Abstract
Objectives:
Guidelines for antiretroviral therapy (ART) initiation have evolved, but consistently note that adherence problems should be considered and addressed. Little is known regarding the reasons providers delay ART initiation in clinically eligible patients.
Methods:
In 2009, we surveyed a probability sample of HIV care providers in 582 outpatient facilities in the United States and Puerto Rico with an open-ended question about nonclinical reasons for delaying ART initiation in otherwise clinically eligible patients.
Results:
Very few providers (2%) reported never delaying ART. Reasons for delaying ART were concerns about patient adherence (68%), patient acceptance (60%), and structural barriers (33%). Provider and practice characteristics were associated with reasons for delaying ART.
Conclusion:
Reasons for delaying ART were consistent with clinical guidelines and were both patient level and structural. Providers may benefit from training and access to referrals for ancillary services to enhance their ability to monitor and address these issues with their patients.
Keywords: antiretroviral therapy, adherence, clinical practice
Introduction
Antiretroviral therapy (ART) for HIV infection prolongs life and prevents HIV transmission.1,2 The US Department of Health and Human Services guidelines for the timing of ART initiation based on pretreatment CD4 count have evolved toward recommendation of treatment for all HIV-infected persons but have consistently included strong recommendations that providers consider and address patient barriers to adherence, as suboptimal adherence can lead to treatment failure and viral resistance.3-5 The guidelines state that providers may choose to defer ART initiation based on factors associated with poor adherence, such as substance use and mental health comorbidities, patient readiness, and other social factors. However, the guidelines also stress the key role of the provider in assessing and identifying areas for intervention needed to improve adherence to ART.3-5 Although there are many studies on the prevalence of ART use,6-9 less is known regarding reasons providers delay ART initiation for clinically eligible patients. Both provider-level factors (profession, years caring for HIV-infected patients, number of HIV-infected patients, having sufficient time or ancillary support to provide counseling) and patient-level factors (drug and alcohol use, homelessness, appointment adherence, and lifestyle factors) have been found to be associated with provider deferral of ART initiation in subpopulations such as youth, injection drug users (IDUs), or persons with schizophrenia.10-13 Assessing barriers to ART initiation for all HIV-infected persons is essential because expanded ART eligibility guidelines, longer life expectancies, and efforts to increase testing, linkage to, and engagement in care are likely to increase the size of the HIV-infected patient population and expand the number of patients for whom providers will consider initiating ART. Characterizing HIV care providers who care for patients experiencing specific barriers to ART initiation may facilitate targeted education and training that gives these providers the resources and support they need to extend the benefits of ART to all of their clinically eligible patients. This analysis addresses the following questions: Do HIV care providers adhere to guidelines regarding ART initiation for clinically eligible patients? What types of nonclinical barriers to ART initiation for otherwise clinically eligible patients do providers experience? What are the patient- and provider-related factors associated with these barriers?
Methods
For the Medical Monitoring Project (MMP) Provider Survey, a probability sample of HIV care providers was selected from outpatient HIV care facilities in 19 states and Puerto Rico that were selected for the second stage of MMP sampling. Sampling methods for MMP have been described in detail elsewhere.14,15 Briefly, MMP is a complex sample, cross-sectional survey where US states and territories are sampled first, followed by facilities providing HIV care, and then by HIV-infected adults (persons aged 18 years and older) who had at least 1 medical care visit during January to April of the given data collection year at participating facilities. All sampled US states and territories participated in MMP, including California (including the separately funded jurisdictions of Los Angeles County and San Francisco), Delaware, Florida, Georgia, Illinois (including Chicago), Indiana, Maryland, Massachusetts, Michigan, Mississippi, New Jersey, New York (including New York City), North Carolina, Oregon, Pennsylvania (including Philadelphia), Puerto Rico, South Carolina, Texas (including Houston), Virginia, and Washington. Of 828 eligible facilities sampled for the second stage of MMP, 582 participated (to avoid biasing the probability sample, nonparticipating facilities were not selectively replaced). For the MMP Provider Survey, the MMP staff obtained a complete list of HIV care providers working at all 582 facilities participating in MMP. In all, 2600 providers were identified, and 1999 were randomly sampled to participate in the MMP Provider Survey. The sample size was chosen based on logistical and funding considerations.
Providers eligible for the survey met the following conditions: (1) provided care to HIV-infected patients aged ≥18 years at a sampled MMP facility at the time of the survey and (2) were physicians (either doctor of medicine or doctor of osteopathic medicine), nurse practitioners (NPs), or physician assistants (PAs) who had completed their formal professional training. Providers were recruited by using a modified version of the Dillman method,16 which entailed mailing individualized recruitment packets to all sampled providers. The recruitment packets included a letter from Centers for Disease Control and Prevention (CDC) explaining the purpose of the survey, instructions for completing the self-administered survey via paper- or Web-based forms, and a US$15 gift card. Nonrespondents were sent 3 additional mailings at set intervals over the following 7 weeks. All data collection was conducted during June 2009–September 2009.
The survey took approximately 15 minutes to complete and consisted of questions concerning provider characteristics, clinic practice characteristics, patient characteristics, HIV treatment referral practices, HIV care and treatment practices, HIV risk-reduction counseling practices, and perceptions of patients’ barriers to HIV care.
