Abstract
Background
Toxocara canis is a nematode that parasitizes dogs, while humans are paratenic hosts. When humans are infected the migrating larvae damage the liver, lungs and even the nervous system. Larva migrans diagnosis is based on immunological techniques; however, the commercial immunodiagnostic kits detect anti-T. canis antibodies which may cross-react with other parasites, mainly nematodes with extra-intestinal migration. Moreover, antibodies do not necessarily reflect an active infection; so detection and quantification of circulating antigens may provide appropriate and timely information for treatment, which prevents irreversible damage. Here we report the standardization of a monoclonal antibody based antigen capture ELISA to diagnose human toxocariasis without cross-reaction.
Methods
We developed anti-T. canis polyclonal antibodies in rabbits and a monoclonal antibody in mouse which did not cross-react with 15 antigens from several parasites. The sandwich ELISA standardization was performed using sera from mice experimentally infected. We tested the method using 29 positive and 58 negative human sera previously typified with a commercial kit, which detects antibodies.
Results
Only 5.0 μg/mL and 10 μg/mL polyclonal antibodies and monoclonal antibody, respectively, were needed in the sandwich ELISA standardization, detecting since 440 pg/mL larva antigens. Nine out of 29 antibody-positive sera were also positive for antigens and no false positive were found. Taking the antibody kit as the reference standard, the sensibility and specificity of the antigen test were 31% and 100%, respectively.
Conclusions
With these tools we established a detection threshold as low as 440 pg/mL antigen. Monoclonal antibody is specific, and did not cross-react with antigens from other parasites. Detection of circulating antigens helps provide appropriate and timely treatment and prevents irreversible damage.
Keywords: Toxocara canis, Larva migrans, Antigen capture
Background
The migration of Toxocara canis larvae is injurious to human beings, because they invade the liver, the lungs or the nervous system [1]. Dogs are definitive hosts, and the parasite successfully infects puppies by uterine, trans-mammary or environmental routes, with prevalence near 100% in some places [2]. In contrast, 12-21% of adult dogs are infected with the parasite [3]. As Toxocara females shed an average of 68,000 eggs/day, dogs are an important source of environmental contamination [4,5]. Children are most susceptible to infection with Toxocara embryonated eggs due to their playing behavior and their tendency to eat dirt. Humans serve as paratenic hosts and the migrating parasite produces: visceral larva migrans (VLM) characterized by hepatic damage and Löffler syndrome with fever, pulmonary inflammatory infiltrate and eosinophilia [6]; ocular larva migrans (OLM) which in severe cases leads to eyesight loss [7]; eosinophilic meningo-encephalitis (EME) [8]; and covert toxocariasis (CT) [9]. Currently, larva migrans is diagnosed by immunological methods, which detect antibodies against excretion-secretion antigens [10]. However, this method has limitations, i.e. there is cross-reactivity with antigens from other parasites [10-12]. For treatment purposes it is important to know if there are circulating antigens. There have been few reports that show the capture of T. canis larvae excretion and secretion antigens (L2TES) as an alternative diagnostic strategy, but with variable results [13-15]. Here, we report the standardization of an ELISA to capture and quantify circulating Toxocara antigens to diagnostic human toxocariasis without cross-reaction.
Methods
Ethical approval
Protocol was approved by the research and ethic committees of National Institute of Pediatrics. All animal procedures were performed in accordance with the guidelines of the Coordinator Commission of the National Institutes of Health of Mexico (Institutos Nacionales de Salud, NOM-062-ZOO-1999).
Toxocara canis larvae
T. canis adults were obtained from the small intestines of puppies euthanized at the Canine Control Centre in Tlalpan, México D.F., as described elsewhere [3]. Parasite females were isolated with a paintbrush or forceps, washed with PBS pH 7.2 and processed for culture in the SGHP medium (Saline, Glucose, Human Plasma) described previously [4]. Toxocara eggs were harvested, concentrated by centrifugation, and incubated for one month until larvae developed, which were induced to hatch following the physiological method described elsewhere [16]. Larvae were purified with Lymphoprep and maintained in RPMI-1640 medium, to collect excretion-secretion antigens (L2TES) in a tube containing protease inhibitors cocktail (Sigma Aldrich, USA); subsequently they were concentrated by centrifugation in Amicon columns (10 KDa cutoff), quantified by the Bradford method, aliquoted and stored at −70°C until use [17].
