Abstract
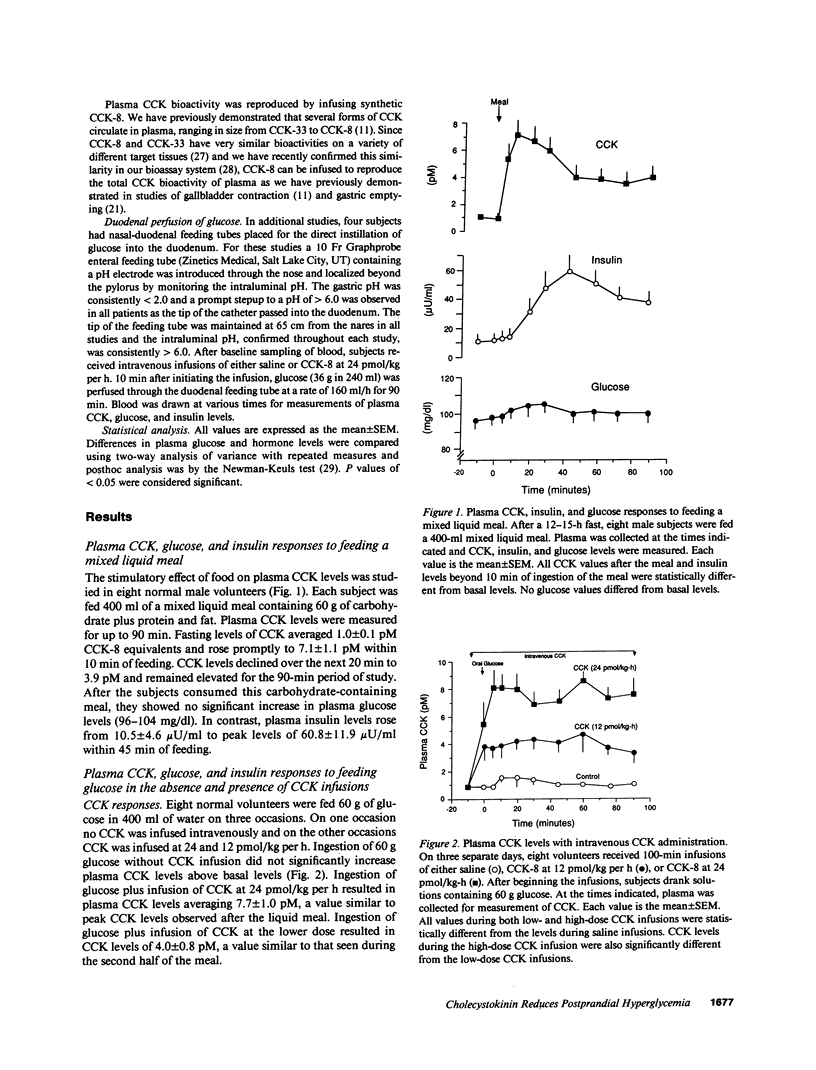
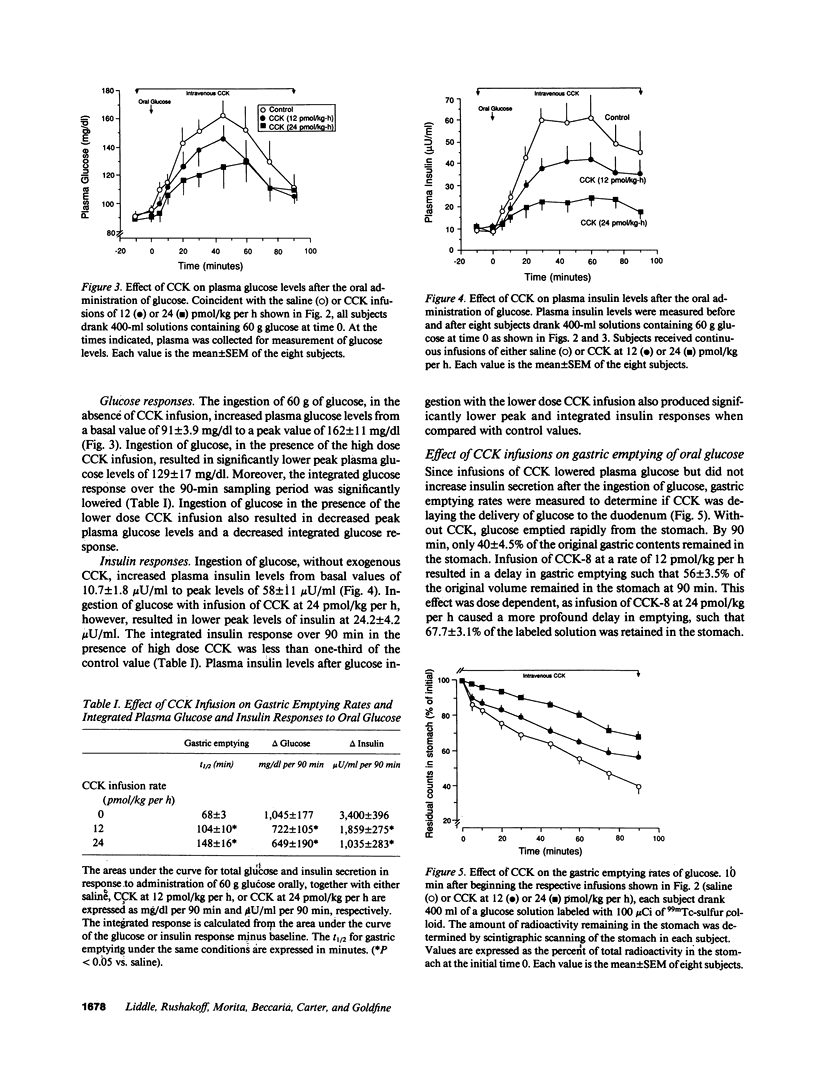
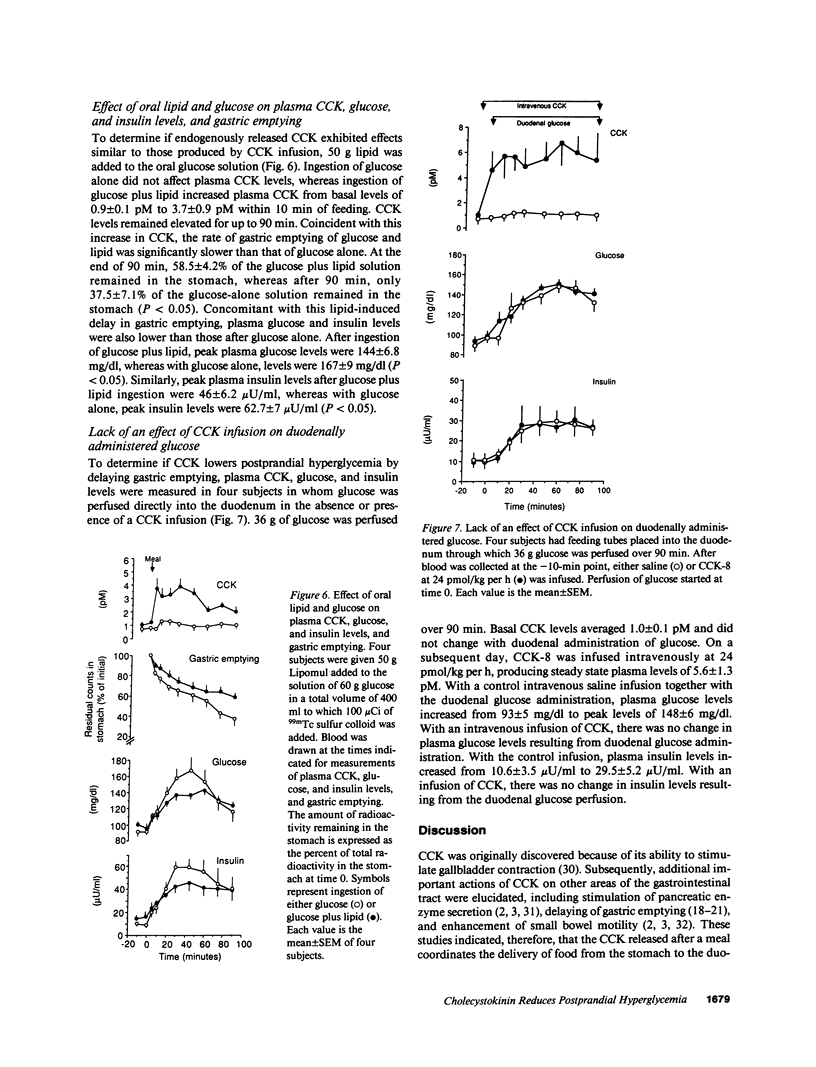
It is known that the ingestion of glucose alone causes a greater increase in plasma glucose levels than ingestion of the same amount of glucose given with other nutrients. Since physiological plasma concentrations of cholecystokinin (CCK) prolong gastric emptying, it is proposed that after a meal, CCK may modify plasma glucose levels by delaying glucose delivery to the duodenum. To evaluate the effect of CCK on oral glucose tolerance, plasma CCK, insulin, and glucose levels and gastric emptying rates were measured in eight normal males before and after the ingestion of 60 g glucose with the simultaneous infusion of either saline or one of two doses of CCK-8 (12 or 24 pmol/kg per h). Gastric emptying rates were measured by gamma camera scintigraphy of technetium 99m sulfur colloid and plasma CCK levels were measured by a sensitive and specific bioassay. Basal CCK levels averaged 1.0 +/- 0.1 pM (mean +/- SEM, n = 8) and increased to 7.1 +/- 1.1 pM after a mixed liquid meal. After glucose ingestion, but without CCK infusion, CCK levels did not change from basal, and the gastric emptying t1/2 was 68 +/- 3 min. Plasma glucose levels increased from basal levels of 91 +/- 3.9 mg/dl to peak levels of 162 +/- 11 mg/dl and insulin levels increased from 10.7 +/- 1.8 microU/ml to peak levels of 58 +/- 11 microU/ml. After glucose ingestion, with CCK infused at 24 pmol/kg per h, plasma CCK levels increased to 8 pM and the gastric emptying t1/2 increased to 148 +/- 16 min. In concert with this delay in gastric emptying, peak glucose levels rose to only 129 +/- 17 mg% and peak insulin levels rose to only 24.2 +/- 4.2 microU/ml. With CCK at 12 pmol/kg per h, similar but less dramatic changes were seen. To demonstrate that endogenous CCK could modify the plasma glucose and insulin responses to oral glucose, oral glucose was given with 50 g of lipid containing long-chain triglycerides. This lipid increased peak CCK levels to 3.7 +/- 0.9 pM. Concomitant with this rise in CCK was a delay