Abstract
Denosumab is a human monoclonal antibody which specifically blocks receptor activator of nuclear factor κB ligand and is a very potent antiresorptive drug. Its efficacy in reducing the risk of vertebral, hip and nonskeletal fracture has been proven in a large prospective, randomized multicenter study of 7808 postmenopausal women with osteoporosis [Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial]. Denosumab causes somewhat greater increases in bone mineral density (BMD) than the class of bisphosphonate antiresorptives. Denosumab also causes an increase in bone mass and bone strength in the spine, ultradistal and diaphysis of the radius, proximal tibia and the hip. Recently long-term treatment with denosumab has been shown to cause a continued almost linear increase in total hip and femoral neck BMD beyond 3 years up to 8 years. In this respect, denosumab seems to differ from the bisphosphonate group in which the rate of improvement of BMD diminishes and for some drugs becomes negative after 3–4 years when the process of secondary mineralization flattens out. This unique property of an antiresorptive drug points towards mechanisms of action which differ from the bisphosphonate group. Both types of antiresorptives decrease cortical porosity but contrary to bisphosphonates the reduction in cortical porosity continues with denosumab which, in addition, also seems to cause a slight continuous modeling-based formation of new bone despite suppression of bone remodeling. The net effect is an increase in cortical thickening and bone mass, and increased strength of cortical bone. This may contribute substantially to the significant further reduction of the nonvertebral fracture risk which was found in the long-term denosumab arm of the FREEDOM extension trial during years 4–7.
Keywords: antiresorptive therapy, bisphosphonate, bone density, bone mass, bone strength, cortical porosity, denosumab, Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months study, receptor activator of nuclear factor κB ligand
Introduction
The registered drugs that are available for the treatment of osteoporosis prevent future fractures through their capacity to increase bone strength. The treatment of osteoporosis to prevent fracture and skeletal-related events in cancer has become possible by the discovery and characterization of one of the most important and specific regulators of bone turnover, the receptor activator of nuclear factor κB ligand (RANKL)–osteoprotegerin (OPG) system and the subsequent development of the first human monoclonal antibody, denosumab, which blocks the RANKL–receptor activator of nuclear factor κB (RANK) pathway. Phase II studies in postmenopausal women with low bone mineral density (BMD) showed a substantial decrease in bone resorption markers and an increase in BMD [McClung et al. 2006; Lewiecki et al. 2007]. This led to a large phase III randomized controlled study in postmenopausal women with osteoporosis [Cummings et al. 2009]. Denosumab is a potent antiresorptive agent which increases bone density and reduces the risk of new vertebral fractures, hip and nonvertebral fractures in postmenopausal women with osteoporosis [Cummings et al. 2009]. The pivotal Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) study has been extended by 7 years. Patients on denosumab treatment thereby are followed for a total of 10 years. The FREEDOM extension study is the largest long-term study with any antiresorptive drug in use for postmenopausal osteoporosis [Papapoulos et al. 2012].
Bone strength are determined by bone mass, area, cortical thickness, and porosity. Bone strength thus has a quantitative component (measured by BMD and other techniques) and a bone microarchitecture component [measured by micro quantitative computed tomography (QCT), high-resolution peripheral QCT and other techniques]. This review article features a short introduction to the RANKL–OPG mechanism and a brief description of denosumab, followed by the results and lessons from the FREEDOM study and its extension with respect to BMD, bone mass and strength.
The RANKL–OPG system
For decades, the molecular signal behind the coupling between bone resorption and bone formation was unknown. However, one of the many valuable results of the human genome project was the identification and cloning of OPG and RANK and its ligand (RANKL) which is a member of the tumor necrosis factor α (TNFα) cytokine superfamily [Anderson et al. 1997; Simonet et al. 1997]. The RANK receptor is expressed on the surface of osteoclasts and osteoclast precursor cells while RANKL is located on the surface of osteoblasts and stromal cells in the bone marrow and to a limited extent also as soluble RANKL [Lacey et al. 1998; Hsu et al. 1999]. RANKL controls osteoclastogenesis by stimulating the differentiation of osteoclast precursor cells and the formation, function and survival of osteoclasts while OPG acts as a decoy receptor for RANKL and inhibits each of these effects [Lacey et al. 1998; Yasuda et al. 1998; Li et al. 2000; Kostenuik, 2005]. The balance between the local concentration of RANKL and OPG in bone tissue is the key mechanism through which systemic hormones, local growth factors and cytokines regulate bone turnover and ultimately the bone mass [Hofbauer and Schoppet, 2004]. Inhibition of RANKL and increased levels of OPG lead to diminished bone resorption, decreased cortical porosity and increase in BMD, bone mass and strength.
Denosumab
Denosumab is a human immunoglobulin G2 monoclonal antibody with high specificity and affinity for RANKL. By binding to RANKL, bone resorption is inhibited. In a prospective, randomized, double-blind phase I trial, serum concentrations of denosumab rose very rapidly during 3 weeks after subcutaneous injection in healthy postmenopausal women and were maintained for up to 9 months [Bekker et al. 2004]. The turnover markers for bone resorption fell within 12 h to a maximal suppression on average of around 84% and then remained suppressed for up to 6 months [Bekker et al. 2004]. Denosumab has no detectable binding to TNFα, TNFβ or TNF-related apoptosis-inducing ligand (TRAIL) and no neutralizing antibodies have been seen in clinical trials.
Pivotal denosumab study in postmenopausal osteoporosis (FREEDOM study)
In the international, randomized, placebo-controlled trial FREEDOM, 7868 postmenopausal women received either 60 mg of denosumab or a placebo injection subcutaneously twice yearly for 36 months [Cummings et al. 2009]. All women received a calcium supplement of at least 1000 mg and 400–800 IU vitamin D according to baseline concentration of serum 25-hydroxy vitamin D. Postmenopausal women between 60 and 90 years of age with a BMD of the lumbar spine or the total hip between −2.5 and −4.0 in T score were included. Women with severe osteoporosis with fractures were excluded as well as women treated with skeletal specific drugs within the past 5 years, bisphosphonate use more for than 3 years or use of bisphosphonate within the 12 months before study initiation. Of the 7868 patients, 60 (30 denosumab and 29 placebo treated) were excluded from all analyses due to inconsistency of data and adherence to the study protocol. There were 3902 women who were treated with denosumab and 3906 women with placebo (mean age ± standard deviation, 72.3 ± 5.2 years) for both groups. A total of 82% of patients completed 36 months of the study (3206 women in the placebo group and 3272 in the denosumab group).
Antifracture efficacy of denosumab
After 3 years, the relative risk of new radiographic vertebral fractures was reduced by 68%, for hip fractures by 40% and for nonvertebral fractures by 20% by denosumab treatment [Cummings et al. 2009]. The absolute reduction of fracture rate was 4.8 %, 0.3% and 1.5% for new vertebral, hip and nonvertebral fracture, respectively. A prespecified and post hoc analysis also showed a significant reduction of new vertebral and hip fracture in women over 75 years of age as well as in other subgroups of women at high risk of fracture [Boonen et al. 2011; McClung et al. 2012]. In women with low bone mass at baseline (T score< –2.5), there was also a lower risk of wrist fracture in the denosumab group [Simon et al. 2013].
