Abstract
Opioid drugs have powerful antidiarrheal effects and many patients taking these drugs for chronic pain relief experience chronic constipation that can progress to opioid-induced bowel dysfunction. Three classes of opioid receptors are expressed by enteric neurons: μ-, δ-, and κ-opioid receptors (MOR, DOR, and KOR). MOR and DOR couple to inhibition of adenylate cylase and nerve terminal Ca2+ channels and activation of K+ channels. These effects reduce neuronal activity and neurotransmitter release. KOR couples to inhibition of Ca2+ channels and inhibition of neurotransmitter release. In the human gastrointestinal tract, MOR, DOR, and KOR link to inhibition of acetylcholine release from enteric interneurons and purine/nitric oxide release from inhibitory motorneurons. These actions inhibit propulsive motility. MOR and DOR also link to inhibition of submucosal secretomotor neurons, reducing active Cl− secretion and passive water movement into the colonic lumen. These effects account for the constipation caused by opioid receptor agonists. Tolerance develops to the analgesic effects of opioid receptor agonists but not to the constipating actions. This may be due to differential β-arrestin-2-dependent opioid receptor desensitization and internalization in enteric nerves in the colon compared with the small intestine and in neuronal pain pathways. Further studies of differential opioid receptor desensitization and tolerance in subsets of enteric neurons may identify new drugs or other treatment strategies of opioid-induced bowel dysfunction.
INTRODUCTION
Opiate drugs have powerful effects on gastrointestinal function, and chronic opiate use for pain relief can cause opioid-induced bowel dysfunction (chronic constipation) and, in extreme cases, narcotic bowel syndrome (1). Other articles in this supplement will address the clinical issues surrounding opiate use and gut function. This review will focus on the physiology of opioid receptors in the gastrointestinal tract and the mechanisms by which opioids alter gut motility and secretion after acute and chronic treatment with opioid receptor agonist drugs.
Opiate receptors in the enteric nervous system
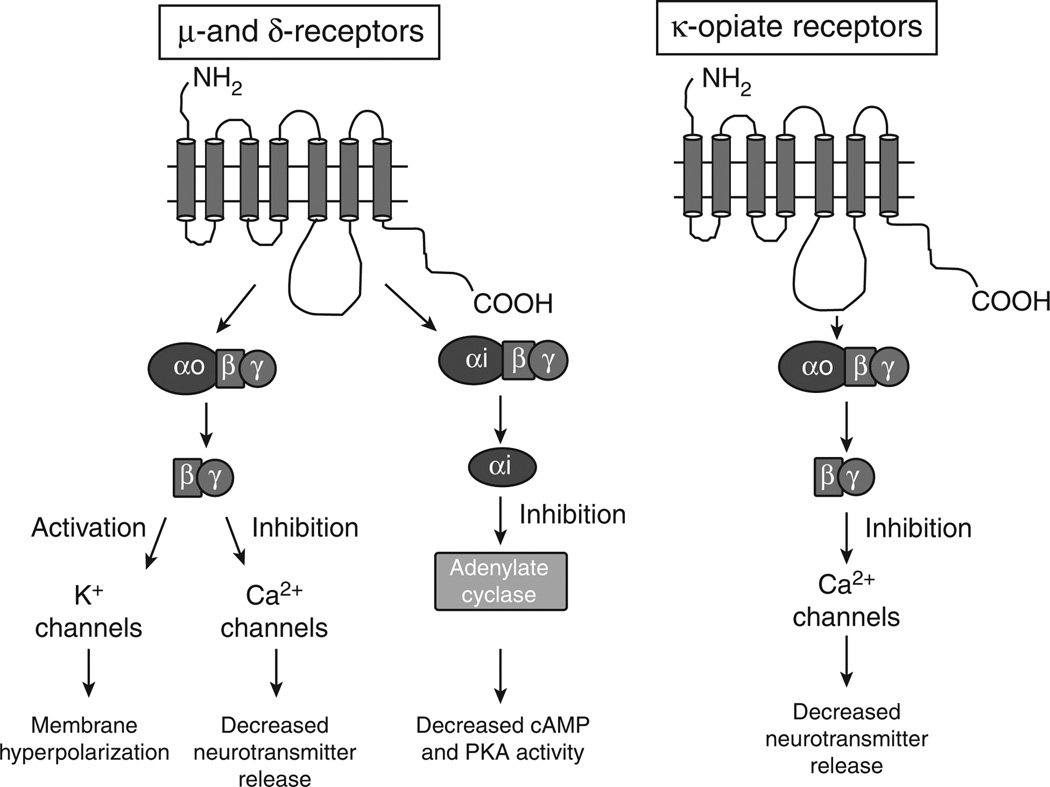
There are three classes of opioid receptors: μ, δ and κ, which are G-protein-coupled receptors (2). All three receptor subtypes are expressed in the gastrointestinal tract where they are localized principally to enteric neurons (3,4). Opioid receptor agonists produce constipation by inhibiting enteric neuron function. μ- and δ-opiate receptors (MOR and DOR) are expressed by enteric neurons and these receptors use common signaling pathways in the enteric nervous system (ENS). Both receptors link to the inhibitory Gi G-protein to cause inhibition of adenylate cyclase, reduced cyclic 3′,5′ adenosine monophosphate, and reduced levels of protein kinase A activation (5,6). This results in reduced neuronal excitability. MOR and DOR also couple to the Go subtype of G-protein, which links MOR and DOR to inhibition of Ca2+ channels and to activation of K+ channels (7–12). Inhibition of Ca2+ channel function will decrease neurotransmitter release from enteric nerve endings, whereas activation of K+ changes causes a hyperpolarization of membrane potential and this reduces the probability of action potential firing by the affected neurons. κ-Opioid receptors (KORs) link via Go to inhibition of nerve terminal Ca2+ channels, also resulting in a decrease in neurotransmitter release (13,14) (see Figure 1). The clinically relevant actions (analgesia, constipation, respiratory depression) of opiate drugs like morphine are mediated predominately by the MOR.