Monoclonal antibody (MoAb) production
Five female BALB/c mice were intraperitoneally inoculated with 500 live Toxocara larvae. Every two weeks a blood sample was collected from the tail vein; the sera were used to evaluate the immune response. Thirty days later, one mouse was euthanized, its spleen was isolated and the cells were fused with the mouse myeloma line X63Ag8.653 at a 5:1 ratio. Hybrid cells were selected following the standard method [18]. Chimeric cells secreting antibodies against T. canis larvae were selected. The cross-reactivity was tested using both excretion-secretion and somatic antigens of T. canis adult. Also Toxocara cati, Ascaris suum, Trichinella spiralis, Ancylostoma caninum, Dipylidium caninum, Fasciola hepatica, Leishmania mexicana, Trypanosoma cruzi, Giardia intestinalis, Trichomonas vaginalis and Acanthamoeba spp. antigens were tested. The controls were hyperimmune and preimmune sera from experimentally infected mouse. One MoAb (termed INP-1E4G4C2) was selected and cloned twice by limiting dilution [19]. The resultant antibody was purified using Montage Antibody Purification Kit with Prosep ®-G (Millipore, USA), typed [20] quantified and stored at −20°C until use.
Polyclonal antibodies (PoAb) production
Two New Zealand rabbits were intraperitoneally injected with five thousand T. canis live larvae. To detect antibody increase, blood samples were obtained from the ear vein weekly. The rabbits were anesthetized and euthanized by bleeding when the titer was1:64,000. Serum was harvested and the IgG fraction was isolated with the Montage Antibody Purification Kit with Prosep ®-G (Millipore, USA), following the manufacturer’s instructions. The protein concentration was determined, and the antibodies were divided into aliquots and stored at −20°C until use.
Determination of the optimal MoAb concentration for ELISA
ELISA plates Immulon 2HB (Dynatech, USA) was coated with 100 μL/well of 0.0 to 40 μg/mL MoAb in borate buffer and incubated overnight at 4°C. The plates were then washed three times and non-specific binding sites were blocked with 1% skimmed milk in PBS-Tween 20 (0.05%) for 30 min at 37°C and then rinsed with 0.9%NaCl −0.05%Tween 20. An anti-mouse IgG-HRP conjugate (Sigma, A4416) was added at a 1:8,000 dilution in PBS-Tween 20, incubated for 2 h at 37°C and washed three times with 0.9%NaCl −0.05%Tween 20. The reaction was revealed with chromogen/substrate solution (0.05 M citrate/citric acid, 0.04 mg O-phenylenediamine and 0.12% H2O2). The reaction was stopped with H2SO4 and the absorbance was measured at 490 nm on an automatic ELISA reader Teca (Sunrise, Switzerland). The MoAb optimal concentration for the ELISA was 10.0 μg/mL (Figure 1).
Figure 1.
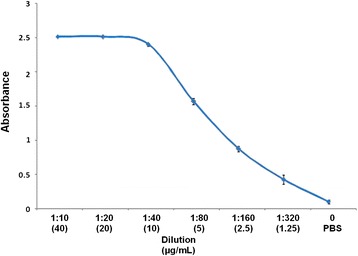
Determination of the optimal 1E4G4C2 MoAb concentration for the ELISA. 96-well polystyrene plate was coated with 0.0, 1.0, 2.5, 5.0, 10, 20 and 40 μg/mL of MoAb; non-specific binding sites were blocked with 1% skimmed milk; anti-mouse IgG-HRP conjugate was added at a 1:8,000 dilution and the reaction was revealed with chromogen/substrate solution. The results are shown as the arithmetic media ± standard deviation from three triplicate assays.
Sandwich ELISA standardization for L2TES detection
The L2TES capture was performed as follows: polystyrene wells were coated overnight at 4°C with 0.1, 1.0, 5.0, 10.0 and 25 μg/mL of PoAb in borate buffer (100 mM boric acid, 0.025 M sodium tetraborate, 75 mM, sodium chloride) pH 8. Unspecific sites were blocked for one hour at room temperature with 1% skimmed milk in PBS-Tween 20. L2TES were added at 0.0, and tenfold increment from 0.0001, until 10 μg/mL and incubated at 37°C for 2 h. Afterwards 10 μg/mL of the MoAb were added and incubated at room temperature. The anti-mouse IgG-HRP (1:8,000) was aggregated and incubated as described before. In every step, the plate was washed three times with NaCl -Tween 20.