Recently, a new property of long-term treatment with an antiresorptive drug has been seen. After 3 years of denosumab treatment in the FREEDOM extension study a further and significant decrease in nonvertebral fractures was observed in year 4 and the period of year 4 to 7 compared with the first 3 years of denosumab treatment [Ferrari et al. 2013]. Also the incidence of wrist fractures was lower in year 4 and 5 of the long-term denosumab group [Bilezikian et al. 2014].
It is of interest to emphasize the impact of denosumab on cortical bone. Radius, tibia and hip regions have been used in various models for studying cortical and trabecular compartments and will be discussed later in this review.
Antifracture efficacy of denosumab versus other antiresorptive therapies
The other available registered antiresorptive drugs, alendronic acid (ALN), risedronic acid (RIS), ibandronate (IBAN) and zoledronic acid (ZOL) for the treatment of postmenopausal osteoporosis including raloxifene (RAL) are also effective with a 3-year relative risk reduction (RRR) for a new vertebral fracture of 47% (ALN), 41% (RIS), 30 % (RAL), 48% (IBAN) [Black et al. 1996; Ettinger et al. 1999; Harris et al. 1999; Chesnut et al. 2004]. The RRR for new vertebral fractures over 3 years of treatment with denosumab was 68% and similar to the RRR of 70% for ZOL in the HORIZON study [Black et al. 2007].
The inclusion patterns of patients with osteoporosis in the pivotal trials have changed since 1993. The trial with ALN Fracture Intervention Trial 1 (FIT1) included patients with severe osteoporosis, all had at least one prevalent vertebral fracture, and a low femoral neck bone mineral density (0.53 ± 0.07 g/cm2) [Black et al. 1996]. At the time the FREEDOM study was designed, effective pharmacologic treatments for osteoporosis were well established and it was no longer ethically acceptable to randomize patients with severe osteoporosis to receive calcium and vitamin D only. This is reflected by the decreasing percentage of included patients with a prevalent vertebral fracture at baseline, which apart from the RAL study with 37%, were 100% (ALN), 80% (RIS), 94% (IBAN) and 62% (ZOL) compared with 68% for denosumab. The change in inclusion pattern is also illustrated in the relatively low 3-year cumulative absolute risk for new vertebral fractures of 7.2% for the placebo group in the FREEDOM study compared with 15.0% (ALN), 16.3% (RIS), 10.1% (RAL) and 9.6% (IBAN) in the other pivotal studies [Black et al. 1996; Ettinger et al. 1999; Harris et al. 1999; Chesnut et al. 2004; Cummings et al. 2009].
The absolute risk reduction for antiresorptive therapies including denosumab is considerably higher for new vertebral fractures (3.5–7.6%) than for hip fractures (0.5–1.3%) [Black et al. 1996, 2007; Ettinger et al. 1999; Harris et al. 1999; McClung et al. 2001; Chesnut et al. 2004; Cummings et al. 2009].
One has to keep in mind, however, that an adequate comparison of the antifracture efficacy of the drugs is impossible due to the differences in study populations.
Bone mineral density
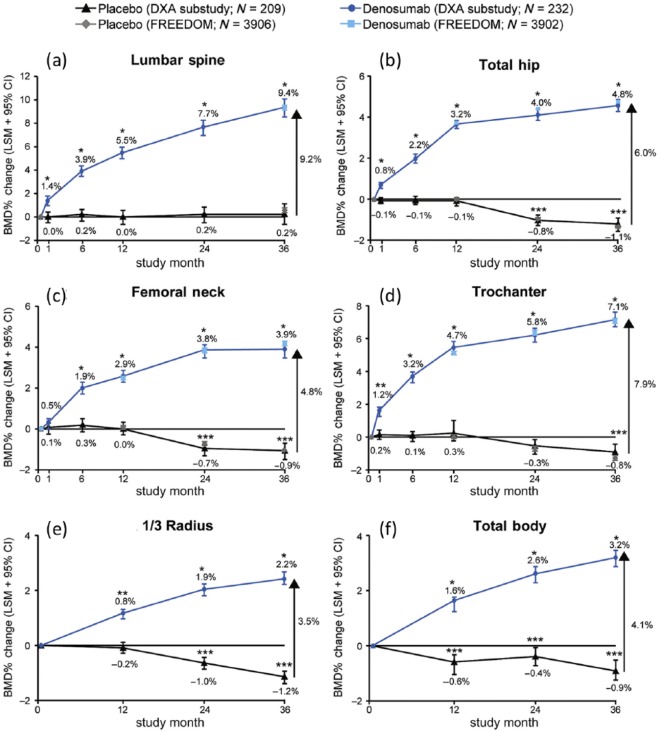
BMD at the lumbar spine and hip was measured by dual X-ray absorptiometry (DXA) at baseline and after 3 years, and annually at the hip [Cummings et al. 2009]. All measured skeletal sites showed steady and significant (p < 0.0001) increases in BMD in the denosumab group, which after 36 months resulted in a relative BMD gain in the lumbar spine of 9.2%, 7.9% at the trochanter, 6.0% at the total hip, 4.8% at the femoral neck and 3.5% at the 1/3 distal radius (Figure 1).
Figure 1.
Percentage change in bone mineral density from baseline (LSM and 95% confidence interval) over time for the placebo group and the denosumab group from the DXA substudy and the total Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial cohorts at the lumbar spine (a), total hip (b), femoral neck (c), trochanter (d), 1/3 radius (e), and total body (f) at 36 months. The denosumab group was given 60 mg subcutaneously every 6 months, and the placebo group received a placebo injection every 6 months *p < 0.0001 and **p < 0.005 for denosumab compared with baseline and placebo. ***p < 0.05 for placebo compared with baseline. (reproduced from Bolognese et al. [2013] with permission).
LSM, least-squares mean.
The BMD gain occurs early. In a subgroup of 441 women, DXA of the lumbar spine and of the hip was also performed at 1, 6, 12 and 24 months [Bolognese et al. 2013]. Significant increases in BMD from baseline were seen after 1 month of denosumab treatment in the lumbar spine (1.4%), trochanter region (1.2%) and total hip (0.8%), and at 6 months in the femoral neck (1.9%), and 0.8% in the distal 1/3 of the radius (Figure 1) [Bolognese et al. 2013]. The placebo group showed no substantial changes over 1 month at these sites (0, 0.2%, –0.1%, respectively) and at 6 months in the femoral neck (0.1%) and the distal radius (–0.1%). The increase in BMD was significantly correlated with reduction in the bone resorption marker serum C-telopeptide of type I collagen (CTX) in the denosumab group [Eastell et al. 2011]. The association between improvements of BMD and a lower risk of fracture is well documented [Wasnich and Miller, 2000; Hochberg et al. 2002; Austin et al. 2012; McClung et al. 2012].
Denosumab versus bisphosphonates regarding BMD
How does denosumab compare with the most used antiresorptive bisphosphonates for the treatment of postmenopausal women with osteoporosis or with low bone mass?
Alendronic acid
In the vertebral fracture arm of the pivotal Fracture Intervention Trial a significant increase in BMD was seen with ALN treatment compared with the placebo group at all sites (p < 0.001). In the lumbar spine, BMD increased 6.2%, at the trochanter 6.1%, in the total hip 4.7%, at the femoral neck 4.1%, and in the proximal forearm 1.6% (Table 1) [Black et al. 1996].
Table 1.