In accordance with the federal human subjects protection regulations at 45 Code of Federal Regulations 46.101c and 46.102d and with the Guidelines for Defining Public Health Research and Public Health Non-Research,17,18 MMP was determined by the National Center for HIV, Viral Hepatitis, STD and TB Prevention’s Office of the Associate Director for Science at the CDC to be a nonresearch, public health surveillance activity used for disease control program or policy purposes. As such, MMP is not subject to human subjects regulations, including federal investigational review board (IRB) review. As an amendment to MMP, the MMP Provider Survey is covered under the same nonresearch determination. Nevertheless, participating project areas and facilities obtained local IRB approval to conduct MMP as required locally. In addition, none of the authors of this article has had access to any information that would directly identify individual persons from whom data were collected. The MMP Provider Survey recruitment letter explained the voluntary nature of the participation in the survey. No formal informed consent was obtained.
Analytic Methods
We used an inductive thematic analysis approach19 to code provider’s written responses to the open-ended survey question, “For patients who are clinically eligible for antiretroviral therapy, what are the main reasons that you might delay initiating antiretroviral therapy?” Three researchers (L.B., E.E.V., and J.L.R.), using a standardized iterative process,20 developed a codebook with thematic codes for nonclinical reasons that providers choose to delay ART initiation; to ensure coding reliability, these researchers then independently coded the question responses. Differences were resolved through discussion and, when needed, modification of coding criteria. Clinical eligibility for ART was not defined for the providers, as providers may disagree on when to prescribe ART and the intent of the question was to assess nonclinical reasons for delaying ART.
Selected provider, practice, and medical care variables, informed by existing literature on barriers to ART initiation,10-13 were assessed to examine their relationship with coded reasons providers gave for delaying ART initiation for clinically eligible persons. HIV provider type was dichotomized as physicians versus NPs or PAs. Provider characteristics assessed included age, gender, race/ethnicity, years caring for HIV-infected patients, and self-assessed knowledge regarding HIV treatment. Practice characteristics included the number of HIV-infected patients cared for per month and the proportion of the provider’s patients who were white, female, men who have sex with men (MSM), and IDUs. Providers were also asked how often they referred patients for ART initiation and whether they felt they had sufficient time to provide HIV care. We used SAS version 9.3 (SAS Institute, Inc, Cary, North Carolina) to calculate frequencies and chi-square tests for association between provider and practice characteristics and reasons providers choose to delay ART. Only factors associated with each reason at P < .10 were entered into separate multivariable logistic regression models (one for each reason) and adjusted odds ratios and 95% confidence intervals are presented.
Results
Of 1999 HIV care providers who were mailed the surveys, 1743 (87%) were eligible. Of those eligible, 734 (42%) completed the survey. Among the 640 providers (87% of providers completed the survey and 37% of all sampled eligible providers) who provided responses to the question about reasons for delaying ART for clinically eligible patients, 15 (2%) said they never delayed ART for clinically eligible persons and 625 (98%) HIV care practitioners provided information on reasons for delaying ART. Among those providing information about reasons for delaying ART, the majority were physicians (69%), were white (74%), had more than 10 years of HIV care experience (69%), and considered themselves to be extremely or very knowledgeable regarding HIV treatment (90%; Table 1).
Table 1.
Provider, Practice, and Medical Care Characteristics of HIV Care Providers Who Provided Reasons for Delaying ART Initiation for Clinically Eligible Patients.a,b
| Characteristics | No. (%) |
|---|---|
| Profession | |
| Physician | 432 (69) |
| Nurse practitioner/physician assistant | 192 (31) |
| Age (years) | |
| 20-34 | 34 (5) |
| 35-49 | 296 (47) |
| 50-64 | 267 (43) |
| 65-88 | 23 (4) |
| Gender | |
| Male | 307 (49) |
| Female | 317 (51) |
| Race/ethnicity | |
| African American | 44 (7) |
| Hispanic | 60 (10) |
| White | 463 (74) |
| Other | 47 (8) |
| Years caring for HIV-infected patients | |
| 0-5 | 84 (13) |
| 6-10 | 102 (16) |
| 11-30 | 434 (69) |
| Knowledgeable regarding HIV treatment? | |
| Extremely/very | 561 (90) |
| Somewhat/not at all | 64 (10) |
| HIV-infected patients/month | |
| 0-20 | 127 (20) |
| 21-50 | 155 (25) |
| 51 or more | 340 (54) |
| White patients | |
| 0%-50% | 462 (74) |
| 51%-100% | 131 (21) |
| Female patients | |
| 0%-50% | 577 (92) |
| 51%-100% | 46 (7) |
| Men who have sex with men | |
| 0%-50% | 248 (40) |
| 51%-100% | 371 (59) |
| Injecting drug users | |
| 0%-50% | 578 (92) |
| 51%-100% | 45 (7) |
| Refer for ART initiation | |
| Never | 542 (87) |
| Sometimes | 45 (7) |
| Always | 34 (5) |
| Sufficient time to provide HIV care | |
| Agree | 399 (63) |
| Neither agree nor disagree | 56 (9) |
| Not agree | 166 (27) |
Abbreviations: ART, antiretroviral therapy; MMP, Medical Monitoring Project.
aN = 625—MMP Provider Survey 2009.
bNumbers may not add to total and percentage may not total 100% because of missing data. Values exclude don’t know responses.
