Abstract
Objective
Focal adhesions (FAs) link the cytoskeleton to the extracellular matrix and as such play important roles in growth, migration and contractile properties of vascular smooth muscle cells (VSMCs). Recently, it has been shown that downregulation of Nox4, a transforming growth factor beta (TGFβ)-inducible, H2O2-producing enzyme, affects the number of FAs. However, the effectors downstream of Nox4 that mediate FA regulation are unknown. The FA resident protein hydrogen peroxide-inducible clone-5 (Hic-5) is H2O2- and TGFβ-inducible, and a binding partner of the heat shock protein (Hsp)27. The objective of this study was to elucidate the mechanism by which Hic-5 and Hsp27 participate in TGFβ-induced, Nox4-mediated VSMC adhesion and migration.
Approach and Results
Through a combination of molecular biology and biochemistry techniques, we found that TGFβ, by a Nox4-dependent mechanism, induces the expression and interaction of Hic-5 and Hsp27, which is essential for Hic-5 localization to FAs. Importantly, we found that Hic-5 expression is required for the TGFβ-mediated increase in FA number, and adhesive forces and migration. Mechanistically, Nox4 downregulation impedes Smad signaling by TGFβ, and Hsp27 and Hic-5 upregulation by TGFβ is blocked in Smad4-deficient cells.
Conclusions
Hic-5 and Hsp27 are effectors of Nox4 required for TGFβ-stimulated FA formation and adhesion strength and migration in VSMC.
Keywords: Vascular Smooth Muscle Cell, Focal Adhesion, Nox4, Hic-5, Hsp27, migration
Nox proteins are the catalytic subunits of a family of NADPH oxidases enzymes.1 Nox homologues are primary sources of reactive oxygen species (ROS) in vascular tissues that participate in numerous physiological and pathophysiological processes.1 In particular, in vascular smooth muscle cells (VSMCs) from rodent conductance arteries, two of these homologues are expressed: Nox1 and Nox4.2 Nox4 localizes to focal adhesions (FAs), stress fibers and the nucleus3 and is upregulated by transforming growth factor beta (TGFβ). Its hydrogen peroxide-producing activity is both causal for, and a consequence of, Smad (Small body size & Mothers Against Decapentaplegic)-dependent pathways.4, 5 Importantly, Nox4 has profound effects on the VSMC cytoskeleton and FAs. In fact, Nox4 is required for the expression of VSMC contractile apparatus proteins6 and is essential for FA formation.7 However, the downstream effectors responsible for the regulation of cytoskeleton dynamics by Nox4, especially those that regulate synthesis and degradation of FA-related proteins, have yet to be identified.