Cumulative bone mineral density (BMD) gain in percentage relative to the placebo group after 3 years of treatment with alendronate (ALN), risedronate (RIS), ibandronate (IBAN), zoledronate (ZOL) or denosumab according to the respective pivotal clinical drug trials.
| No of patients Plbo/Trmnt | 3-year cumulative BMD gain relative to the placebo group (%) |
|||||
|---|---|---|---|---|---|---|
| LS | Hip (total) | FN | Troc | Radius (distal) | ||
| ALN [Black et al. 1996] | 1005/1022 | 6.2 | 4.7 | 4.1 | 6.1 | 1.6* |
| RIS [Harris et al. 1999] | 855/813/811$ | 4.3 | – | 2.8 | 4.0 | 1.2‡ |
| IBAN [Chesnut et al. 2004] | 975/977 | 4.4–5.2§ | 3.6–4.1§ | 3.0–3.4§ | 5.4–5.7§ | – |
| ZOL [Black et al. 2007] | 3861/3875¶ | 6.7 | 6.0 | 5.1 | – | – |
| Denosumab [Cummings et al. 2009] | 3906/3902# | 9.2 | 6.0 | 4.8 | 7.9 | 3.5 |
Proximal radius.
Placebo/20 mg dose scheme/2.5 mg daily, respectively.
Midshaft radius.
20 mg dose scheme/2.5 mg daily.
Data from the DXA subgroup which comprised 272 women treated with ZOL and 270 placebo treated.
Data from 232 denosumab and 209 placebo treated women in the DXA substudy.
FN, femoral neck; LS, lumbar spine; Plbo, placebo-treated group; Trmnt, treatment group; Troc, trochanter region.
Denosumab has been directly compared with ALN with respect to changes in BMD as an endpoint in a randomized multicenter double-blind study [Brown et al. 2009]. In that study, 593 women were evaluated for efficacy after 12 months of treatment with denosumab 60 mg subcutaneously every 6 months plus a placebo tablet once weekly, and 586 women received ALN 70 mg orally once a week plus a placebo injection every 6 months; they completed the study and were evaluated for BMD. Denosumab significantly increased BMD compared with ALN at the total hip at 12 months (3.5% versus 2.6%; p < 0.0001). In addition, significantly greater increases in BMD were observed with denosumab treatment at all measured skeletal sites. The differences in favor of denosumab treatment were significant (p < 0.0002) at the various skeletal sites at 12 months and were 0.6% at the femoral neck, 1.0% at the trochanter, 1.1% at the lumbar spine and 0.6% at the 1/3 distal radius [Brown et al. 2009]. The number of patients who adhered to denosumab treatment, however, was higher than the number of patients who continued to take oral ALN 70 mg once weekly [Kendler et al. 2011].
Risedronic acid
From the pivotal US multicenter Vertebral Efficacy With Risedronate Therapy (VERT) study, BMD data are available from a subgroup of 821 postmenopausal women with osteoporosis who received RIS 5 mg oral tablet daily for 3 years, and from 820 placebo-treated women [Harris et al. 1999]. Treatment with denosumab showed considerable higher BMD gain at all skeletal sites after 3 years compared with RIS (Table 1) [Harris et al. 1999].
Ibandronate
Treatment of postmenopausal women with osteoporosis in the pivotal BONE study with continuous oral IBAN (2.5 mg daily) or intermittent oral IBAN 20 mg every other day for 12 doses every 3 months showed increases in BMD after 3 years (versus placebo) at the lumbar spine, total hip, femoral neck and trochanter regions. The distal radius was not measured [Chesnut et al. 2004]. The BMD gain was lower at all measured sites compared with denosumab (Table 1).
Denosumab has also been compared with IBAN in an open-label, randomized, multicenter study of postmenopausal women with low adherence to bisphosphonate treatment. There were 417 postmenopausal women who were treated with denosumab and 416 postmenopausal women who were treated with 150 mg IBAN orally every month for 12 months. Significantly (p < 0.001 at all sites) greater BMD gains from baseline were observed with denosumab compared with IBAN at the lumbar spine (4.1% versus 2.0%), total hip (2.3% versus 1.1%) and femoral neck (1.7% versus 0.7%) [Recknor et al. 2013].
Risedronic acid and ibandronate
Direct comparison between denosumab and RIS or IBAN has been performed in an open-label, randomized, multicenter study of postmenopausal women with low bone density who had discontinued or demonstrated suboptimal adherence to ALN therapy. There were 402 women who were switched from ALN to oral RIS 150 mg monthly and 422 women who were switched from ALN to denosumab 60 mg twice every 6 months, and they were evaluated by BMD after 12 months. Denosumab significantly increased (p < 0.0001) BMD compared with RIS at the lumbar spine (3.4% versus 1.1%), total hip (2.0% versus 0.5%) and femoral neck (1.4% versus 0%) [Roux et al. 2014].
Zoledronic acid
No head-to-head comparison between treatment with denosumab and ZOL has been performed. However, BMD data are available from the pivotal 3-year HORIZON fracture trial in which 3889 postmenopausal women were randomized to 5 mg ZOL intravenously every 12 month and 3876 women to placebo injections [Black et al. 2007]. BMD of the lumbar spine, total hip and femoral neck were measured in a subgroup of women comprising 272 treated with ZOL and 270 with placebo. The increase in BMD of the lumbar spine in the ZOL treatment group was lower (6.7%) compared with the denosumab group (9.2%) (Table 1). However, when the BMD results from this study are compared with the FREEDOM study, changes in BMD of the total hip (6.0% versus 6.0%) and femoral neck (4.8% versus 5.1%) are similar in the two studies after ZOL treatment (Table 1) [Black et al. 2007; Cummings et al. 2009].
Although differences exist when comparing studies of postmenopausal women with osteoporosis or low BMD, it appears that denosumab demonstrates superior increases in bone density over 1–3 years compared with bisphosphonate-treated individuals, with the possible exception of hip measurements between denosumab and ZOL. A recent meta-analysis also supports this statement [Mandema et al. 2014].
BMD, long-term data from denosumab treatment
The 3-year FREEDOM trial has been extended 7 years to a planned total study period of 10 years (FREEDOM extension study). After the end of the third year, 2343 women from the original denosumab group agreed to continue treatment with denosumab 60 mg twice yearly (long-term group), and 2207 women of the former placebo group accepted switching to denosumab after the end of their 3-year period (crossover group) [Papapoulos et al. 2012]. Six years of data showed that BMD in the long-term denosumab group, during years 3–6, continued to increase in the lumbar spine by 4.9% (the cumulative gain since the start of the FREEDOM study was 15.2%), in the total hip by 1.8% (cumulative gain 7.5%), in the femoral neck by 1.7% (cumulative 6.7%) and in the distal 1/3 of the radius by 0.6% (cumulative gain 2.7%). The crossover group increased BMD at all sites during years 3–6, similar to the denosumab group in the first 3 years of the original FREEDOM study [Bone et al. 2013].
After 8 years, 1386 patients remained in the long-term denosumab group and 1296 patients remained in the crossover denosumab group of the FREEDOM extension study [Papapoulos et al. 2013]. A further increase in BMD of the lumbar spine and total hip after 8 years of treatment was observed [Papapoulos et al. 2013]. The BMD gain after year 3 was almost linear in the lumbar spine. This observation corroborates the 8-year BMD data from the considerably smaller phase II study in women with low bone mass [McClung et al. 2013a].