Reasons for Delaying ART
Responses to the question about reasons for delaying ART fell into 3 broad categories, namely, provider concerns about patient adherence (adherence concerns mentioned by 68%), patient acceptance of ART (acceptance concerns mentioned by 60%), and provider concerns about structural barriers to ART use (structural concerns mentioned by 33%). Within each category, subthemes were identified (Table 2). All themes and subthemes identified were either directly associated with poor adherence (eg, substance use) or indirectly associated with adherence via inconsistent access to ART (eg, insurance) and were thus consistent with recommended guidelines regarding nonclinical factors providers should consider when initiating ART.
Table 2.
Reasons for Delaying Antiretroviral Therapy for Clinically Eligible Patients.a
| Themes | Subthemesb | No. (%) |
|---|---|---|
| Adherence concerns | 424 (68) | |
| Substance abuse | 216 (35) | |
| Mental health | 178 (28) | |
| General nonadherence (anticipated or history of) | 148 (24) | |
| Chaos/instability (lifestyle and social situation) | 106 (17) | |
| Appointment adherence | 63 (10) | |
| Acceptance concerns | 378 (60) | |
| Readiness/refusal to start/commit | 347 (56) | |
| Denial/fear/lack of knowledge | 34 (5) | |
| Structural concerns | 209 (33) | |
| Cost/insurance/medications | 122 (20) | |
| Homelessness/unstable housing | 81 (13) | |
| Otherc | 23 (4) |
Abbreviation: MMP, Medical Monitoring Project.
aN = 625—MMP Provider Survey 2009.
bParticipants could have identified more than one subtheme.
cOther includes incarceration, referral to other care source, and transportation.
Within adherence concerns, providers mentioned specific factors that would lead them to have concerns about patient adherence sufficient that they would not prescribe ART to an otherwise eligible patient. One factor was substance abuse, which was mentioned by 35% of providers. Some providers specifically mentioned “active” drug use, implying they would not prescribe ART to patients whose substance abuse issues were not being adequately treated. Adherence concerns due to mental health problems (28%) were also mentioned, and some providers specified that their concerns were specific to “uncontrolled” psychiatric issues. General concerns about a patient’s likelihood of adherence were also mentioned (24%), sometimes based on a history of nonadherence and at other times on the provider’s expectation of patient nonadherence (“someone I know won’t adhere to taking meds regularly”). Another subtheme was concerns about adherence due to chaos or instability in a patient’s lifestyle or social situation (17%). Finally, some providers stated they would have concerns about adherence to ART if patients were nonadherent to their appointments (10%).
Within patient acceptance concerns, more than half (56%) of the providers cited patient lack of “readiness” to take or commit to ART (eg, “patient not ready to commit to lifelong therapy”). Also categorized in this subtheme were mentions of patient refusal to start ART (eg, “patient does not want to take pills”). A smaller number of providers (5%) cited patient “denial” about their positive HIV status, fear of taking ART, or lack of knowledge about ART as barriers to prescribing.
Within structural concerns, 20% of providers mentioned barriers to prescribing ART related to the cost of medication or lack of insurance or coverage for medications (eg, “access to funding for medications”). Some providers characterized these issues as short-term delays (eg, “patient waiting for AIDS Drug Assistance Program [ADAP] or Medicaid approval”), whereas others cited program eligibility barriers that could result in longer term delay of ART (eg, “ADAP in Georgia will not pay for it unless CD4 cells/mm3 count is less than 350”). A smaller number of providers (10%) mentioned barriers related to patient homelessness or unstable housing (eg, “arrange housing for homeless” and “unstable living arrangements”). Finally, a few providers (4%) cited other structural barriers, such as delays related to the transfer of patients into or out of the correctional system or waiting for consultation with a specialist before prescribing ART.
Figure 1 illustrates the nonclinical barriers to ART initiation cited by providers. Nearly all providers (94%) mentioned either adherence or acceptance concerns, and 12% of providers mentioned all 3 barriers (Figure 1).
Figure 1.
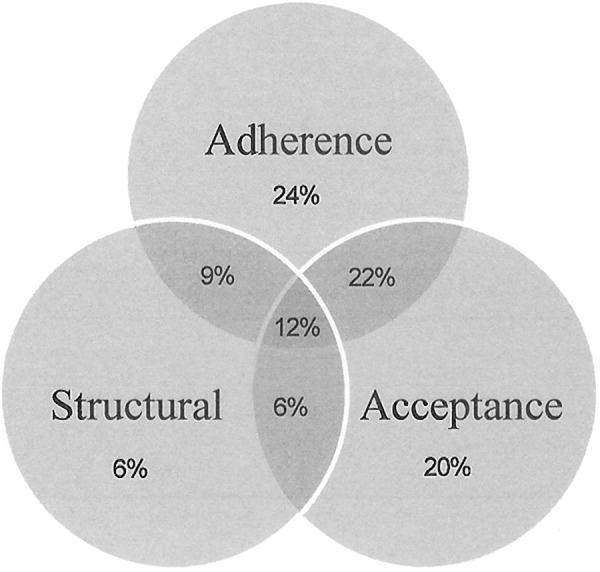
Reasons for delaying antiretroviral therapy for clinically eligible patients, N = 625—MMP Provider Survey 2009.
Factors Associated with Reasons for Delaying ART
Factors associated with different reasons for delaying ART varied (Table 3). Multivariable modeling indicated that provider age <50 compared to ≥65, white compared to African American or other provider race/ethnicity, and having 51 or more patients per month compared to less than 21 patients per month were independently associated with citing adherence concerns. Being an NP or PA compared to a physician, having more than 5 years of experience caring for HIV-infected patients, having more than 50% of MSM patients, and never referring for ART initiation were independently associated with identifying acceptance concerns. White compared to Hispanic provider race/ethnicity was the only factor independently associated with mentioning structural concerns.