Figure 1.
Signaling pathways activated by opioid receptor agonists in the enteric nervous system. μ- and δ-opioid receptors couple to inhibition of adenylate cyclase via the inhibitory G-protein, Gi. These receptors also couple to activation of K+ channels and inhibition of Ca2+ channels via a Go-linked pathway. These mechanisms suppress enteric neuronal activity. κ-Opioid receptors link to inhibition of Ca2+ channels on enteric nerve terminals. This would inhibit neurotransmitter release. These pathways contribute to the constipating effects caused by opioid receptor agonists. cAMP, cyclic 3′,5′ adenosine monophosphate; PKA, protein kinase A.
Cellular and molecular actions of opioid drugs in the ENS
Mechanisms responsible for opioid drug-induced changes in motility
Gut motility is controlled in large part by the myenteric plexus division of the ENS. The myenteric plexus controls contractions and relaxations of the longitudinal and circular muscle layers by releasing excitatory (principally, acetylcholine, and substance P) (15) and inhibitory neuromuscular transmitters (ATP/βNAD, nitric oxide, and vasoactive intestinal peptide) (15–17). Myenteric motor neuronal activity is controlled by inter neurons whose activity coordinates the timing of contraction and relaxation required for production of propulsive motility patterns such as the peristaltic reflex. Interneurons use acetylcholine as the primary excitatory neurotransmitter, but ATP and 5-HT also contribute to fast excitatory interneuronal synaptic transmission (18).
Electrophysiological methods are the principal tools used to investigate the cellular mechanism of opiate action on enteric neurons. Morphine causes membrane hyperpolarization that is associated with a decrease in input resistance (12,14). The reversal potential for the hyperpolarization occurred at the K+ equilibrium potential, indicating that the opiate receptor coupled to K+ channel activation. MOR, DOR, and KOR also link to inhibition of Ca2+ channel function (10,11,14). Opioid receptor-mediated inhibition of the Ca2+ channel function also uses direct coupling of the receptor to the G-protein βγ subunit, which inhibits opening of the Ca2+ channel (10,11). Inhibition of the nerve terminal Ca2+ channel function will suppress neurotransmitter release and alter gut motility. Studies of small and large intestinal preparations maintained in vitro have been used to identify the sites of action of opioid drugs on gut motility. Studies on the human colon have shown that morphine acts at MOR and DOR to inhibit inhibitory neuromuscular transmission (19). This will lead to an increase in smooth muscle tone and a decrease in propulsive motility. This is an important mechanism for the constipating effects of morphine and other opioid receptor agonist drugs. In addition, suppression of inhibitory neuromuscular transmission is likely responsible for the cramps caused by opioid drugs.