Detection of L2TES in human sera
We tested the method using 29 positive and 58 negative serum samples previously tested with a commercial kit (Sciemedx, USA) which detects antibodies against T. canis larvae antigens. A fraction of each sample was treated with EDTA to dissociate immune complexes; briefly, samples were diluted 1:1 with 1.0 M EDTA, pH 7.5, boiled for 10 minutes and centrifuged at 12,000 x g/5 minutes, and the supernatant was used in the test [21]. Another serum fraction was used diluted with PBS instead of EDTA. ELISA was performed with the PoAb at 5 μg/mL, 100 μL of sera with or without treatment with EDTA, the MoAb at 10 μg/mL and anti-mouse IgG-HRP diluted 1:8,000.
In all cases, the cut-off value was obtained adding three times the standard deviation to the mean absorbance value of the negative samples. The experiments were repeated three times.
Western blot
Electrophoresis was performed in a Mini-Protean II (BioRad, USA) on 4-20% polyacrylamide slab gradient gels (BioRad,USA), with Kaleidoscope prestained standards (BioRad,USA) and 100 μg L2TES/well; electrophoresis was at 150 V for 2 h. Proteins were transferred to a PVDF membrane Immobilon (Millipore, USA) at 60 V for 1.5 h in Mini Trans-Blot (BioRad, USA). Nonspecific sites were blocked overnight with 5% skimmed milk in PBS-Tween 20 at 4°C. The blot was then incubated 2 h with INP-1E4G4C2 MoAb at room temperature and washed with PBS-Tween 20 (0.05%). The anti-mouse IgG-HRP conjugate, diluted 1:1,000 was then added and incubated for 2 h at room temperature. After three washes with PBS-Tween 20 (0.05%) and two with PBS, the substrate/chromogen solution (30 mg 3,3`-Diaminobenzidine, and 6 μL 30% H2O2 in 60 mL PBS) was added and the reaction was stopped with distilled water.
Results
The INP-1E4G4C2 is an IgG1 isotype MoAb that did not show cross-reactivity with other parasite antigens tested, including somatic antigens from T. canis adults. Only L2TES and larval somatic antigen gave high absorbance values; in contrast, the hyperimmune mouse serum recognized several parasites (Table 1). MoAb optimal concentration was 10 μg/mL because the absorbance inflection point was at 5.0 μg/mL (Figure 1). Sandwich ELISA standardization was with 5.0 μg/mL and 10 μg/mL of the polyclonal and monoclonal antibodies, and was detected since 440 pg/mL of L2TES (Figure 2).
Table 1.
Detection of cross-reactions of INP-1E4G4C2 MoAb against antigens from several parasites
| Absorbance at 490 nm | |||
|---|---|---|---|
| Antigens | INP-1E4G4C2 MoAb | Hyperimmune serum | Preimmune serum |
| Toxocara canis L2TES | 2.5 | 2.5 | 0.116 |
| Toxocara canis larvae somatic antigen | 2.5 | 2.5 | 0.137 |
| Toxocara canis ATES | 0.27 | 2.5 | 0.146 |
| Toxocara canis adult somatic antigen | 0.287 | 1.842 | 0.204 |
| Toxocara cati | 0.201 | 1.089 | 0.085 |
| Ascaris suum | 0.285 | 0.825 | 0.099 |
| Ancylostoma caninum | 0.226 | 1.132 | 0.12 |
| Trichinella spiralis | 0.26 | 0.898 | 0.042 |
| Fasciola hepatica | 0.139 | 0.388 | 0.141 |
| Dipilidium caninum | 0.147 | 0.269 | 0.116 |
| Giardia intestinalis | 0.138 | 0.301 | 0.191 |
| Trypanosoma cruzi | 0.131 | 0.292 | 0.107 |
| Leishmania spp | 0.089 | 0.218 | 0.12 |
| Trichomonas vaginalis | 0.091 | 0.163 | 0.129 |
| Acantamoeba spp | 0.135 | 0.296 | 0.149 |
Cut-off: 0.35; L2TES: Larvae 2 Toxocara excretion-secretion antigens. ATES: Secretion excretion antigens from Toxocara canis adult.
Figure 2.