Table 3.
Provider, Practice, and Medical Care Characteristics Associated with Reasons for Delaying Antiretroviral Therapy for Clinically Eligible Patients.a,b
| Provider/Patient/HIV Care Characteristics | Adherence Concerns | Acceptance Concerns | Structural Concerns | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | χ2 P Value | aOR (95% CI)b | P Value | No. (%) | χ2 P Value | aOR (95% CI)b | P Value | No. (%) | χ2 P Value | aOR (95% CI)b | P Value | |
| Profession | .10 | .08 | .02 | .21 | ||||||||
| Physician | 284 (66) | 251 (58) | Referent | 138 (32) | ||||||||
| NP/PA | 139 (72) | 126 (65) | 1.6 (1.1-2.5) | 71 (37) | ||||||||
| Age (years) | <.01 | <.01 | .51 | .04 | .05 | |||||||
| 20-34 | 27 (79) | 5.3 (1.5-19.1) | 22 (64) | 11 (32) | 1.3 (0.7-2.5) | |||||||
| 35-49 | 214 (72) | 3.8 (1.5-9.8) | 169 (57) | 115 (39) | 1.2 (0.4-2.8) | |||||||
| 50-64 | 170 (64) | 2.1 (0.9-5.5) | 168 (63) | 73 (27) | 0.7 (0.3-1.7) | |||||||
| 65-88 | 10 (43) | Referent | 14 (61) | 8 (35) | Referent | |||||||
| Gender | .22 | .56 | .07 | .34 | ||||||||
| Male | 201 (65) | 182 (60) | 92 (30) | 0.8 (0.6-1.2) | ||||||||
| Female | 222 (70) | 195 (62) | 117 (37) | Referent | ||||||||
| Race/ethnicity | <.01 | <.01 | .65 | .01 | .03 | |||||||
| African American | 25 (57) | 0.5 (0.2-0.9) | 28 (64) | 20 (45) | 1.2 (0.6-2.2) | |||||||
| Hispanic | 37 (62) | 0.5 (0.3 -1.0) | 32 (53) | 11 (18) | 0.3 (0.2-0.8) | |||||||
| White | 330 (71) | Referent | 284 (61) | 154 (33) | Referent | |||||||
| Other | 24 (51) | 0.3 (0.2-0.7) | 28 (60) | 20 (42) | 1.3 (0.7-2.5) | |||||||
| Years caring for HIV-infected patients | .43 | <.01 | <.01 | .68 | ||||||||
| 0-5 | 62 (74) | 37 (44) | Referent | 27 (32) | ||||||||
| 6-10 | 69 (68) | 64 (63) | 2.4 (1.3-4.4) | 38 (37) | ||||||||
| 11-30 | 289 (67) | 275 (63) | 2.3 (1.4-3.9) | 143 (33) | ||||||||
| Knowledgeable regarding HIV treatment? | <.01 | .12 | <.01 | .93 | .47 | |||||||
| Extremely/very | 397 (71) | 1.8 (0.9-3.7) | 349 (62) | 1.0 (0.5-2.1) | 185 (33) | |||||||
| Somewhat/not at all | 27 (42) | Referent | 29 (38) | Referent | 24 (37) | |||||||
| HIV-infected patients/month | <.01 | .03 | <.01 | .27 | .10 | |||||||
| 0-20 | 66 (52) | Referent | 63 (50) | Referent | 41 (32) | |||||||
| 21-50 | 105 (68) | 1.6 (0.9-2.8) | 92 (59) | 1.4 (0.8-2.4) | 42 (27) | |||||||
| 51 or more | 250 (73) | 1.9 (1.2-3.3) | 221 (65) | 1.5 (0.9-2.4) | 125 (37) | |||||||
| Proportion of white patients | .44 | .25 | .07 | .11 | ||||||||
| 0%-50% | 316 (68) | 283 (61) | 162 (35) | 1.4 (0.9-2.3) | ||||||||
| 51%-100% | 85 (65) | 73 (55) | 35 (27) | Referent | ||||||||
| Proportion of female patients | .27 | .13 | .03 | .11 | ||||||||
| 0%-50% | 396 (69) | 353 (61) | 186 (32) | 0.6 (0.3-1.1) | ||||||||
| 51%-100% | 28 (61) | 23 (50) | 22 (48) | Referent | ||||||||
| Proportion of patients who are men who have sex with men | .60 | <.01 | .01 | .77 | ||||||||
| 0%-50% | 172 (69) | 134 (54) | Referent | 85 (34) | ||||||||
| 51%-100% | 250 (67) | 240 (66) | 1.5 (1.1-2.2) | 123 (33) | ||||||||
| Injecting drug users | .12 | .49 | .99 | |||||||||
| 0%-50% | 398 (69) | 351 (61) | 193 (33) | |||||||||
| 51%-100% | 26 (58) | 25 (56) | 15 (33) | |||||||||
| Refer ART initiation | <.01 | .38 | <.01 | .02 | .36 | |||||||
| Never | 383 (71) | 1.3 (0.7-2.4) | 341 (63) | 2.1 (1.1-3.9) | 178 (33) | |||||||
| Sometimes/always | 39 (49) | Referent | 34 (43) | Referent | 30 (38) | |||||||
| Sufficient time to provide HIV care | ||||||||||||
| Agree | .09 | .30 | .70 | .11 | ||||||||
| Neither agree nor disagree | 261 (65) | 0.7 (0.5-1.1) | 244 (61) | 122 (31) | ||||||||
| Not agree | 38 (68) | 0.9 (0.5-1.9) | 31 (55) | 22 (39) | ||||||||
| 124 (75) | Referent | 101 (61) | 64 (39) | |||||||||
| Total | 424 (68) | 378 (60) | 209 (33) | |||||||||
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; NP/PA, nurse practitioner/physician assistant; MMP, Medical Monitoring Project.