In addition to motorneurons and interneurons, there is a third type of neuron in the ENS that functions as an intrinsic primary afferent or sensory neuron. These neurons have cell bodies in the myenteric and submucosal ganglia and they are bipolar neurons with an axon projecting into the mucosal villi and a second axon synapsing with interneurons or motorneurons in submucosal or myenteric ganglia. These sensory neurons are the first neurons in reflex pathways activated by mucosal stimulation. These axon terminals in the mucosa express chemical and mechanical sensitive ion channels that respond to chemical and mechanical stimulation of the mucosa. Action potentials propagate along the axon back through the cell bodies and then down the axon that ramifies in the submucosal or meyenteric ganglia. These sensory neurons express a Ca2+-activated K+ channel that mediates a long-lasting action potential after-hyperpolarization. This after-hyperpolarization limits the firing rate of the neurons. Morphine increases the duration but not the peak amplitude of the after-hyperpolarization, indicating that the opiate receptor is linked to a mechanism that controls the time course of the increase in intracellular Ca2+ following each action potential (14,20). In the same neurons, morphine shortened the action potential duration by decreasing the Ca2+ -dependent portion of the action potential, and this decrease in Ca2+ entry should shorten, not lengthen, the duration of the after-hyperpolarization.
The studies described above have focused largely on myenteric neurons from the guinea-pig small intestine. As gene knockout mice have become an important research tool, there have been electrophysiological studies of myenteric neurons from the mouse that have revealed additional mechanisms of opiate action (21). These studies used murine myenteric neurons maintained in primary culture along with whole-cell patch clamp methods to study the ionic basis of the inhibitory effects of morphine. These studies revealed that morphine could reduce myenteric neuronal activity by coupling to inhibition of voltage-gated Na+ channels. This effect would reduce action potential firing and suppress interneuronal signaling and signaling to the muscle layers, although the opioid receptor subtype(s) and signaling mechanism were not identified (21).
Mechanisms responsible for opioid drug-induced changes in intestinal secretion
Opioid receptor agonists can inhibit colon water and electrolyte secretion and this effect can contribute to opioid-induced constipation. Secretomotor neurons are localized in the submucosal plexus, and the axons of these neurons project into the mucosal layer where they come into close apposition with epithelial cells (15). The epithelial cells express several types of chloride channels, including ClC2 (22). Submucosal secretomotor neurons release acetylcholine and VIP, which activate muscarinic and VIP receptors on the basolateral surface of the enterocytes (15), which can activate epithelial cell chloride channels. Chloride moves from the enterocyte cytoplasm into the gut lumen. H2O follows chloride via an osmotic mechanism. Opioid drugs activating MOR and DOR on secretomotor neurons suppress acetylcholine and VIP release, resulting in a decrease in chloride secretion and osmotic water movement (8,22).