Denosumab versus bisphosphonates, long-term BMD data
There are very limited published data on BMD with respect to long-term follow up of treatment with bisphosphonates in postmenopausal women with osteoporosis. Long-term BMD data are available from women treated with ALN (10 years), RIS (7 years) or ZOL (6 years) [Bone et al. 2004; Mellstrom et al. 2004; Black et al. 2006, 2012].
Alendronic acid
BMD of the lumbar spine and hip BMD continued to increase after 10 years of oral ALN treatment in postmenopausal women with osteoporosis with 5 mg (n = 78) or 10 mg daily (n = 86) or placebo (n = 83) [Bone et al. 2004]. During years 6–10, the lumbar spine BMD increased by 3.7%, total hip by 0.8% and femoral neck by 0.9%. The lumbar spine BMD after 10 years showed a cumulative gain from baseline of 13.7 %, total hip 6.7% and femoral neck 5.4% in the 10 mg ALN daily group [Bone et al. 2004]. Very similar BMD gains were also observed in the larger, fracture intervention trial long-term extension (FLEX) study comprising 333 women treated with 10 mg ALN daily for 10 years and 437 placebo-treated women [Black et al. 2006].
Risedronic acid
BMD data from 83 women treated with RIS 5 mg daily for 7 years in the Vertebral Efficacy With Risedronate Therapy MultiNational (VERT-MN) extension study showed a significant cumulative gain of 11.5% in the lumbar spine from baseline at study start [Mellstrom et al. 2004]. However, the BMD change during years 5–7 of 1.8% was insignificant. There was no placebo group for comparison. The total hip BMD increased to 3.9% after 7 years but without an apparent increase from years 3 to 7 [Mellstrom et al. 2004].
Zoledronic acid
In women who participated in the Horizon PFT extension study and who continued infusion of ZOL 5 mg intravenously every 12 months (n = 616), BMD increased by 12.1% in the lumbar spine and 4.5% in the femoral neck compared with baseline [Black et al. 2012]. During years 3–6, BMD increased by 3.2% in the lumbar spine and by 0.24% in the femoral neck but fell in the total hip (–0.36%) and in the distal radius (–0.12%) [Black et al. 2012]. In addition, BMD in the femoral neck fell between years 4.5 and 6. This pattern is different from that observed with denosumab treatment beyond 3 years (Figure 2) [Bone et al. 2013]. Denosumab continued to increase cortical bone BMD in the total hip and femoral neck beyond 3 years while ZOL did not show further gain at these sites during years 3–6. These changes by denosumab may be important for nonvertebral fracture risk on long-term use.
Figure 2.
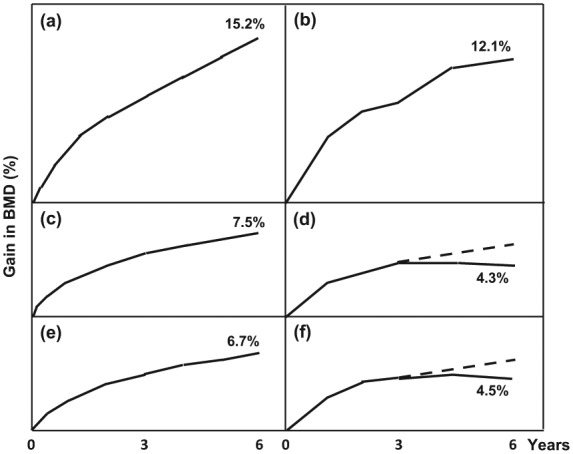
Development of bone mineral density (BMD) in the lumbar spine (a, b), total hip (c, d) and femoral neck (e, f) during 6 years of treatment of postmenopausal women with denosumab 60 mg subcutaneously every 6 months (left column: a, c, e) or zoledronate 5 mg intravenously every 12 months (right column: b, d, f) (modified after Bone et al. [2013] and Black et al. [2006]). A dashed line for the hypothetical BMD development during denosumab treatment in years 3–6 is inserted in (d) and (f). Please note that the patient groups in the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) and the Horizon study differ in several respects, including the degree of severity of osteoporosis, which makes the likely BMD change if denosumab had been used in (d) and (f) speculative.
Taken together, the information (which is limited due to the differences in numbers of patients and follow-up periods etc. in the ALN and RIS studies) points towards a limited BMD gain after 3–5 years of bisphosphonate use. Data from the Horizon PFT extension study point towards a continued rise in BMD in predominantly trabecular bone such as lumbar spine but very limited BMD gain in predominantly cortical bone such as in the femoral neck [Black et al. 2012]. This is a clear difference compared with long-term treatment with denosumab when the BMD in predominantly cortical tissue continues to increase substantially beyond 3 years, such as in the femoral neck [Bone et al. 2013]. Denosumab therefore seems to have a different effect on BMD in cortical bone compared with the bisphosphonates with prolonged treatment. This difference is especially apparent and most reliable when denosumab is compared with the results from the ZOL study, which has the highest number of study participants among the long-term studies of bisphosphonates.
BMD after discontinuation of denosumab
What happens to bone turnover when the treatment with denosumab is stopped? In a randomized phase III trial of postmenopausal osteoporosis, 256 women with low bone mass were treated with denosumab 60 mg twice yearly for 24 months (DEFEND trial) and then followed for an additional 24 months [Bone et al. 2011]. After discontinuation of denosumab at 24 months, the bone resorption measured by CTX increased within 3 months to reach a maximum at 6 months. The BMD fell at all measured sites and returned to just above pretreatment baseline within 12 months in the lumbar spine and to baseline in the total hip. The percentage fall in BMD of the lumbar spine was significantly associated with the peak increase of CTX [Bone et al. 2011]. In the following 12 months, BMD of the lumbar spine remained slightly above pretreatment baseline while the total hip BMD continued to fall slightly below. Two years of treatment with denosumab thus resulted in a BMD gain of the lumbar spine of around 0.5% and an insignificant loss of around 0.5% in the total hip at the end of the study (48 months). The BMD of the radius showed similar changes to the total hip, although they were somewhat delayed.
BMD at all measured sites was significantly higher at 48 months compared with the placebo-treated group [Bone et al. 2011]. Such an increase in bone density could possibly explain the lack of increase in fracture incidence during the 2 years off denosumab treatment [Brown et al. 2013]. Low fracture occurrence was also observed in the phase II study of women with low bone mass in which 3% of the patients in both groups sustained a new fracture during the follow-up period between 24 and 48 months [Bone et al. 2011].
Taken together, the gain in BMD during 2 years of treatment stopped and BMD returned to approximate pretreatment values relatively fast within around 12 months. However, discontinuation of treatment did not increase the fracture risk in this relatively limited observation period and small number of participants in these trials.
Bone mass
QCT measurements have the advantage over DXA because assessment of BMD can be based on three-dimensional measurements whereby bone volume, its density [volumetric (BMD)] and mineral content (BMC) can be determined with good accuracy. Not only does BMD increase with denosumab treatment in trabecular bone tissue of the spine, hip and radius, as discussed above, but recent data have also shown an important gain in bone mass in cortical compartments. A short review of the various studies supporting the cortical effects of denosumab in terms of bone mass, cortical porosity and cortical thickness is given in this section.