in gastric emptying and a lowering of plasma glucose and insulin values. To confirm that CCK reduced hyperglycemia by its effect on gastric motility, 36 g glucose was perfused directly into the duodenum through a nasal-duodenal feeding tube in four subjects. With duodenal perfusion of glucose, there was no change in plasma CCK levels, but plasma glucose levels increased from basal levels of 93+/-5 to 148+/-6 mg/dl and insulin levels rose from 10.6+/-3.5 to 29.5+/-5.2 microU/ml. When CCK was infused at 24 pmol/kg per h, neither the plasma glucose nor insulin responses to the duodenal administration of glucose were modified. Thus we conclude that CCK, in physiological concentrations, delays gastric emptying, slows the delivery of glucose to the duodenum, and reduces postprandial hyperglycemia. These data indicate, therefore, that CCK has a significant role in regulating glucose homeostasis in human.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anika M. S. Effects of cholecystokinin and caerulein on gastric emptying. Eur J Pharmacol. 1982 Nov 19;85(2):195–199. doi: 10.1016/0014-2999(82)90465-4. [DOI] [PubMed] [Google Scholar]
- Bantle J. P., Laine D. C., Castle G. W., Thomas J. W., Hoogwerf B. J., Goetz F. C. Postprandial glucose and insulin responses to meals containing different carbohydrates in normal and diabetic subjects. N Engl J Med. 1983 Jul 7;309(1):7–12. doi: 10.1056/NEJM198307073090102. [DOI] [PubMed] [Google Scholar]
- Barker G. R., Cochrane G. M., Corbett G. A., Dufton J. F., Hunt J. N., Roberts S. K. Glucose, glycine and diglycine in test meals at stimuli to a duodenal osmoreceptor slowing gastric emptying. J Physiol. 1978 Oct;283:341–346. doi: 10.1113/jphysiol.1978.sp012504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman D. N. Effects of meal temperature and volume on the emptying of liquid from the human stomach. J Physiol. 1982 Oct;331:461–467. doi: 10.1113/jphysiol.1982.sp014383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondar R. J., Mead D. C. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem. 1974 May;20(5):586–590. [PubMed] [Google Scholar]