Cellular and molecular mechanisms of tolerance to the effects of opioid receptor agonists
Chronic morphine treatment for the relief of pain results in tolerance whereby increased doses are required to maintain response. There are several mechanisms of tolerance to the actions of opioid receptor agonists, and these differ in their time course as tolerance develops. The development of cellular tolerance following activation of G-protein-coupled receptors has been attributed in part to the desensitization and internalization of the activated receptor by the class of proteins termed arrestins (23,24). In particular, β-arrestin-2 has been reported to have an integral role as a scaffolding protein for the internalization of G-protein-coupled receptor through clathrin-coated pits and subsequent intracellular trafficking to the endosome (2,25,26). When an agonist binds to an opiate receptor, it activates the G-protein-dependent signaling mechanisms described above. The activated receptor becomes a substrate for a G-protein receptor kinase, which can phosphorylate specific amino acids found on the intracellular portions of the receptor. Receptor phosphorylation results in acute receptor desensitization that develops within 1 min of receptor activation. The receptor can either be dephosphorylated and reactivated or β-arrestin-2 can be recruited to the phosphorylated receptors, prolonging desensitization by signaling for receptor internalization into the endosome. The receptor can be dephosphorylated in the endosome causing β-arrestin-2 to fall off the receptor, which signals for receptor recycling back to the plasma membrane for another round of opioid agonist receptor activation (2,25,26). This canonical pathway for receptor desensitization does not follow for all opioid agonists. Morphine, for example, produces profound tolerance, yet results in markedly less internalization. Several theories have been proposed to explain these differences. This includes different signaling pathways of MOR phosphorylation by low- and high-efficacy opioid agonists. Morphine-induced analgesic tolerance can be reversed by protein kinase C inhibitors, suggesting that protein kinase C and not G-protein receptor kinase phosphorylation mediates morphine-induced tolerance, whereas the tolerance induced by the high-efficacy opioid agonist DAMGO is mediated via G-protein receptor kinase (27). MOR internalization occurs in myenteric neurons after MOR agonist treatment in vivo (28) and in vitro (29). This response was highly agonist-dependent with morphine being a very poor activator of the MOR internalization mechanism and this could contribute to the development of opioid-induced bowel dysfunction as described below.
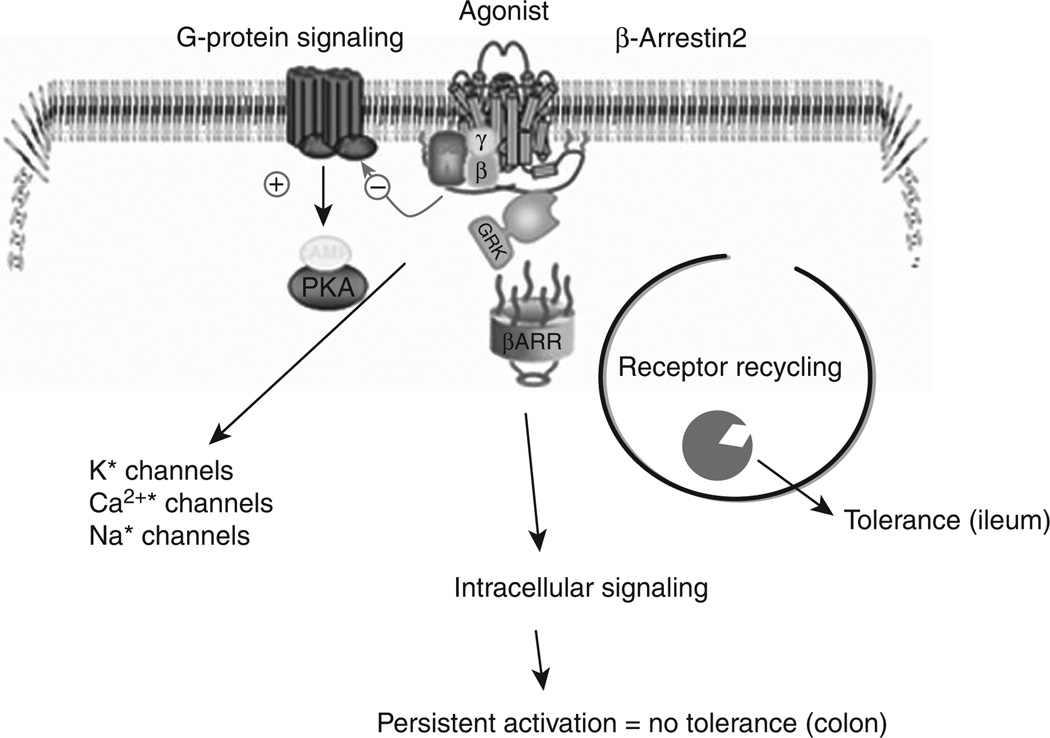
One of the challenges associated with treating chronic pain with opiate receptor agonists is that tolerance develops to the analgesic but not to the constipating effects of agonists. Recent studies suggest that this may be due to differences in receptor desensitization pathways involving β-arrestin-2 in the ENS. Tolerance developed to repeated administration of morphine in the small intestine but not in the colon (see Figure 2) (30). However, tolerance could be induced in the colon of β-arrestin-2 knockout mice (31). In the ileum, prolonged exposure to morphine resulted in decreased β-arrestin-2 expression. This was not observed in the colon, implicating that the decrease in β-arrestin-2 associates with tolerance development in the ileum but not in the colon. In contrast, tolerance to the analgesic effects of morphine upon chronic administration is abrogated in the β-arrestin-2 knockout mice (32). When acutely administered, morphine reduces constipation in the β-arrestin-2 knockout mice (33). These seemingly opposite effects of the role of β-arrestin-2 in tolerance development of the central nervous system pain pathways and in the ENS indicate that cellular mechanisms affecting opioid-induced constipation upon