Three-year QCT data of the spine and hip from a prespecified QCT substudy of women from the FREEDOM trial showed a significant improvement of vBMD in denosumab-treated subjects at 12, 24 and 36 months in the lumbar spine, total hip and femoral neck regions [McClung et al. 2013b]. After 36 months, vBMD in the denosumab group had increased by 22% in the lumbar spine, 8% in the total hip and 6% in the femoral neck compared with placebo. The BMC of the total hip also increased significantly, mainly due to the cortical compartment [McClung et al. 2013b].
Hip
Using hip scans by QCT from the same QCT substudy of FREEDOM and a special software program (Medical Imaging Analysis Framework) it has been possible to separate the hip scan into a trabecular, subcortical and cortical compartment [Genant et al. 2013]. Denosumab treatment for 36 months significantly increased total hip vBMD and BMC by 7.9% and 7.4%, respectively, relative to the placebo group. All three subcompartments of the hip also increased significantly where the largest absolute gain from baseline occurred in cortical BMC (456 mg compared with 247 mg in the trabecular and 108 mg in the subcortical compartment) [Genant et al. 2013].
Radius
Three-year data from the prespecified DXA and QCT substudies of the FREEDOM trial also showed significantly increased BMD, vBMD and BMC with denosumab treatment not only in trabecular bone but also in cortical bone in the radius [Simon et al. 2013].
Two-year QCT data of the radius from the phase III DEFEND trial of 336 postmenopausal women with low bone mass treated with denosumab 60 mg twice yearly showed a significant increase in vBMD and BMC in both trabecular and cortical compartments, and cortical thickness in the proximal forearm [Genant et al. 2010].
Radius and tibia
The effect of denosumab on cortical bone (and trabecular bone) has also been investigated in a randomized, multicenter, double-blind, phase II study of postmenopausal women with low bone mass (−2.0 to −3.0 in T score) and without fractures or vertebral deformities [Seeman et al. 2010]. Morphologic changes were assessed using high-resolution peripheral QCT (HR-pQCT) at the distal radius and distal tibia. In the placebo group total, cortical and trabecular vBMD, and cortical thickness decreased (from −2.1% to −0.8%) at the distal radius after 12 months. Denosumab significantly (p < 0.001) improved these variables (from 0.3% to 3.4%) versus placebo. Denosumab also increased total, cortical and trabecular vBMD, and cortical thickness of the distal tibia. The changes in total and cortical vBMD were significantly greater at 12 months in the denosumab group compared with ALN (p < 0.02) [Seeman et al. 2010].
The authors suggested that the differences in total and cortical bone were due to different actions of the two drugs on the bone multicellu-lar unit (BMU) level [Seeman et al. 2010]. Denosumab showed greater suppression of resorption and likely lower birthrate of the BMU and of new resorptive cavities, plus inhibition of resorption in already existing cavities thereby increasing the bone density to which remineralization of existing formation sites contributes. In addition, the improvement in cortical thickness of the radius and tibia which was seen by denosumab treatment could be due to an increase in cortical area achieved by a decrease in the cortical porosity [Seeman et al. 2010]. The diistribution of the denosumab and the bisphosphonate class of antiresorptive drugs within the bone as well as their effects on precursors and mature osteoclasts thus may explain their different skeletal effects [Baron et al. 2011].
Cortical porosity and thickness
The investigators have pursued the concept of decreased cortical porosity by denosumab treatment [Zebaze et al. 2014a]. Both ALN and denosumab decreased cortical porosity of the distal radius by HR-pQCT at 12 months. Denosumab showed a more pronounced effect on diminishing cortical porosity during 6–12 months than ALN, however the effect levelled out during this period [Zebaze et al. 2014a]. Recently also decreased cortical porosity of the hip by denosumab treatment has been reported [Zebaze et al. 2014b].
Qualitative assessment by micro QCT of iliac crest bone biopsies from a small subgroup of FREEDOM study participants showed significantly higher cortical vBMD in the denosumab group (866 g/cm2) compared with the placebo group (851 g/cm2, p < 0.02) at 24 months [Reid et al. 2010]. Also the percentage of cortical porosity was significantly lower in the denosumab-treated group (3.64%) compared with the placebo group (4.58%, p = 0.01).
In an animal model, denosumab treatment of ovariectomized cynomolgus monkeys for 16 months caused a profound decrease in bone turnover markers for resorption (CTX) and formation (BSALP) [Ominsky et al. 2011].The treatment period corresponds to 4 years in an adult human regarding bone remodeling. Despite decreased bone turnover, significant increases were found in BMC and vBMD with denosumab treatment in both trabecular and cortical bone, and in total vBMD measured by pQCT compared with sham-operated monkeys. Cortical vBMD increased both in the cortical part of the diaphysis of the radius and proximal tibia, as did cortical area BMD of the diaphysis of the radius [Ominsky et al. 2011].
Recently, continuous modeling-based bone formation despite suppression of bone remodeling has been reported from the same study [Ominsky et al. 2014]. Denosumab thus caused increased cortical thickness of the hip due to modeling-based slight formation of new bone, which was most apparent in the superior endocortex and inferior periosteum, and may explain the sustained increase in hip BMD [Ominsky et al. 2013].
Cortical porosity: denosumab versus ZOL and teriparatide
The actions of denosumab on cortical porosity is clearly different from ZOL. ZOL treatment with 5 mg intravenously once yearly over 18 months increased cortical thickness of the tibia but did not decrease cortical porosity from baseline in the tibia and the radius in an open-labelled prospective clinical study as estimated by HR-pQCT [Hansen et al. 2013]. Denosumab also differed from teriparatide [parathyroid hormone (PTH) 1–34] and PTH 1–84 which both increase the cortical porosity at the radius by 37% versus 32% and by 27% versus 22% at the tibia [Hansen et al. 2013].
Although recent data from cynomolgus monkeys indicate some anabolic actions of denosumab in the superior endocortex and inferior periost, this process seems rather weak. Intermittent teriparatide or PTH 1–84, however, causes a rapid and profound increase in cortical and intratrabecular remodeling and modeling with increased cortical porosity and intratrabecular tunneling, increased periostal and endocortical bone apposition and cortical thickness [Oxlund et al. 1993; Jiang et al. 2003; Recker et al. 2009; Baron and Hesse, 2012; Ominsky et al. 2013].
All taken together, denosumab treatment not only improved BMD, vBMD and BMC of trabecular bone in the lumbar spine, hip, tibia and radius but also and most importantly of the cortical compartments of the forearm, tibia and hip. The cortical effects of denosumab seem to be fairly unique for an antiresorptive drug, such as increased cortical thickness and continued decrease in cortical porosity, properties most important for vBMD and bone strength. These may contribute to the further decrease in nonvertebral fractures reported after 4 years of long-term denosumab treatment and in the crossover group during years 4–7, and the decrease in wrist fractures during years 4–5 after long-term treatment in the FREEDOM extension study [Bilezikian et al. 2014; Ferrari et al. 2013].
Strength
Radius
Increased bone mass in the forearm with denosumab treatment is also reflected in an increased strength of the forearm [estimated by polar moment of inertia (PMI)] which has been observed in the FREEDOM and DEFEND studies [Simon et al. 2013; Genant et al. 2010]. The positive effects on bone strength (PMI) of the radius seem greater with denosumab than with ALN treatment (p < 0.001) [Seeman et al. 2010]. Increased bone strength with denosumab treatment in itself may also be explained in part by a decreased cortical porosity, as earlier studies have shown a reverse relation between increased cortical porosity and exponentially decreased bone strength [Yeni et al. 1997].