- Christian P. E., Datz F. L., Sorenson J. A., Taylor A. Technical factors in gastric emptying studies. J Nucl Med. 1983 Mar;24(3):264–268. [PubMed] [Google Scholar]
- Creutzfeldt W., Ebert R. New developments in the incretin concept. Diabetologia. 1985 Aug;28(8):565–573. doi: 10.1007/BF00281990. [DOI] [PubMed] [Google Scholar]
- Dupre J., Curtis J. D., Unger R. H., Waddell R. W., Beck J. C. Effects of secretin, pancreozymin, or gastrin on the response of the endocrine pancreas to administration of glucose or arginine in man. J Clin Invest. 1969 Apr;48(4):745–757. doi: 10.1172/JCI106032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre J., Ross S. A., Watson D., Brown J. C. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973 Nov;37(5):826–828. doi: 10.1210/jcem-37-5-826. [DOI] [PubMed] [Google Scholar]
- Eng J., Du B. H., Pan Y. C., Chang M., Hulmes J. D., Yalow R. S. Purification and sequencing of a rat intestinal 22 amino acid C-terminal CCK fragment. Peptides. 1984 Nov-Dec;5(6):1203–1206. doi: 10.1016/0196-9781(84)90188-8. [DOI] [PubMed] [Google Scholar]
- Eysselein V. E., Reeve J. R., Jr, Shively J. E., Hawke D., Walsh J. H. Partial structure of a large canine cholecystokinin (CCK58): amino acid sequence. Peptides. 1982 Jul-Aug;3(4):687–691. doi: 10.1016/0196-9781(82)90171-1. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Raper H. S. Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. J Physiol. 1943 Jun 30;102(1):115–125. doi: 10.1113/jphysiol.1943.sp004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansen K. Effects of cholecystokinin (CCK)-4, nonsulfated CCK-8, and sulfated CCK-8 on pancreatic somatostatin, insulin, and glucagon secretion in the dog: studies in vitro. Endocrinology. 1984 May;114(5):1770–1775. doi: 10.1210/endo-114-5-1770. [DOI] [PubMed] [Google Scholar]
- Hunt J. N. Does calcium mediate slowing of gastric emptying by fat in humans? Am J Physiol. 1983 Jan;244(1):G89–G94. doi: 10.1152/ajpgi.1983.244.1.G89. [DOI] [PubMed] [Google Scholar]