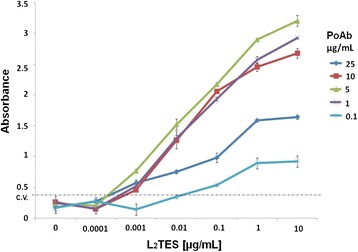
Determination PoAb optimal concentration to L2TES capture. 96-well polystyrene plate was coated with several concentrations of PoAb, non-specific binding sites were blocked with 1% skimmed milk; added different concentrations; 10 μg/mL of INP-1E4G4C2 MoAb, anti-mouse IgG-HRP conjugate at a 1:8,000 dilution and the reaction was revealed with chromogen/substrate solution. The reaction was stopped with H2SO4 and the absorbance was measured at 490 nm. The results are shown as the arithmetic media ± standard deviation from three triplicate assays.
With this technique we found from 470 pg/mL to 10 ng/mL of antigen in 9 out of 29 (sensitivity = 31%) positive sera previously diagnosed with a commercial kit that detects antibodies. In none of the 58 negative samples the antigen was detected (specificity = 100%) (Figure 3). We were able to detect antigens only in samples treated with EDTA.
Figure 3.
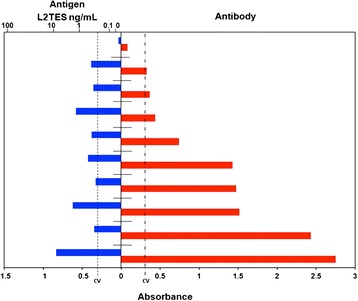
Detection of L2TES in human sera. 96-well polystyrene plate was coated with PoAb at 5 μg/mL, sera previously treatment with EDTA 100 μL, the MoAb at 10 μg/mL and anti-mouse IgG-HRP diluted 1:8,000.The reaction was revealed with chromogen/substrate solution. The reaction was stopped with H2SO4 and the absorbance was measured at 490 nm. The results are shown as the arithmetic media ± standard deviation from three triplicate assays.
The western blot revealed that INP-1E4G4C2 MoAb recognized three bands of 130, 205 and >205 KDa, respectively (Figure 4).
Figure 4.
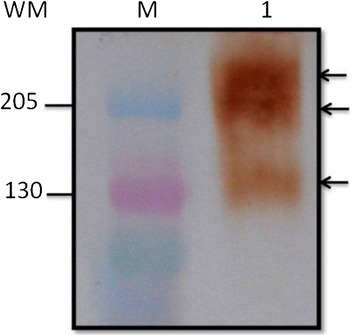
Western blot of L2TES with INP-1E4G4C2 MoAb Electrophoresis was performed on 4–20% polyacrylamide slab gradient gels former and separated at 150 V for 2 h. Proteins were transferred to a PVDF membrane immobilon. Nonspecific sites were blocked overnight with 5% skimmed milk in PBS-tween 20 at 4°C, incubated 2 h with INP-1E4G4C2 MoAb at room temperature and washed three times. Added and incubated for 2 h at room temperature with anti-mouse IgG-HRP conjugate (diluted 1:1,000) and washed three times; the substrate/chromogen solution was added and the reaction was stopped with distilled water. (M) kaleidoscope prestained standards. (1) 100 μg L2TES/well.
Discussion
Toxocara larva migrans diagnosis is not easy, because the methods are based on the detection of antibodies against the parasite, which do not determine the infection status and may give rise to false positive results, due to cross-reactions with other parasites, especially nematodes. With the intention to develop a technique for L2T.canis circulating antigens detection, we obtained one monoclonal antibody against L2T. canis antigens; which neither identified T. canis adult excretion-secretion and somatic antigens, nor presented cross-reactivity with other nematodes such as: T. cati, A. suum, A. caninum or T. spiralis (Table 1).
We used an approach of antigen capture by polyclonal antibodies instead of monoclonal, which gave higher analytical sensitivity (440 pg/mL of L2TES) than those described by Yokoi and Ishiyama, who used MoAbs to coat the plate and were able to capture antigen from 4.0 ng/mL and 5.0 ng/mL, respectively [14,15].