prolonged use may also involve differences in receptor signaling mechanisms. Recent studies have identified at least 31 splice variants of the OPRM-1 gene that encodes for the MOR, largely identified in the central nervous system (34). Alternative splicing event occurs in humans and rodents, and points toward the multitude of mechanisms that relate to tolerance development to different opioid agonists. Differences in the carboxy terminal end of the isoforms result in differential cellular signaling and may explain why cross-tolerance to analgesia does not occur among different opioid agonists (35). The presence of differences in MOR mediating the central inhibition of gastrointestinal transit was suggested by earlier pharmacological studies with MOR antagonist naloxonazine (36) where anti-transit effects of intrathecally administered morphine were blocked by the antagonist but not when morphine was delivered at supra-spinal levels via intracerebroventricular injections. This was in contrast to the inhibition by naloxanazine of the analgesic effects. More recently, Mori et al. (37) suggested the differential activation of MOR at central and peripheral sites by morphine, oxycodone, and fentanyl. Thus, identifying the specific isoforms in the gastrointestinal tract will be important to establish new receptor targets for treating opioid-induced bowel dysfunction.
Figure 2.
Activation of μ-opioid receptor induces G-protein and β-arrestin-2 signaling pathways. G-protein activation allows for signaling to inhibition of adenylate cyclase and modulation of ion channels. Activation of the β-arrestin-2 pathway, in chronic activation, results in tolerance due to β-arrestin-2-mediated receptor degradation in the ileum, while maintained β-arrestin-2 levels prevent tolerance development in the colon. PKA, protein kinase A.
A significant development in opioid pharmacology has been the identification of biased agonism. This stems from the realization that a drug’s efficacy to stimulate G-protein activation can be distinguished from its efficacy for β-arrestin-2 recruitment (38). As stated above, in the β-arrestin-2 knockout mice, acutely administered morphine reduces constipation (33), indicating that persistent opiate receptor signaling in the colon via β-arrestin-2 (leading to decreased propulsive motility and colonic secretion) could be an important contributor to the development of opioid-induced constipation. Thus, opioid agonists that do not recruit β-arrestin-2 would result in reduced constipation. TRV130 is a G-protein-biased ligand with less β-arrestin-2 recruitment compared with morphine, with higher potency toward analgesic effects and reduced constipation (39). The potential role of these biased ligands in long-term use is unclear.
SUMMARY AND CONCLUSIONS
Morphine and other opioid receptor agonists cause constipation by disrupting neurotransmission in the ENS. This causes a reduction in propulsive motility and colonic chloride secretion. Morphine and other agonists act at MOR, DOR, and KOR to inhibit Ca2+ and Na+ channels and activate K+ channels on enteric neurons. Receptor desensitization is a key component regulating opiate receptor signaling in the nervous system, and β-arrestin binding to activated opiate receptors is a key event in causing receptor internalization and desensitization. There are differences in β-arrestin signaling that are important for tolerance development to the analgesic effects of morphine and other opioid receptor agonists. However, β-arrestin signaling does not link to opioid receptor desensitization and tolerance in the colon. This is likely one cellular/molecular mechanism responsible for opioid-induced bowel dysfunction. Development of opioid receptor agonists with biased agonism and identification of receptor isoforms in the gastrointestinal tract are potential areas that require further studies.
ACKNOWLEDGMENTS
We thank John Ferguson for editorial assistance in preparing the manuscript for publication.