- Hunt J. N., Stubbs D. F. The volume and energy content of meals as determinants of gastric emptying. J Physiol. 1975 Feb;245(1):209–225. doi: 10.1113/jphysiol.1975.sp010841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levant J. A., Kun T. L., Jachna J., Sturdevant R. A., Isenberg J. I. The effects of graded doses of C-terminal octapeptide of cholecystokinin on small intestinal transit time in man. Am J Dig Dis. 1974 Mar;19(3):207–209. doi: 10.1007/BF01072536. [DOI] [PubMed] [Google Scholar]
- Liddle R. A., Elashoff J., Reeve J. R., Jr Relative bioactivities of cholecystokinins-8 and -33 on rat pancreatic acini. Peptides. 1986 Sep-Oct;7(5):723–727. doi: 10.1016/0196-9781(86)90085-9. [DOI] [PubMed] [Google Scholar]
- Liddle R. A., Goldfine I. D., Rosen M. S., Taplitz R. A., Williams J. A. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985 Apr;75(4):1144–1152. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle R. A., Goldfine I. D., Williams J. A. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology. 1984 Sep;87(3):542–549. [PubMed] [Google Scholar]
- Liddle R. A., Morita E. T., Conrad C. K., Williams J. A. Regulation of gastric emptying in humans by cholecystokinin. J Clin Invest. 1986 Mar;77(3):992–996. doi: 10.1172/JCI112401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCINTYRE N., HOLDSWORTH C. D., TURNER D. S. NEW INTERPRETATION OF ORAL GLUCOSE TOLERANCE. Lancet. 1964 Jul 4;2(7349):20–21. doi: 10.1016/s0140-6736(64)90011-x. [DOI] [PubMed] [Google Scholar]
- Moran T. H., McHugh P. R. Cholecystokinin suppresses food intake by inhibiting gastric emptying. Am J Physiol. 1982 May;242(5):R491–R497. doi: 10.1152/ajpregu.1982.242.5.R491. [DOI] [PubMed] [Google Scholar]
- Mutt V., Jorpes J. E. Structure of porcine cholecystokinin-pancreozymin. 1. Cleavage with thrombin and with trypsin. Eur J Biochem. 1968 Oct 17;6(1):156–162. doi: 10.1111/j.1432-1033.1968.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Otsuki M., Sakamoto C., Yuu H., Maeda M., Morita S., Ohki A., Kobayashi N., Terashi K., Okano K., Baba S. Discrepancies between the doses of cholecystokinin or caerulein-stimulating exocrine and endocrine responses in perfused isolated rat pancreas. J Clin Invest. 