It has been suggested that several monoclonal antibodies can detect more than one band and that recognize more than one epitope [15]. The INP-1E4G4C2 MoAb detected three bands of L2TES, perhaps the capture of pg/mL quantities of L2TES was possible because the MoAb recognized several proteins which share epitopes. Yokoi [14] obtained a monoclonal antibody (IgG1) that did not cross-react with three parasites analyzed (A. suum, D. immitis and T. canis adult). Another study reported two monoclonal antibodies: one (IgM) that only identified T. canis excretion-secretion antigens, and other (IgG1) that distinguish T. canis and T. cati excretion-secretion antigens [13].
When we tested the method using antibody-positive samples, as defined by a commercial kit, we found circulating antigens in 31.0% of serum samples and none among 58 negative samples. These data suggest that 9 children had circulating antigens and perhaps larvae migration. It is likely that another 20 cases had antibodies against Toxocara from past infections, although it cannot be ruled out that they were individuals harboring other parasites, because the ELISA kit is unable to distinguish past infections from active infection and gives cross-reactions. Based on these data, we believe that the INP-1E4G4C2 MoAb could serve as a useful tool to demonstrate the presence of L2T.canis circulating antigens, and therefore, to establish the infection status of the host. Moreover, we considered it is necessary to test the sandwich ELISA in more samples from patients.
Conclusions
With these tools we established a detection threshold as low as 440 pg/mL antigen. Monoclonal antibody is specific, and did not cross-react with antigens from other parasites. Detection of circulating antigens helps provide appropriate and timely treatment and prevents irreversible damage.
Acknowledgements
This work was supported in part by federal funds of Instituto Nacional de Pediatría (protocol 10/032), and federal founds of monoclonal antibody laboratory and rapid test laboratory of Instituto de Diagnóstico y Referencia Epidemiológicos.
Abbreviations
- ATES
Secretion excretion antigens from Toxocara canis adult
- CI
Confidence interval
- CT
Covert toxocariasis
- EDTA
Ethylenediaminetetraacetic acid
- ELISA
Enzyme-Linked ImmunoSorbent Assay
- EME
Eosinophilic meningo-encephalitis
- H2O2
Hydrogen peroxide
- H2SO4
Sulfuric acid
- IgG-HRP
Horseradish peroxidase conjugated IgG
- INP-1E4G4C2 MoAc
Monoclonal antibody produced in Instituto Nacional de Pediatría clone 1E4G4C2
- L2T.canis
larvae 2 of Toxocara canis
- L2TES
Larvae 2 Toxocara excretion-secretion antigens
- MoAb
Monoclonal antibody
- NaCl
Sodium chloride
- NaCl Tween 20
Solution of sodium chloride with tween 20
- OLM
Ocular larva migrans
- PBS
Phosphate buffer solution
- PBS-Tween 20
Phosphate buffer solution with tween 20
- PoAb
Polyclonal antibodies
- PVDF
Polyvinylidene difluoride
- RPMI-1640
Roswell Park Memorial Institute medium 1640
- SGHP
Medium of Saline, Glucose, Human Plasma
- Tween 20
Polyoxyethylene Sorbitan Monolaurate
- VLM
Visceral larva migrans
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ARC conceived the study, samples collection, performed the experiments, analyzed the data and drafted the manuscript; YMF, MEME, AML were involved in the production of monoclonal and polyclonal antibodies; DC, SCS participated in study implementation and manuscript revision; MNMG, MPM conceived and designed the study, analyzed the data and wrote the manuscript. All authors read and approved the final version of the manuscript.
Contributor Information
Aarón Rodríguez-Caballero, Email: bioarca@gmail.com.
Mario Noé Martínez-Gordillo, Email: marionmgordillo@yahoo.com.
Yolanda Medina-Flores, Email: medinafy@yahoo.com.
María Edith Medina-Escutia, Email: medinaeme@yahoo.com.
Antonio Meza-Lucas, Email: anmez@hotmail.com.
Dolores Correa, Email: mariadol@yahoo.com.
Silvia Caballero-Salazar, Email: caballerosalazars@gmail.com.
Martha Ponce-Macotela, Email: macotelam@yahoo.com.