Hip
Biomechanical testing of bone specimens from denosumab-treated ovariectomized cynomolgus monkeys for 16 months has confirmed increased bone strength of the femoral neck and spine which was highly correlated with bone mass [Ominsky et al. 2011]. The increased strength may be explained by a decreased remodeling and cortical porosity and increased cortical thickness [Kostenuik et al. 2011; Ominsky et al. 2011].
A large number of osteoporotic hip fractures occur in the trochanter region due to falls, and denosumab treatment reduced the number of hip fractures by 40% in the FREEDOM study [Cummings et al. 2009]. Interestingly, recent data derived from three-dimensional cortical bone mapping point towards strengthening of the femoral cortex and lateral trochanter region by denosumab treatment. In a substudy of 80 denosumab-treated women in the FREEDOM trial a significant increase in femoral cortical density and thickness by 5.4% compared with placebo was already evident at 12 months [Poole et al. 2014]. Also, increased cortical mass surface density and cortical thickness of up to 12% occurred at the lateral femoral trochanter region in the denosumab group relative to the placebo-treated group after 36 months [Poole et al. 2014]. One third of the increase in the femoral cortical mass came from increasing cortical density, and two thirds from increasing cortical thickness relative to placebo [Poole et al. 2014].
Hip and spine
Recent data from a subgroup of postmenopausal women from the FREEDOM study comprising 51 women treated with denosumab and 48 women receiving placebo showed increased strength in the hip and spine [Keaveny et al. 2014]. Finite element analysis (FEA) scans of the hip and spine obtained with QCT were used to estimate hip and spine strength at 12 and 36 months. Compared with baseline, the FEA indicated that the hip strength had increased significantly (p < 0.0001) at 12 months by 5.3% and by 8.6% at 36 months in the denosumab-treated group. In the placebo group, on the contrary, hip strength was unchanged at 12 months and was decreased by 5.6% at 36 months.
At the spine, the strength was similarly significantly increased for the denosumab group by 18.2% at 36 months while it decreased by 4.2% in the placebo group. Relative to the placebo group, 3 years of denosumab treatment increased the strength in the hip and spine by 14.3% (p < 0.0001) and 22.4% (p < 0.0001), respectively [Keaveny et al. 2014]. The strength associated with both the trabecular and cortical bone improved in the denosumab group but diminished at both sites in the placebo group [Keaveny et al. 2014]. In denosumab-treated cynomolgus monkeys, vertebral bone strength also improved by 50% according to biomechanical tests and by 46% on finite element analysis [Lee et al. 2014].
Taken together, both phase II and phase III studies of denosumab treatment have shown an increased strength of trabecular and more importantly of cortical bone in the 1/3 distal radius, diaphysis of the radius and proximal tibia, spine and femoral cortex and at the trochanter region [Genant et al. 2010; Seeman et al. 2010; Ominsky et al. 2011; Simon et al. 2013; Keaveny et al. 2014; Poole et al. 2014].
Increased strength of trabecular and cortical bone is also reflected by the significantly decreased incidence of spine, hip fractures and nonvertebral fractures following denosumab treatment over 3 years in postmenopausal women with osteoporosis and in post hoc analysis of subgroups in the FREEDOM study [Cummings et al. 2009; Boonen et al. 2011; McClung et al. 2012; Simon et al. 2013]. The positive effects of denosumab on the improvements of cortical strength even seem to continue after 3 years [Ferrari et al. 2013]
Adverse effects
During the 36 months of the FREEDOM study there was no difference in the number of total adverse, serious or fatal events or events leading to discontinuation of participants in the study [Cummings et al. 2009]. Eczema was seen in 3.0% and 1.7% (p < 0.001) of the denosumab and placebo groups, respectively. Cellulitis, including erysipelas, as a serious adverse event occurred significantly more with denosumab (0.3%) compared with placebo (<0.1%) [Cummings et al. 2009]. However, the rate of all adverse events including eczema and cellulitis did not change with long-term use of denosumab [Bone et al. 2013]. Osteonecrosis of the jaw (ONJ) or atypical femur fracture (AFF) was not seen during the 3-year study period [Cummings et al. 2009]. At the 6-year FREEDOM extension study follow up, six adjudicated cases of ONJ and one case of AFF was reported [Bone et al. 2013]. As of September 2014, the company postmarketing survey has registered 32 adjudicated cases of ONJ, 4 cases of AFF, 8 cases of severe hypocalcemia and 5 cases of anaphylaxis based on 1,252,566 patient years of denosumab use in osteoporosis [Geller et al. 2014]. It has been speculated that denosumab may have beneficial effects on arterial calcification. However, denosumab does not influence the progression of aortic calcification or incidence of adverse cardiovascular events over 3 years in postmenopausal women with osteoporosis and high cardiovascular risk [Samelson et al. 2014].
Summary
Denosumab is a human monoclonal antibody which specifically blocks RANKL and thereby becomes a very potent antiresorptive drug. Its efficacy in reducing the risk of vertebral, hip and nonskeletal fracture has been proven in a large prospective, randomized multicenter study of 7808 postmenopausal women with osteoporosis (FREEDOM trial). Denosumab causes somewhat greater increases in BMD than the class of bisphosphonate antiresorptives. Denosumab causes an increase in bone mass and bone strength in the spine, ultradistal and diaphysis of the radius, proximal tibia and the hip. Recently long-term treatment with denosumab has been shown to cause a continued almost linear increase in spine, total hip and femoral neck BMD beyond 3 years and up to 8 years. In this respect, denosumab seems to differ from the bisphosphonate group in which the rate of improvement of BMD diminishes and for some drugs becomes negative after 2–3 years when the process of secondary mineralization flattens out.
This unique property of an antiresorptive drug points toward mechanisms of action of denosumab that differ from the bisphosphonate group. Both types of drugs decrease cortical porosity but contrary to bisphosphonates the reduction in cortical porosity continues with denosumab which, in addition, also seems to cause a continuous modeling-based formation of new bone despite suppression of bone remodeling. The net effect is an increase in cortical thickening, bone mass and strength of cortical bone which most likely substantially contributes to a significant further reduction of the risk of nonvertebral fracture as observed in the long-term arm of the FREEDOM extension trial during years 4–7.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: OT has been lecturer and consultant for the Swedish National Board of Health and Welfare and The Swedish Medical Products Agency. OT has received fees from Amgen, GSK, Lilly, Nycomed, MEDA and Takeda as lecturer, consultant and/or scientific advisor.