Financial support: Akbarali has received grant support from NIH DA024009 and DK046367. Galligan has received grant support from NIH DK094932. An independent medical education grant from Takeda Pharmaceuticals was provided to support the development of this supplement.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: James J. Galligan, MD.
Specific author contributions: Galligan contributed to the idea, wrote the manuscript, and provided critical revisions. Akbarali contributed to interpreting data and in the drafting of the manuscript. All authors have seen and approved the final paper.
Potential competing interests: J.J.G. has served as an expert witness for Faegre & Benson on litigation related to Zelnorm. The sponsor did not review the manuscript before publication, nor did they provide input into the content of the supplement. The authors declare no competing interests.
REFERENCES
- 1.Grunkemeier DM, Cassara JE, Dalton CB, et al. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol. 2007;5:1126–1139. doi: 10.1016/j.cgh.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams JT, Ingram SL, Henderson G, et al. Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65:223–254. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl 2):17–28. doi: 10.1111/j.1743-3150.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 4.Poonyachoti S, Portoghese PS, Brown DR. Characterization of opioid receptors modulating neurogenic contractions of circular muscle from porcine ileum and evidence that delta- and kappa-opioid receptors are coexpressed in myenteric neurons. J Pharmacol Exp Ther. 2001;297:69–77. [PubMed] [Google Scholar]
- 5.Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154:384–396. doi: 10.1038/bjp.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu JG, Anand KJ. Protein kinases modulate the cellular adaptations associated with opioid tolerance and dependence. Brain Res Brain Res Rev. 2001;38:1–19. doi: 10.1016/s0165-0173(01)00057-1. [DOI] [PubMed] [Google Scholar]
- 7.Mihara S, North RA. Opioids increase potassium conductance in submucous neurones of guinea-pig caecum by activating delta-receptors. Br J Pharmacol. 1986;88:315–322. doi: 10.1111/j.1476-5381.1986.tb10207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.North RA, Williams JT, Surprenant A, et al. Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci USA. 1987;84:5487–5491. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatsumi H, Costa M, Schimerlik M, et al. Potassium conductance increased by noradrenaline, opioids, somatostatin, and G-proteins: whole-cell recording from guinea pig submucous neurons. J Neurosci. 1990;10:1675–1682. doi: 10.1523/JNEUROSCI.10-05-01675.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surprenant A, Shen KZ, North RA, et al. Inhibition of calcium currents by noradrenaline, somatostatin and opioids in guinea-pig submucosal neurones. J Physiol. 1990;431:585–608. doi: 10.1113/jphysiol.1990.sp018349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen KZ, Surprenant A. Noradrenaline, somatostatin and opioids inhibit activity of single HVA/N-type calcium channels in excised neuronal membranes. Pflugers Arch. 1991;418:614–616. doi: 10.1007/BF00370580. [DOI] [PubMed] [Google Scholar]
- 12.Morita K, North RA. Opiate activation of potassium conductance in myenteric neurons: inhibition by calcium ion. Brain Res. 1982;242:145–150. doi: 10.1016/0006-8993(82)90504-2. [DOI] [PubMed] [Google Scholar]
- 13.Cherubini E, Morita K, North RA. Opioid inhibition of synaptic transmission in the guinea-pig myenteric plexus. Br J Pharmacol. 1985;85:805–817. doi: 10.1111/j.1476-5381.1985.tb11079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherubini E, North RA. Mu and kappa opioids inhibit transmitter release by different mechanisms. Proc Natl Acad Sci USA. 1985;82:1860–1863. doi: 10.1073/pnas.82.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 16.Jin JG, Murthy KS, Grider JR, et al. Stoichiometry of neurally induced VIP release, NO formation, and relaxation in rabbit and rat gastric muscle. Am J Physiol. 1996;271:G357–G369. doi: 10.1152/ajpgi.1996.271.2.G357. [DOI] [PubMed] [Google Scholar]
- 17.Hwang SJ, Durnin L, Dwyer L, et al. β-Nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology. 2011;140:608–617. doi: 10.1053/j.gastro.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galligan JJ, LePard KJ, Schneider DA, et al. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst. 2000;81:97–103. doi: 10.1016/s0165-1838(00)00130-2. [DOI] [PubMed] [Google Scholar]