References
- 1.Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003;16(2):265–72. doi: 10.1128/CMR.16.2.265-272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schantz PM, Biagi FF. Coexistence of Toxocara and Toxascaris in dogs in Mexico city. J Parasitol. 1968;54(1):185–6. doi: 10.2307/3276910. [DOI] [PubMed] [Google Scholar]
- 3.Ponce-Macotela M, Peralta-Abarca GE, Martínez-Gordillo MN. Giardia intestinalis and other zoonotic parasites: prevalence in adult dogs from the southern part of Mexico city. Vet Parasitol. 2005;113(1–2):1–4. doi: 10.1016/j.vetpar.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez-Caballero A, Luna-Ochoa RI, Ponce-Macotela M, Peralta-Abarca GE, Martínez-Gordillo MN. A simple and inexpensive in vitro method for retrieving fertilized Toxocara canis eggs. Parasitol Res. 2007;101(3):829–32. doi: 10.1007/s00436-007-0539-2. [DOI] [PubMed] [Google Scholar]
- 5.Traversa D. Pet roundworms and hookworms: a continuing need for global worming. Parasit Vectors. 2012;5(91):1–19. doi: 10.1186/1756-3305-5-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akuthota P, Weller PF. Eosinophilic pneumonias. Clin Microbiol Rev. 2012;25(4):649–60. doi: 10.1128/CMR.00025-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schantz PM, Weis PE, Pollard ZF, White MC. Risk factors for toxocaral ocular larva migrans: a case control study. Am J Pub Health. 1980;70(12):1269–72. doi: 10.2105/AJPH.70.12.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidal JE, Sztajnbok J, Seguro AC. Eosinophilic meningoencephalitis due to Toxocara canis: case report and review of the literature. Am J Trop Med Hyg. 2003;69(3):341–3. [PubMed] [Google Scholar]
- 9.Nathwani D, Laing RB, Currie PF. Covert toxocariasis -a cause of recurrent abdominal pain in childhood. Br J Clin Pract. 1992;46(4):271. [PubMed] [Google Scholar]
- 10.Jacquier P, Gottstein B, Stingelin Y, Eckert J. Immunodiagnosis of toxocariosis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. J Clin Microbiol. 1991;29(9):1831–5. doi: 10.1128/jcm.29.9.1831-1835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muñoz-Guzmán MA, Del Río-Navarro BE, Valdivia-Anda G, Alba-Hurtado F. The increase in seroprevalence to Toxocara canis in asthmatic children is related to cross-reaction with Ascaris suum antigens. Allergol Immunopathol. 2010;38(3):115–21. doi: 10.1016/j.aller.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Romero Núñez C, Mendoza Martínez GD, Yañez Arteaga S, Ponce Macotela M, Bustamante Montes P, Ramírez Durán N. Prevalence and risk factors associated with Toxocara canis infection in children. ScientificWorldJournal. 2013;9(2013):572089. doi: 10.1155/2013/572089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson BD, Burkot TR, Gillespie SH, Kennedy MW, Wambai Z, Maizels RM. Detection of circulating parasite antigen and specific antibody in Toxocara canis infections. Clin Exp Immunol. 1988;74(2):236–41. [PMC free article] [PubMed] [Google Scholar]
- 14.Yokoi K, Kobayashi F, Sakai J, Usui M, Tsuji M. Sandwich ELISA detection of excretory secretory antigens of Toxocara canis larvae using a specific monoclonal antibody. Southeast Asian J Trop Med Public Health. 2002;33(1):33–7. [PubMed] [Google Scholar]
- 15.Ishiyamna S, Ono K, Rai SK, Uga S. Method for detecting circulating Toxocara canis antigen and its application in human serum samples. Nepal Med Coll J. 2009;11(1):9–13. [PubMed] [Google Scholar]
- 16.Ponce-Macotela M, Rodríguez-Caballero A, Peralta-Abarca GE, Martínez-Gordillo MN. A simplified method for hatching and isolating Toxocara canis larvae to facilitate excretory-secretory antigen collection in vitro. Vet Parasitol. 2011;175(3–4):382–5. doi: 10.1016/j.vetpar.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(7):248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 18.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–7. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 19.Galfrè G, Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- 20.Bowman DD, Mika-Grieve M, Grieve RB. Circulating excretory-secretory antigen levels and specific antibody responses in mice infected with Toxocara canis. Am J Trop Med Hyg. 1987;36(1):75–82. doi: 10.4269/ajtmh.1987.36.75. [DOI] [PubMed] [Google Scholar]
- 21.Gillespie H, Bidwell D, Voller A, Robertson BD, Maizels RM. Diagnosis of human toxocariasis by antigen capture enzyme linked immunosorbent assay. J Clin Pathol. 1993;46(86):551–4. doi: 10.1136/jcp.46.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]