References
- Anderson D., Maraskovsky E., Billingsley W., Dougall W., Tometsko M., Roux E., et al. (1997) A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390: 175–179. [DOI] [PubMed] [Google Scholar]
- Austin M., Yang Y., Vittinghoff E., Adami S., Boonen S., Bauer D., et al. (2012) Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fractures. J Bone Miner Res 27: 687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R., Ferrari S., Russell R.G. (2011) Denosumab and Bisphosphonates: Different Mechanisms of Action and Effects. Bone 48: 677–692. [DOI] [PubMed] [Google Scholar]
- Baron R., Hesse E. (2012) Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J Clin Endocrinol Metab 97: 311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekker P., Holloway D., Rasmussen A., Murphy R., Martin S., Leese P., et al. (2004) A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 19: 1059–1066. [DOI] [PubMed] [Google Scholar]
- Bilezikian J., Benhamou C., Lin C., Brown J., Daizadeh N., Ebeling P., et al. (2014) Denosumab restores cortical bone loss at the distal radius associated with aging and reduces wrist fracture risk: analyses from the FREEDOM extension cross-over group. J Bone Miner Res 29(Suppl. 1): abstract #1047. Available at: http://www.asbmr.org/education/2014-abstracts (accessed 22 September 2014). [Google Scholar]
- Black D., Cummings S., Karpf D., Cauley J., Thompson D., Nevitt M., et al. (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348: 1535–1541. [DOI] [PubMed] [Google Scholar]
- Black D., Schwartz A., Ensrud K., Cauley J., Levis S., Quandt S., et al. (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-Term Extension (FLEX): a randomized trial. JAMA 296: 2927–2938. [DOI] [PubMed] [Google Scholar]
- Black D., Delmas P., Eastell R., Reid I., Boonen S., Cauley J., et al. (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356: 1809–1822. [DOI] [PubMed] [Google Scholar]
- Black D., Reid I., Boonen S., Bucci-Rechtweg C., Cauley J., Cosman F., et al. (2012) The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the Horizon-Pivotal Fracture Trial (PFT). J Bone Miner Res 27: 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognese M., Teglbjaerg C., Zanchetta J., Lippuner K., McClung M., Brandi M., et al. (2013) Denosumab significantly increases DXA BMD at both trabecular and cortical sites: results from the FREEDOM study. J Clin Densitom 16: 147–153. [DOI] [PubMed] [Google Scholar]
- Bone H., Bolognese M., Yuen C., Kendler D., Miller P., Yang Y., et al. (2011) Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 96: 972–980. [DOI] [PubMed] [Google Scholar]
- Bone H., Chapurlat R., Brandi M., Brown J., Czerwinski E., Krieg M., et al. (2013) The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab 98: 4483–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone H., Hosking D., Devogelaer J., Tucci J., Emkey R., Tonino R., et al. (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350:1189–1199. [DOI] [PubMed] [Google Scholar]
- Boonen S., Adachi J., Man Z., Cummings S., Lippuner K., Torring O., et al. (2011) Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab 96: 1727–1736. [DOI] [PubMed] [Google Scholar]
- Brown J., Prince R., Deal C., Recker R., Kiel D., De Gregorio L., et al. (2009) Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 24: 153–161. [DOI] [PubMed] [Google Scholar]
- Brown J., Roux C., Torring O., Ho P., Beck Jensen J., Gilchrist N., et al. (2013) Discontinuation of denosumab and associated fracture incidence: analysis from the fracture reduction evaluation of denosumab in osteoporosis every 6 months (FREEDOM) trial. J Bone Miner Res 28: 746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut C., Skag A., Christiansen C., Recker R., Stakkestad J., Hoiseth A., et al. (2004) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. Journal of Bone and Mineral Research 19: 1241–1249. [DOI] [PubMed] [Google Scholar]
- Cummings S., San Martin J., McClung M., Siris E., Eastell R., Reid I., et al. (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361: 756–765. [DOI] [PubMed] [Google Scholar]
- Eastell R., Christiansen C., Grauer A., Kutilek S., Libanati C., McClung M., et al. (2011) Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J Bone Miner Res 26: 530–537. [DOI] [PubMed] [Google Scholar]
- Ettinger B., Black D., Mitlak B., Knickerbocker R., Nickelsen T., Genant H., et al. (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282: 637–645. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Adachi J., Zapalowski C., Miller P., Reginster J., Törring O., et al. (2013) Further reduction in the nonvertebral fracture rate is observed following 3 years of denosumab treatment: results with up to 7 years in the FREEDOM extension. J Bone Miner Res 28(Suppl. 1): abstract #1017. Available at: http://www.asbmr.org/education/2013-abstracts (accessed 23 August 2014). [Google Scholar]
- Geller M., Wagman R., Ho P., Siddhanti S., Stehman-Breen C., Watts N., et al. (2014) Findings from denosumab (Prolia(R)) post-marketing safety surveillance for atypical femur fracture, osteonecrosis of the jaw, severe symptomatic hypocalcemia, and anaphylaxis. J Bone Miner Res 29(Suppl. 1): abstract FR0388. Available at: http://www.asbmr.org/education/2014-abstracts (accessed 6 October 2014). [Google Scholar]
- Genant H., Engelke K., Hanley D., Brown J., Omizo M., Bone H., et al. (2010) Denosumab improves density and strength parameters as measured by QCT of the radius in postmenopausal women with low bone mineral density. Bone 47: 131–139. [DOI] [PubMed] [Google Scholar]
- Genant H., Libanati C., Engelke K., Zanchetta J., Hoiseth A., Yuen C., et al. (2013) Improvements in hip trabecular, subcortical, and cortical density and mass in postmenopausal women with osteoporosis treated with denosumab. Bone 56: 482–488. [DOI] [PubMed] [Google Scholar]
- Hansen S., Hauge E., Beck Jensen J., Brixen K. (2013) Differing effects of PTH 1–34, PTH 1–84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-PQCT. J Bone Miner Res 28:736–745. [DOI] [PubMed] [Google Scholar]
- Harris S., Watts N., Genant H., McKeever C., Hangartner T., Keller M., et al. (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA 282: 1344–1352. [DOI] [PubMed] [Google Scholar]
- Hochberg M., Greenspan S., Wasnich R., Miller P., Thompson D., Ross P. (2002) Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab 87: 1586–1592. [DOI] [PubMed] [Google Scholar]
- Hofbauer L., Schoppet M. (2004) Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 292: 490–495. [DOI] [PubMed] [Google Scholar]
- Hsu H., Lacey D., Dunstan C., Solovyev I., Colombero A., Timms E., et al. (1999) Tumor necrosis factor receptor family member rank mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA 96: 3540–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Zhao J., Mitlak B., Wang O., Genant H., Eriksen E. (2003) Recombinant human parathyroid hormone (1–34) [Teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res 18: 1932–1941. [DOI] [PubMed] [Google Scholar]
- Keaveny T., McClung M., Genant H., Zanchetta J., Kendler D., Brown J., et al. (2014) Femoral and vertebral strength improvements in postmenopausal women with osteoporosis treated with denosumab. J Bone Miner Res 29: 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler D., McClung M., Freemantle N., Lillestol M., Moffett A., Borenstein J., et al. (2011) Adherence, preference, and satisfaction of postmenopausal women taking denosumab or alendronate. Osteoporos Int 22: 1725–1735. [DOI] [PubMed] [Google Scholar]
- Kostenuik P. (2005) Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Current Opinion in Pharmacology 5:618–625. [DOI] [PubMed] [Google Scholar]
