (2005) Effective repair of a fresh osteochondral defect in the rabbit knee joint by articulated joint distraction following subchondral drilling. J Orthop Res 23: 909–915. [DOI] [PubMed] [Google Scholar]
- Kan A., Tabin C. (2013) C-Jun is required for the specification of joint cell fates. Genes Dev 27: 514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson C., Thornemo M., Henriksson H., Lindahl A. (2009) Identification of a stem cell niche in the zone of ranvier within the knee joint. J Anat 215: 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelling S., Kruegel J., Irmer M., Path J., Sadowski B., Miro X., et al. (2009) Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell 4: 324–335. [DOI] [PubMed] [Google Scholar]
- Koyama E., Shibukawa Y., Nagayama M., Sugito H., Young B., Yuasa T., et al. (2008) A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol 316: 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafeber F., Intema F., Van Roermund P., Marijnissen A. (2006) Unloading joints to treat osteoarthritis, including joint distraction. Curr Opin Rheumatol 18: 519–525. [DOI] [PubMed] [Google Scholar]
- Leong D., Hardin J., Cobelli N., Sun H. (2011) Mechanotransduction and cartilage integrity. Ann N Y Acad Sci 1240: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Xie T. (2005) Stem cell niche: structure and function. Annu Rev Cell Dev Biol 21: 605–631. [DOI] [PubMed] [Google Scholar]
- Li T., Longobardi L., Myers T., Temple J., Chandler R., Ozkan H., et al. (2013) Joint TGF-beta type II receptor-expressing cells: ontogeny and characterization as joint progenitors. Stem Cells Dev 22: 1342–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longobardi L., Li T., Myers T., O’rear L., Ozkan H., Li Y., et al. (2012) TGF-beta type II receptor/MCP-5 axis: at the crossroad between joint and growth plate development. Dev Cell 23: 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Otsuki S., Grogan S., Sah R., Terkeltaub R., D’lima D. (2010) Cartilage cell clusters. Arthritis Rheum 62: 2206–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludin A., Sela J., Schroeder A., Samuni Y., Nitzan D., Amir G. (2013) Injection of vascular endothelial growth factor into knee joints induces osteoarthritis in mice. Osteoarthritis Cartilage 21: 491–497. [DOI] [PubMed] [Google Scholar]
- Marijnissen A., Van Roermund P., Van Melkebeek J., Schenk W., Verbout A., Bijlsma J., et al. (2002) Clinical benefit of joint distraction in the treatment of severe osteoarthritis of the ankle: proof of concept in an open prospective study and in a randomized controlled study. Arthritis Rheum 46: 2893–2902. [DOI] [PubMed] [Google Scholar]
- Martin P. (1997) Wound healing –aiming for perfect skin regeneration. Science 276: 75–81. [DOI] [PubMed] [Google Scholar]
- Morales T. (2007) Chondrocyte moves: clever strategies? Osteoarthritis Cartilage 15: 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morito T., Muneta T., Hara K., Ju Y., Mochizuki T., Makino H., et al. (2008) Synovial fluid-derived mesenchymal stem cells increase after intra-articular ligament injury in humans. Rheumatology 47: 1137–1143. [DOI] [PubMed] [Google Scholar]
- Mundy C., Yasuda T., Kinumatsu T., Yamaguchi Y., Iwamoto M., Enomoto-Iwamoto M., et al. (2011) Synovial joint formation requires local EXT1 expression and heparan sulfate production in developing mouse embryo limbs and spine. Dev Biol 351: 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgill D., Bayer L. (2013) Negative pressure wound therapy: past, present and future. Int Wound J 10(Suppl. 1): 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra D., Sandell L. (2012) Antiangiogenic and anticancer molecules in cartilage. Expert Rev Mol Med 14: e10. [DOI] [PubMed] [Google Scholar]
- Pazin D., Gamer L., Cox K., Rosen V. (2012) Molecular profiling of synovial joints: use of microarray analysis to identify factors that direct the development of the knee and elbow. Dev Dyn 241: 1816–1826. [DOI] [PubMed] [Google Scholar]
- Polk B., Frey M. (2012) Mucosal restitution and repair. In: Johnson L., Ghisban F., Kaunitz J., Merchant J., Said H., Wood J. (eds), Physiology of the Gastrointestinal Tract, 5th edn, Vol. 1 London: Academic Press, pp. 1147–1168. [Google Scholar]
- Poole A. (2005) Cartilage in health and disease. In: Koopman W., Moreland L. (eds), Arthritis & Allied Conditions: A Textbook of Rheumatology, 15th edn. Philadelphia, PA: Lippincott Williams, Wilkins, pp. 223–269. [Google Scholar]
- Poole A., Rosenberg L., Reiner A., Ionescu M., Bogoch E., Roughley P. (1996) Contents and distribution of proteoglycans decorin and biglycan in normal and osteoarthritic human articular cartilage.J Orthopaed Res 14: 681–689. [DOI] [PubMed] [Google Scholar]