aN = 625—MMP Provider Survey 2009.
bFactors associated with each type of concern at P < .10 in bivariate analyses using chi-square tests were entered into separate multivariable logistic regression models and aORs and 95% CIs are presented.
Discussion
Among a national sample of HIV providers from a large number of diverse care facilities, we found that reasons for delaying ART for clinically eligible patients were consistent with current guidelines, which strongly recommend that barriers to patient adherence be addressed before prescribing ART and on an ongoing basis after ART initiation.3-5 Providers reported concerns about patient adherence, patient refusal or lack of readiness to start treatment, and structural barriers to accessing treatment among their patients as reasons to delay ART for otherwise clinically eligible patients. Provider and practice characteristics were associated with reasons for delaying ART, and these findings provide information that can be used to direct appropriate training and education to providers to assist them in addressing specific barriers among their patients.
Concerns about patient adherence were cited by more than two-thirds of providers as reasons to delay ART.21 Providers identified mental health, substance abuse, appointment adherence, and unstable lifestyle as reasons for concerns about patient adherence, which have all been found to be associated with nonadherence to ART or poorer health outcomes.4 However, research has shown that providers have poor ability to predict which patients will be adherent.22 For patients not taking ART, administration of a brief medication beliefs assessment has been found to predict future adherence in ART-naive populations.23 Providers must balance concerns about adherence with the benefits of starting treatment so that they do not exclude persons who may clinically benefit from ART. However, in order to correctly assess those who are most likely to be adherent to ART, providers must have adequate supports to monitor and address nonadherence among patients taking ART.
Recent guidelines for improving ART adherence strongly recommend routine collection of self-reported adherence from all HIV-infected patients taking ART,24 and tools have been developed to assist providers in implementing these recommendations and linking patients to adherence support services.25 The Centers for Disease Control and Prevention has identified 10 efficacious evidence-based adherence interventions,26 although more operational research may help answer important questions about which adherence interventions are best in specific populations and settings.27 To complement these intervention efforts, providers now have expanded options for once-daily ART regimens and fixed-dose combinations to reduce pill burden that are recommended to improve adherence among persons taking ART.24
Being less than 50 years of age, white, and seeing more than 50 HIV-infected patients per month were provider characteristics found to be independently associated with citing adherence concerns. This might reflect differences in how these providers view patient behaviors or, alternately, that these providers have patients who are more likely to be nonadherent. Regarding the former, unmeasured generational variation in how providers assess patients might account for the association between provider age and adherence concerns. Regarding the latter, we found that white providers were more likely than nonwhite providers to have majority patient populations who are more likely to be adherent (ie, non-IDU, white, and MSM).28 Thus, differences in patient demographic factors between white and nonwhite providers likely do not account for the association between provider race and adherence concerns. We did not collect information that would allow us to assess racial differences among providers in patient assessment, but others have found an association between provider and patient race and ART prescription.29 Further exploration might provide insights into the role of cross-cultural competency in prescribing practices among white providers. Having a large number of HIV-infected patients might be associated with practicing at large public HIV care facilities, such as those funded by the Ryan White HIV/AIDS Program. Because these facilities may have a higher proportion of patients with mental health and substance abuse comorbidities, which are associated with nonadherence,4 it is not surprising that providers in these settings would more often cite adherence concerns as reasons to delay ART. Regardless of the reasons for the association between these characteristics and adherence concerns, younger providers, white providers, and those who see more than 50 patients per month may benefit from enhanced training on the adherence screening and intervention options mentioned earlier, which could allow them to better identify patients likely to be nonadherent and provide them with the resources and tool to address adherence concerns with their patients.
The majority of providers also mentioned patient acceptance barriers to prescribing ART, citing patient lack of readiness for ART and refusal of ART. These concerns were commonly cited, but there are few studies of how providers address these barriers and whether the methods they use are effective. Problems with trust, acceptance, and readiness to start ART may become more prevalent as providers adopt guidelines to consider ART initiation for patients with higher CD4 counts. Also, increased testing, linkage, and engagement have the potential to increase the numbers of patients seen at an earlier stage of illness who are not experiencing the physical symptoms of HIV infection, which has been found to be associated with decreased engagement in care and reluctance to take ART.30
Regarding readiness to take ART, Grimes and Grimes reviewed the treatment guidelines of 5 internationally recognized expert panels and 5 review articles on readiness, trust, and adherence and concluded that readiness is not adequately defined or measured and that evidence is lacking that readiness predicts future adherence.31 Moreover, after review of the published evidence they conclude that there are no clinically useful interventions to improve readiness. More work in this area could help determine whether readiness can be accurately assessed in the clinical setting and how best to improve readiness among patients.