- 19.Bauer AJ, Sarr MG, Szurszewski JH. Opioids inhibit neuromuscular transmission in circular muscle of human and baboon jejunum. Gastroenterology. 1991;101:970–976. doi: 10.1016/0016-5085(91)90723-x. [DOI] [PubMed] [Google Scholar]
- 20.Tokimasa T, Morita K, North A. Opiates and clonidine prolong calcium-dependent after-hyperpolarizations. Nature. 1981;294:162–163. doi: 10.1038/294162a0. [DOI] [PubMed] [Google Scholar]
- 21.Smith TH, Grider JR, Dewey WL, et al. Morphine decreases enteric neuron excitability via inhibition of sodium channels. PLoS One. 2012;7:e45251. doi: 10.1371/journal.pone.0045251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fei G, Raehal K, Liu S, et al. Lubiprostone reverses the inhibitory action of morphine on intestinal secretion in guinea pig and mouse. J Pharmacol Exp Ther. 2010;334:333–340. doi: 10.1124/jpet.110.166116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohse MJ, Benovic JL, Codina J, et al. β-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 24.Wilden U, Hall SW, Kuhn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci USA. 1986;83:1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sternini C. Receptors and transmission in the brain-gut axis: potential for novel therapies. III. Mu-opioid receptors in the enteric nervous system. Am J Physiol. 2001;281 :G8–G15. doi: 10.1152/ajpgi.2001.281.1.G8. [DOI] [PubMed] [Google Scholar]
- 26.Claing A, Laporte SA, Caron MG, et al. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol. 2002;66:61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 27.Bailey CP, Smith FL, Kelly E, et al. How important is protein kinase C in mu-opioid receptor desensitization and morphine tolerance? Trends Pharmacol Sci. 2006;27:558–565. doi: 10.1016/j.tips.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Sternini C, Spann M, Anton B, et al. Agonist-selective endocytosis of mu opioid receptor by neurons in vivo. Proc Natl Acad Sci USA. 1996;93:9241–9246. doi: 10.1073/pnas.93.17.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patierno S, Anselmi L, Jaramillo I, et al. Morphine induces μ opioid receptor endocytosis in guinea pig enteric neurons following prolonged receptor activation. Gastroenterology. 2011;140:618–626. doi: 10.1053/j.gastro.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross GR, Gabra BH, Dewey WL, et al. Morphine tolerance in the mouse ileum and colon. J Pharmacol Exp Ther. 2008;327:561–572. doi: 10.1124/jpet.108.143438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang M, Maguma HT, Smith TH, et al. The role of β- arrestin2 in the mechanism of morphine tolerance in the mouse and guinea pig gastro intestinal tract. J Pharmacol Exp Ther. 2012;340:567–576. doi: 10.1124/jpet.111.186320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bohn LM, Gainetdinov RR, Lin FT, et al. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 33.Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314:1195–1201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- 34.Pan YX. Diversity and complexity of the mu opioid receptor gene: alternative pre-mRNA splicing and promoters. DNA Cell Biol. 2005;24:736–750. doi: 10.1089/dna.2005.24.736. [DOI] [PubMed] [Google Scholar]
- 35.Pasternak GW. Incomplete cross tolerance and multiple mu opioid peptide receptors. Trends Pharmacol Sci. 201;22:67–70. doi: 10.1016/s0165-6147(00)01616-3. [DOI] [PubMed] [Google Scholar]
- 36.Heyman JS, Williams CL, Burks TF, et al. Dissociation of opioid antinociception and central gastrointestinal propulsion in the mouse: studies with naloxonazine. J Pharmacol Exp Ther. 1988;245:238–243. [PubMed] [Google Scholar]
- 37.Mori T, Shibasaki Y, Matsumoto K, et al. Mechanisms that underlie mu-opioid receptor agonist-induced constipation: differential involvement of mu-opioid receptor sites and responsible regions. J Pharmacol Exp Ther. 2013;347:91–99. doi: 10.1124/jpet.113.204313. [DOI] [PubMed] [Google Scholar]
- 38.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 39.DeWire SM, Yamashita DS, Rominger DH, et al. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 2013;344:708–717. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]