- Pretzel D., Linss S., Rochler S., Endres M., Kaps C., Alsalameh S., et al. (2011) Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res Ther 13: R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M., Sandell L. (2014) Regeneration of articular cartilage in healer and non-healer mice. Matrix Biol 39: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M., Schmidt E., Mcalinden A., Cheverud J., Sandell L. (2013) Molecular insight into the association between cartilage regeneration and ear wound healing in genetic mouse models: targeting new genes in regeneration. G3 (Bethesda) 3: 1881–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer F., Kwoh C., Hannon M., Green S., Jakicic J., Boudreau R., et al. (2012) Risk factors for magnetic resonance imaging-detected patellofemoral and tibiofemoral cartilage loss during a six-month period: The Joints on Glucosamine Study. Arthritis Rheum 64: 1888–1898. [DOI] [PubMed] [Google Scholar]
- Roemer F., Zhang Y., Niu J., Lynch J., Crema M., Nevitt M., et al. (2009) Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over 30-month period in the Multicenter Osteoarthritis Study. Radiology 252: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell L. (2012) Etiology of osteoarthritis: genetics and synovial joint development. Nat Rev Rheumatol 8: 77–89. [DOI] [PubMed] [Google Scholar]
- Schindler O. (2011) Current concepts of articular cartilage repair. Acta Orthop Belg 77: 709–726. [PubMed] [Google Scholar]
- Schluck T., Nienhaus U., Aegerter-Wilmsen T., Aegerter C. (2013) Mechanical control of organ size in the development of the drosophila wing disc. PLoS One 8: e76171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol D., McCabe D., Choe H., Zheng H., Yu Y., Jang K., et al. (2012) Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum 64: 3626–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnoli A., O’Rear L., Chandler R., Granero-Molto F., Mortlock D., Gorska A., et al. (2007) TGF-beta signaling is essential for joint morphogenesis. J Cell Biol 177: 1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata P., Mou H., Pardo-Saganta A., Zhao R., Prabhu M., Law B., et al. (2013) Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 503: 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiku M., Shah R., Allison G. (2000) Evidence linking chondrocyte lipid peroxidation to cartilage matrix protein degradation. Possible role in cartilage aging and the pathogenesis of osteoarthritis. J Biol Chem 275: 20069–20076. [DOI] [PubMed] [Google Scholar]
- Tumaneng K., Russell R., Guan K. (2012) Organ size control by Hippo and TOR pathways. Curr Biol 22: R368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertel B. (1995) The ins and outs of aggrecan. Trends Cell Biol 5: 458–464. [DOI] [PubMed] [Google Scholar]
- Verzijl N., Degroot J., Thorpe S., Bank R., Shaw J., Lyons T., et al. (2000) Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem 275: 39027–39031. [DOI] [PubMed] [Google Scholar]
- Vincent T. (2013) Targeting mechanotransduction pathways in osteoarthritis: a focus on the pericellular matrix. Curr Opin Pharmacol 13: 449–454. [DOI] [PubMed] [Google Scholar]
- Watt F., Ismail H., Didangelos A., Peirce M., Vincent T., Wait R., et al. (2013) Src and fibroblast growth factor 2 independently regulate signaling and gene expression induced by experimental injury to intact articular cartilage. Arthritis Rheum 65: 397–407. [DOI] [PubMed] [Google Scholar]
- Wiegant K., Van Roermund P., Intema F., Cotofana S., Eckstein F., Mastbergen S., et al. (2013) Sustained clinical and structural benefit after joint distraction in the treatment of severe knee osteoarthritis. Osteoarthritis Cartilage 21: 1660–1667. [DOI] [PubMed] [Google Scholar]
- Wilusz R., Sanchez-Adams J., Guilak F. (2014) The structure and function of the pericellular matrix of articular cartilage. Matrix Biol 39: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V., Gurtner G., Longaker M. (2013) Wound healing: a paradigm for regeneration. Mayo Clin Proc 88: 1022–1031. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Cigan A., Marrero L., Lopreore C., Liu S., Ge D., et al. (2011) Expression of doublecortin reveals articular chondrocyte lineage in mouse embryonic limbs. Genesis 49: 75–82. [DOI] [PubMed] [Google Scholar]
- Zhao B., Tumaneng K., Guan K. (2011) The Hippo Pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol 13: 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W., Li Y., Li L., Zhang W., Wang S., Zheng X. (2013) YAP-mediated regulation of the chondrogenic phenotype in response to matrix elasticity. J Mol Histol 44: 587–595. [DOI] [PubMed] [Google Scholar]