1979 Mar;63(3):478–484. doi: 10.1172/JCI109325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz D., Merimee T. J., Maffezzoli R., Burgess J. A. Patterns of hormonal release after glucose, protein, and glucose plus protein. Lancet. 1966 Aug 27;2(7461):454–456. doi: 10.1016/s0140-6736(66)92767-x. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. Immunochemical studies on cholecystokinin. I. Development of sequence-specific radioimmunoassays for porcine triacontatriapeptide cholecystokinin. J Biol Chem. 1978 Jun 10;253(11):4016–4021. [PubMed] [Google Scholar]
- Rushakoff R. J., Goldfine I. D., Carter J. D., Liddle R. A. Physiological concentrations of cholecystokinin stimulate amino acid-induced insulin release in humans. J Clin Endocrinol Metab. 1987 Sep;65(3):395–401. doi: 10.1210/jcem-65-3-395. [DOI] [PubMed] [Google Scholar]
- Solomon T. E., Yamada T., Elashoff J., Wood J., Beglinger C. Bioactivity of cholecystokinin analogues: CCK-8 is not more potent than CCK-33. Am J Physiol. 1984 Jul;247(1 Pt 1):G105–G111. doi: 10.1152/ajpgi.1984.247.1.G105. [DOI] [PubMed] [Google Scholar]
- Szecówka J., Lins P. E., Efendić S. Effects of cholecystokinin, gastric inhibitory polypeptide, and secretin on insulin and glucagon secretion in rats. Endocrinology. 1982 Apr;110(4):1268–1272. doi: 10.1210/endo-110-4-1268. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Ketterer H., Dupré J., Eisentraut A. M. The effects of secretin, pancreozymin, and gastrin on insulin and glucagon secretion in anesthetized dogs. J Clin Invest. 1967 Apr;46(4):630–645. doi: 10.1172/JCI105565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verspohl E. J., Ammon H. P., Williams J. A., Goldfine I. D. Evidence that cholecystokinin interacts with specific receptors and regulates insulin release in isolated rat islets of Langerhans. Diabetes. 1986 Jan;35(1):38–43. doi: 10.2337/diab.35.1.38. [DOI] [PubMed] [Google Scholar]
- Yamagishi T., Debas H. T. Cholecystokinin inhibits gastric emptying by acting on both proximal stomach and pylorus. Am J Physiol. 1978 Apr;234(4):E375–E378. doi: 10.1152/ajpendo.1978.234.4.E375. [DOI] [PubMed] [Google Scholar]