Patient refusal to take ART was another common subtheme. Recent studies of ART refusal among patients are limited, but in a clinical trial assessing the effectiveness of early ART initiation for reducing sexual transmission of HIV, investigators found that 19% of participants in the control arm declined to start ART even after early treatment was shown to be effective for reducing sexual transmission and was recommended for them by study investigators and that not being “ready” to start ART was the most common reason for refusal.32 Other work has found that patients not in HIV care frequently mention concerns about taking medications.33 Among patients who are receiving HIV care, lower acceptance of ART has been found to be associated with concerns about side effects, mistrust of medications and health care providers, perceived effect of medications on quality of life, and a preference for alternative medicine and self-care.34-42 Additional work may be helpful to understand what motivates patients to take ART, particularly when they are healthy. At least 1 study has found that the majority of persons with CD4 counts above 349 cells/mm3 who were not taking ART were interested in starting ART to prevent HIV transmission to partners.43
Nurse practitioners and PAs were more likely than physicians to mention patient acceptance barriers to ART initiation, which might reflect differences in patient–provider interaction. It may also be the case that patients are more comfortable refusing ART when offered by providers who are not doctor of medicine. Providers with majority MSM patients were also more likely to cite acceptance barriers. This could be due to differing communication styles among providers in MSM-focused versus non-MSM-focused facilities, or because MSM are more vocal about acceptance barriers to ART use than other populations. Regardless, our findings suggest that non-MD providers and those working in MSM-focused facilities might benefit from training that provides them with the skills needed to discuss and resolve patient acceptance barriers to ART.
A third of providers mentioned structural barriers to ART initiation, including concerns about payment for ART and homelessness. Although providers may be less able to directly address these types of barriers, referrals to case managers can help alleviate structural barriers for patients.44 For example, case managers can facilitate access to programs such as the ADAP, which provides medications for those without other coverage, and the Housing Opportunities for Persons with AIDS program, which provides housing assistance and related supportive services. Although as of June 2013, 15 states had current or anticipated ADAP cost-containment measures, including 3 states with waiting lists (http://www.nastad.org/docs/ADAP_Watch/ADAP-Watch-June-2013.html), the implementation of the Affordable Care Act (ACA) might increase access to medications for more persons through Medicaid expansion.45 Ensuring that providers have access to case management referrals may allow them to feel more comfortable prescribing ART to unstably housed persons, who according to studies can be adherent with proper support.46
In multivariable analysis, we found Hispanic providers were less likely to cite structural barriers as reasons to delay ART. Because the Hispanic population and ADAP funding and eligibility vary considerably among US states (http://www.pewhispanic.org/2013/08/29/mapping-the-latino-population-by-state-county-and-city/, http://kff.org/hivaids/fact-sheet/aids-drug-assistance-programs/), this finding may reflect geographic differences across states rather than differences in how structural barriers are perceived by Hispanic providers compared to non-Hispanic providers, although we did not collect data that would allow us to fully assess this.
Our analysis is subject to limitations. First, our response rate of 42% is lower than optimal, although it is comparable to or higher than other recent studies of HIV care providers.10,12,13 Despite low response, our sample was drawn from a population-based frame and includes providers from a large number of facilities with diverse characteristics (eg, public and private, small, and large), which gives us confidence in our findings. Another limitation is that the survey did not assess the number of patients for whom providers encounter barriers to ART initiation or their perceptions about the relative importance of those barriers. However, because the reasons we identified were so commonly reported, this suggests these issues are seen regularly, although possibly affecting few patients, as most patients sampled through MMP were prescribed ART.47 Finally, in response to the evidence of the clinical and prevention benefits of early initiation of ART, US treatment guidelines in the years since the provider survey was administered have steadily moved toward recommending ART be offered to all patients regardless of disease stage,4 and more tolerable single-tablet regimens have been developed. In light of these changes, compared to the providers we surveyed in 2009, providers practicing now may be more willing to prescribe ART to patients about whom they have adherence or acceptance concerns. However, although providers may now initiate ART for a higher proportion of their patients, this does not necessarily mean they will no longer consider patient adherence or acceptance when making these decisions. The guidelines continue to note that adherence, patient willingness to take ART, and other psychosocial factors are among the reasons providers may choose to postpone therapy, indicating these concerns should still be considered by providers. In fact, as changes in clinical guidelines for ART initiation increase the numbers of persons whom providers consider clinically eligible for ART, the issues described in this analysis may be more prevalent, as healthier persons may be less motivated to take ART.33
Conclusions
HIV care providers reported reasons to delay ART initiation that were consistent with current clinical guidelines and were both patient level and structural; hence, multilevel strategies to address patient barriers to taking ART are most likely to be effective. These barriers may become more prevalent as we move toward universal ART prescription (which may increase the number of patients with less motivation to take ART) and the transformation of health care through the ACA (which may decrease structural barriers to medication coverage). Additionally, effectively addressing provider concerns about patient adherence is dependent upon correctly assessing who is likely to be nonadherent to ART. Our findings suggest provider training and support in the following areas may be beneficial: (1) adherence assessment and intervention, particularly for patients with substance use and mental health problems, (2) psychosocial support to enhance readiness to start treatment, and (3) linkage to case managers and social services to overcome patient structural barriers.