- Kostenuik P., Smith S., Jolette J., Schroeder J., Pyrah I., Ominsky M. (2011) Decreased bone remodeling and porosity are associated with improved bone strength in ovariectomized cynomolgus monkeys treated with denosumab, a fully human RANKL antibody. Bone 49: 151–161. [DOI] [PubMed] [Google Scholar]
- Lacey D., Timms E., Tan H., Kelley M., Dunstan C., Burgess T., et al. (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93: 165–176. [DOI] [PubMed] [Google Scholar]
- Lee D., Hoffmann P., Varela A., Kostenuik P., Ominsky M., Keaveny T. (2014) Finite element analysis accurately reflects the improvements in vertebral strength with denosumab in ovariectomized cynomolgus monkeys. J Bone Miner Res 29(Suppl. 1): abstract #SA0388. Available at: http://www.asbmr.org/education/2014-abstracts (accessed 6 October 2014). [Google Scholar]
- Lewiecki E., Miller P., McClung M., Cohen S., Bolognese M., Liu Y., et al. (2007) Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res 22: 1832–1841. [DOI] [PubMed] [Google Scholar]
- Li J., Sarosi I., Yan X., Morony S., Capparelli C., Tan H., et al. (2000) RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA 97: 1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandema J., Zheng J., Libanati C., Perez Ruixo J. (2014) Time course of bone mineral density changes with denosumab compared with other drugs in postmenopausal osteoporosis: a dose-response based meta-analysis. J Clin Endocrinol Metab 99: 3746–3755. [DOI] [PubMed] [Google Scholar]
- McClung M., Boonen S., Törring O., Roux C., Rizzoli R., Bone H., et al. (2012) Effect of denosumab treatment on the risk of fractures in subgroups of women with postmenopausal osteoporosis. J Bone Miner Res 27: 211–218. [DOI] [PubMed] [Google Scholar]
- McClung M., Geusens P., Miller P., Zippel H., Bensen W., Roux C., et al. (2001) Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group.N Engl J Med 344: 333–340. [DOI] [PubMed] [Google Scholar]
- McClung M., Lewiecki E., Cohen S., Bolognese M., Woodson G., Moffett A., et al. (2006) Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354: 821–831. [DOI] [PubMed] [Google Scholar]
- McClung M., Lewiecki E., Geller M., Bolognese M., Peacock M., Weinstein R., et al. (2013a) Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporos Int 24: 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung M., Zanchetta J., Hoiseth A., Kendler D., Yuen C., Brown J., et al. (2013b) Denosumab densitometric changes assessed by quantitative computed tomography at the spine and hip in postmenopausal women with osteoporosis. J Clin Densitom 16: 250–256. [DOI] [PubMed] [Google Scholar]
- Mellstrom D., Sorensen O., Goemaere S., Roux C., Johnson T., Chines A. (2004) Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 75: 462–468. [DOI] [PubMed] [Google Scholar]
- Ominsky M., Libanati C., Boyce R., Kostenuik P., Baron R., Wagman R., et al. (2013) Continuous modeling-based bone formation: a novel mechanism that could explain the sustained increases in hip bone mineral density (BMD) with denosumab treatment.J Bone Miner Res 28:(Suppl. 1): abstract #LB-MO30. Available at: http://www.asbmr.org/education/2013-abstracts (accessed 23 August 2014). [Google Scholar]
- Ominsky M., Libanati C., Boyce R., Kostenuik P., Baron R., Wagman R., et al. (2014) Continuous modelling-based bone formation could explain sustained increases in hip bone mineral density with denosumab treatment. Presented at the European Calcified Tissue Society Conference 2014 Abstract #pp355. Available at: http://www.bone-abstracts.org/ba/0003/ba0003pp355.htm (accessed 5 July 2014). [Google Scholar]
- Ominsky M., Stouch B., Schroeder J., Pyrah I., Stolina M., Smith S., et al. (2011) Denosumab, a fully human RANKL antibody, reduced bone turnover markers and increased trabecular and cortical bone mass, density, and strength in ovariectomized cynomolgus monkeys. Bone 49: 162–173. [DOI] [PubMed] [Google Scholar]
- Oxlund H., Ejersted C., Andreassen T., Torring O., Nilsson M. (1993) Parathyroid hormone (1–34) and (1–84) stimulate cortical bone formation both from periosteum and endosteum. Calcif Tissue Int 53: 394–399. [PubMed] [Google Scholar]
- Papapoulos S., Chapurlat R., Libanati C., Brandi M., Brown J., Czerwinski E., et al. (2012) Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J Bone Miner Res 27: 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K., Treece G., Gee A., Brown J., McClung M., Wang A., et al. (2014) Denosumab rapidly increases cortical bone in key locations of the femur: a 3D bone mapping study in women with osteoporosis. J Bone Miner Res 4 August (epub ahead of print). [DOI] [PubMed] [Google Scholar]
- Recker R., Bare S., Smith S., Varela A., Miller M., Morris S., et al. (2009) Cancellous and cortical bone architecture and turnover at the iliac crest of postmenopausal osteoporotic women treated with parathyroid hormone 1–84. Bone 44: 113–119. [DOI] [PubMed] [Google Scholar]
- Recknor C., Czerwinski E., Bone H., Bonnick S., Binkley N., Palacios S., et al. (2013) Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy: a randomized open-label trial. Obstet Gynecol 121: 1291–1299. [DOI] [PubMed] [Google Scholar]
- Reid I., Miller P., Brown J., Kendler D., Fahrleitner-Pammer A., Valter I., et al. (2010) Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies. J Bone Miner Res 25: 2256–2265. [DOI] [PubMed] [Google Scholar]
- Roux C., Hofbauer L., Ho P., Wark J., Zillikens M., Fahrleitner-Pammer A., et al. (2014) Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label study. Bone 58: 48–54. [DOI] [PubMed] [Google Scholar]
- Samelson E., Miller P., Christiansen C., Daizadeh N., Grazette L., Anthony M., et al. (2014) RANKL inhibition with denosumab does not influence 3-year progression of aortic calcification or incidence of adverse cardiovascular events in postmenopausal women with osteoporosis and high cardiovascular risk. J Bone Miner Res 29: 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E., Delmas P., Hanley D., Sellmeyer D., Cheung A., Shane E., et al. (2010) Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 25: 1886–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J., Recknor C., Moffett A., Jr, Adachi J., Franek E., Lewiecki E., et al. (2013) Impact of denosumab on the peripheral skeleton of postmenopausal women with osteoporosis: bone density, mass, and strength of the radius, and wrist fracture. Menopause 20: 130–137. [DOI] [PubMed] [Google Scholar]
- Simonet W., Lacey D., Dunstan C., Kelley M., Chang M., Luthy R., et al. (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89: 309–319. [DOI] [PubMed] [Google Scholar]
- Wasnich R., Miller P. (2000) Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab 85: 231–236. [DOI] [PubMed] [Google Scholar]
- Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., et al. (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95: 3597–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeni Y., Brown C., Wang Z., Norman T. (1997) The influence of bone morphology on fracture toughness of the human femur and tibia. Bone 21: 453–459. [DOI] [PubMed] [Google Scholar]
- Zebaze R., Libanati C., Austin M., Ghasem-Zadeh A., Hanley D., Zanchetta J., et al. (2014a) Differing effects of denosumab and alendronate on cortical and trabecular bone. Bone 59: 173–179. [DOI] [PubMed] [Google Scholar]
- Zebaze R., Libanati C., McClung M., Zanchetta J., Kendler D., Höiseth A., et al. (2014b) Denosumab treatment in women with osteoporosis reduces hip cortical porosity. Presented at the European Calcified Tissue Society Conference 2014 Abstract #pp354. Available at: http://www.bone-abstracts.org/ba/0003/ba0003pp355.htm (accessed 28 August 2014). [Google Scholar]