Acknowledgments
We thank the participating MMP providers, project areas, and Provider and Community Advisory Board members. We also acknowledge the contributions of the Clinical Outcomes Team and Behavioral and Clinical Surveillance Branch at CDC. MMP is funded by the Centers for Disease Control and Prevention.
Footnotes
Authors’ Note: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Medical Monitoring Project Provider Survey is funded by the Centers for Disease Control and Prevention.
References
- 1. The HIV-CAUSAL Collaboration. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-Defining illness in HIV-Infected persons in developed countries: an observational study. Ann Intern Med. 2011;154 (8):509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365 (6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Web site http://aidsinfo.nih.gov/contentfiles/adultandadolescentgl001226.pdf Published November 3, 2008 Accessed October 27, 2014.
- 4. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services. Web site http://aidsinfo.nih.gov/contentfiles/adultandadolescentgl003371.pdf Published February 12, 2013 Accessed October 27, 2014.
- 5. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Web site http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf Published May 1, 2014 Accessed October 27, 2014.
- 6. Fleishman JA, Yehia BR, Moore RD, Gebo KA, Agwu AL. Disparities in receipt of antiretroviral therapy among HIV-infected adults (2002-2008). Med Care. 2012;50 (5):419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hanna DB, Buchacz K, Gebo KA, et al. Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001-2009. Clin Infect Dis. 2013;56 (8):1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pence BW, Ostermann J, Kumar V, Whetten K, Thielman N, Mugavero MJ. The influence of psychosocial characteristics and race/ethnicity on the use, duration, and success of antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47 (2):194–201. [DOI] [PubMed] [Google Scholar]
- 9. Zhang S, McGoy SL, Dawes D, Fransua M, Rust G, Satcher D. The potential for elimination of racial-ethnic disparities in HIV treatment initiation in the Medicaid population among 14 southern states. PLoS One. 2014;9 (4):e96148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Himelhoch S, Powe NR, Breakey W, Gebo KA. Schizophrenia, AIDS and the decision to prescribe HAART: results of a national survey of HIV clinicians. J Prev Interv Community. 2007;33 (1-2):109–120. [DOI] [PubMed] [Google Scholar]
- 11. Loughlin A, Metsch L, Gardner L, Anderson-Mahoney P, Barrigan M, Strathdee S. Provider barriers to prescribing HAART to medically-eligible HIV-infected drug users. AIDS Care. 2004;16 (4):485–500. [DOI] [PubMed] [Google Scholar]
- 12. Westergaard RP, Ambrose BK, Mehta SH, Kirk GD. Provider and clinic-level correlates of deferring antiretroviral therapy for people who inject drugs: a survey of North American HIV providers. J Int AIDS Soc. 2012;15 (1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gagliardo C, Murray M, Saiman L, Neu N. Initiation of antiretroviral therapy in youth with HIV: a U.S.-Based provider survey. AIDS Patient Care STDS. 2013;27 (9):498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blair J, McNaghten A, Frazier E, Skarbinski J, Huang P, Heffelfinger J. Clinical and behavioral characteristics of adults receiving medical care for HIV infection—medical monitoring project, United States, 2007. MMWR Surveill Summ. 2011;60 (11):1–20. [PubMed] [Google Scholar]
- 15. Frankel MR, McNaghten AD, Shapiro MF, et al. A probability sample for monitoring the HIV-infected population in care in the U.S. and in selected states. Open AIDS J. 2012;6 (suppl 1: M21):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dillman DA. Mail and Telephone Surveys: The Total Design Method. New York: John Wiley; 1978. [Google Scholar]
- 17. Protection of Human Subjects, US Federal Code Title 45 Part 46. Web site Published July 14, 2009. http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html Published July 14, 2009 Accessed February 4, 2014.
- 18. Centers for Disease Control and Prevention. Distinguishing Public Health Research and Public Health Nonresearch. Web site http://www.cdc.gov/od/science/integrity/docs/cdc-policy-distinguishing-public-health-research-nonresearch.pdf Published July 29, 2014 Accessed February 4, 2014.
- 19. Guest GS, MacQueen KM, Namey EE. Applied Thematic Analysis. Thousand Oaks, CA: Sage Publications, Inc.; 2012. [Google Scholar]
- 20. MacQueen K, McLellan-Lemal E, Bartholow K, Milstein B. Team-based Codebook Development: Structure, Process, and Agreement. In: Guest G, MacQueen KM, eds. Handbook for Team-based Qualitative Research. Lanham, MD: Altamira Press; 2008:119–124. [Google Scholar]
- 21. Wong MD, Cunningham WE, Shapiro MF, et al. Disparities in HIV treatment and physician attitudes about delaying protease inhibitors for nonadherent patients. J Gen Intern Med. 2004;19 (4):366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bangsberg DR, Hecht FM, Clague H, et al. Provider assessment of adherence to HIV antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;26 (5):435–442. [DOI] [PubMed] [Google Scholar]
- 23. Reynolds NR, Testa MA, Marc LG, et al. Factors influencing medication adherence beliefs and self-efficacy in persons naive to antiretroviral therapy: a multicenter, cross-sectional study. AIDS Behav. 2004;8 (2):141–150. [DOI] [PubMed] [Google Scholar]
- 24. Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156(11):817–833, W–284, W–285, W–286, W–287, W–288, W–289, W–290, W–291, W–292, W–293, W–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amico KR, Zuniga JM, Wilson IB, Gross R, Young B. Provider guidance for linking patients to antiretroviral therapy adherence interventions: recommendations from an IAPAC advisory committee on adherence monitoring and support. J Int Assoc Provid AIDS Care. 2013;12 (2):79–83. [DOI] [PubMed] [Google Scholar]
- 26. Charania MR, Marshall KJ, Lyles CM, et al. Identification of evidence-based interventions for promoting HIV medication adherence: findings from a systematic review of U.S.-based studies, 1996-2011. AIDS Behav. 2014;18 (4):646–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herbst JH, Glassman M, Carey JW, et al. Operational research to improve HIV prevention in the United States. J Acquir Immune Defic Syndr. 2012;59 (5):530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beer L, Skarbinski J. Nationally Representative Estimates of Self-reported Adherence to Antiretroviral Therapy in the United States—Medical Monitoring Project, 2009. Paper presented at: 8th International Conference on HIV Treatment and Prevention Adherence; June, 2013; Miami, FL. [Google Scholar]
- 29. King WD, Wong MD, Shapiro MF, Landon BE, Cunningham WE. Does racial concordance between HIV-positive patients and their physicians affect the time to receipt of protease inhibitors? J Gen Intern Med. 2004;19 (11):1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beer L, Fagan JL, Valverde E, Bertolli J. Health-related beliefs and decisions about accessing HIV medical care among HIV-infected persons who are not receiving care. AIDS Patient Care STDS. 2009;23 (9):785–792. [DOI] [PubMed] [Google Scholar]
- 31. Grimes RM, Grimes DE. Readiness, trust, and adherence: a clinical perspective. J Int Assoc Provid AIDS Care. 2013;12 (3):185–194. [DOI] [PubMed] [Google Scholar]
- 32. Batani J, Brum T, Calvet G, et al. Acceptance of ART in the Delay Arm after Notification of Interim Study Results: Data from HPTN 052. Poster presented at: 20th Conference on Retroviruses and Opportunistic Infections (Poster 550); March 2013; Atlanta, GA. [Google Scholar]
- 33. Beer L, Fagan JL, Garland P, et al. Medication-related barriers to entering HIV care. AIDS Patient Care STDS. 2012;26 (4):214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alfonso V, Bermbach N, Geller J, Montaner JS. Individual variability in barriers affecting people's decision to take HAART: a qualitative study identifying barriers to being on HAART. AIDS Patient Care STDS. 2006;20 (12):848–857. [DOI] [PubMed] [Google Scholar]
- 35. Altice FL, Mostashari F, Friedland GH. Trust and the acceptance of and adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28 (1):47–58. [DOI] [PubMed] [Google Scholar]
- 36. Cooper V, Buick D, Horne R, et al. Perceptions of HAART among gay men who declined a treatment offer: preliminary results from an interview-based study. AIDS Care. 2002;14 (3):319–328. [DOI] [PubMed] [Google Scholar]
- 37. Gold RS, Hinchy J, Batrouney CG. The reasoning behind decisions not to take up antiretroviral therapy in Australians infected with HIV. Int J STD AIDS. 2000;11 (6):361–370. [DOI] [PubMed] [Google Scholar]
- 38. Gold RS, Ridge DT. “I will start treatment when I think the time is right”: HIV-positive gay men talk about their decision not to access antiretroviral therapy. AIDS Care. 2001;13 (6):693–708. [DOI] [PubMed] [Google Scholar]
- 39. Horne R, Cooper V, Gellaitry G, Date HL, Fisher M. Patients’ perceptions of highly active antiretroviral therapy in relation to treatment uptake and adherence: the utility of the necessity-concerns framework. J Acquir Immune Defic Syndr. 2007;45 (3):334–341. [DOI] [PubMed] [Google Scholar]
- 40. Kremer H, Ironson G, Schneiderman N, Hautzinger M. To take or not to take: decision-making about antiretroviral treatment in people living with HIV/AIDS. AIDS Patient Care STDS. 2006;20 (5):335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mostashari F, Riley E, Selwyn PA, Altice FL. Acceptance and adherence with antiretroviral therapy among HIV-infected women in a correctional facility. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18 (4):341–348. [DOI] [PubMed] [Google Scholar]
- 42. Murray LK, Semrau K, McCurley E, et al. Barriers to acceptance and adherence of antiretroviral therapy in urban Zambian women: a qualitative study. AIDS Care. 2009;21 (1):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dombrowski JC, Harrington RD, Fleming M, Golden MR. Letter to the Editor: treatment as prevention: are HIV clinic patients interested in starting antiretroviral therapy to decrease HIV transmission? AIDS Patient Care STDS. 2010;24 (12):747–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sherer R, Stieglitz K, Narra J, et al. HIV multidisciplinary teams work: support services improve access to and retention in HIV primary care. AIDS Care. 2002;14 (suppl 1):S31–S44. [DOI] [PubMed] [Google Scholar]
- 45. McManus KA, Engelhard CL, Dillingham R. Current challenges to the United states’ AIDS drug assistance program and possible implications of the affordable care act. AIDS Res Treat. 2013;2013:350169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bamberger JD, Unick J, Klein P, Fraser M, Chesney M, Katz MH. Helping the urban poor stay with antiretroviral HIV drug therapy. Am J Public Health. 2000;90 (5):699–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Centers for Disease Control and Prevention. Vital signs: HIV prevention through care and treatment--United States. MMWR Morb Mortal Wkly Rep. 2011;60 (47):1618–1623. [PubMed] [Google Scholar]