FAs are specialized sites through which the cytoskeleton connects to the extracellular matrix. They are highly organized dynamic structures with rich signaling machinery capable of executing decisions about the cellular responses to a variety of stimuli. For instance, in VSMCs, FA turnover regulates migratory8 and biomechanical9 properties and thus is likely to impact vascular diseases. The components of FAs are diverse and include signaling proteins as well as scaffolding molecules such as Paxillin10 and the hydrogen peroxide (H2O2)-inducible clone-5 (Hic-5)11 that interact with Vinculin in a Rho GTPase-regulated manner.12
Hic-5, first identified as an H2O2 and TGFβ-inducible gene,13 is now recognized as a member of the Paxillin family of proteins and functions as an adapter in FAs.11 In VSMCs, Hic-5 expression has been functionally implicated in the regulation of migration and injury-induced neointima formation14 and in the cellular responses to uni-axial stretch.15, 16
The heat shock protein (Hsp) 27 is a chaperone protein that confers cellular tolerance to a variety of stresses including heat and oxidative stress17 and its expression is increased by H2O2.18 Importantly, Hsp27 interacts with the actin cytoskeleton and has been shown to participate in the stabilization of FAs in response to heat shock stress.19 Interestingly, it has also been identified as a Hic-5 binding protein.20
In the current study, we hypothesized that Hic-5 and Hsp27 are downstream effectors of Nox4 that are required for proper FA assembly in VSMCs. We found that TGFβ induces the expression of both Hic-5 and Hsp27 via a Nox4- and Smad- dependent mechanism, Hsp27 helps Hic-5 translocate to the FA, and Hic-5 expression is absolutely required for TGFβ-induced, Nox4-mediated FA formation.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
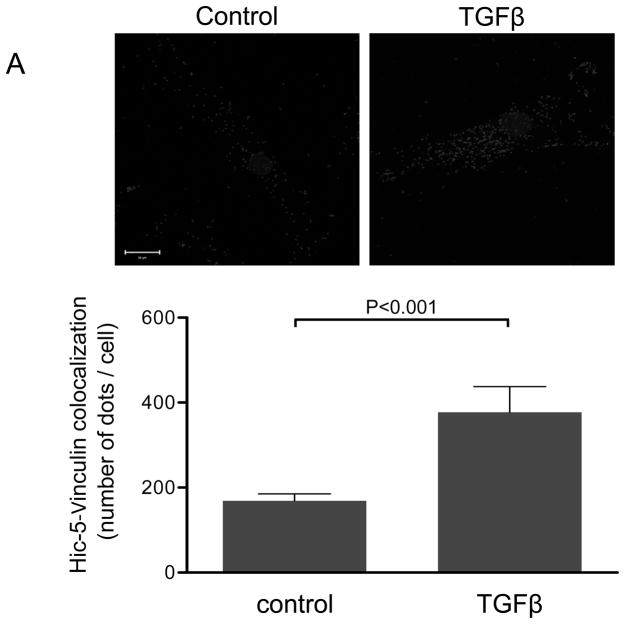
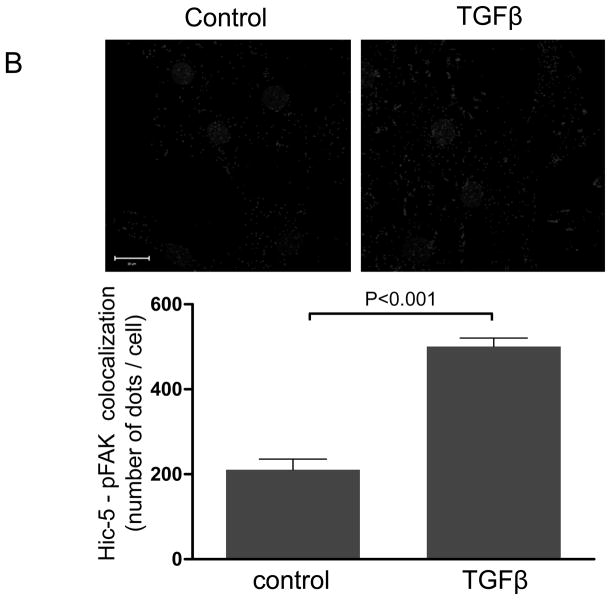
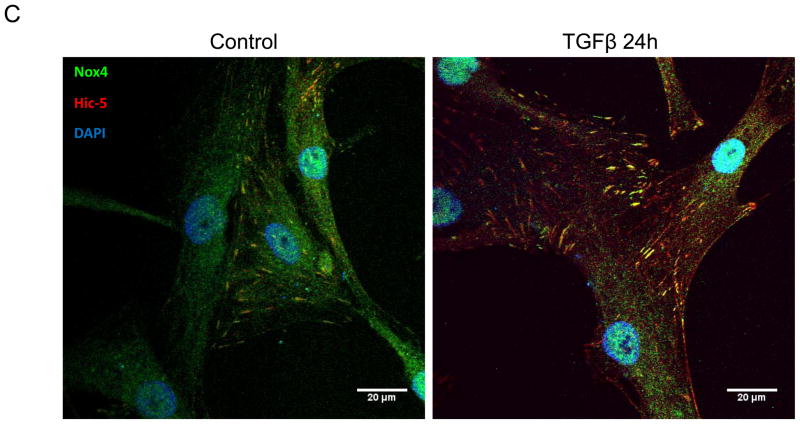
We have previously shown that downregulation of Nox4 or its activator Poldip2 significantly reduces the number of FAs in VSMCs. 7 We first confirmed that, as previously reported for other cell types,14–16, 21, 22 Hic-5 localizes to FAs in VSMC. Using an in situ proximity ligation assay, we found that in HASMCs Hic-5 co-localizes with Vinculin and p-FAK, as shown by the characteristic punctuated staining indicative of protein interaction (Figures 1A and B respectively). Hic-5 localization to FAs was increased more than two times in the presence of TGFβ, a well-established Nox4 agonist5, 23 and a positive regulator of FAs in VSMCs.24 Since Hic-5 is a FA localized protein and previous reports showed that Nox4 also localizes to the FA, we evaluated if Hic-5 colocalizes with Nox4. Indeed, we found that both proteins colocalize in FA in an association that is increased after TGFβ treatment (Figure 1C). For these studies, we used a custom made antibody against Nox4 (characterized in Supplemental Figure IA).
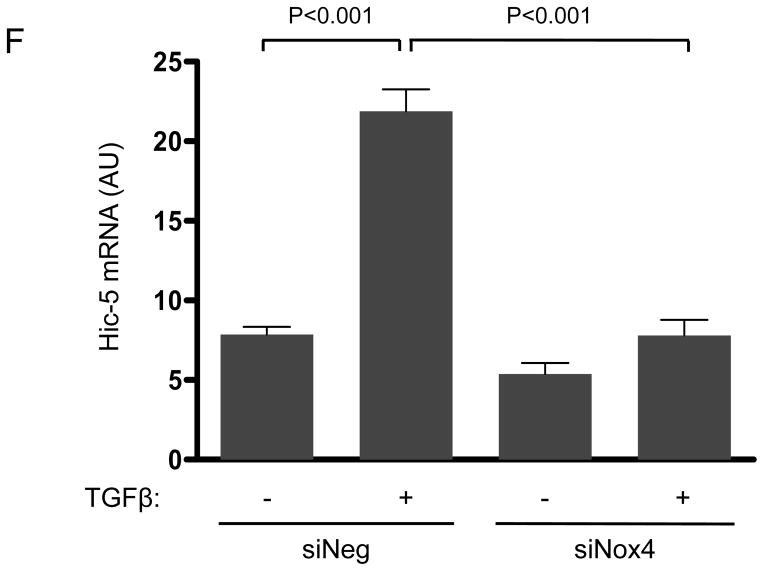
Figure 1. The focal adhesion protein Hic-5 is regulated by TGFβ via a Nox4-dependent mechanism.
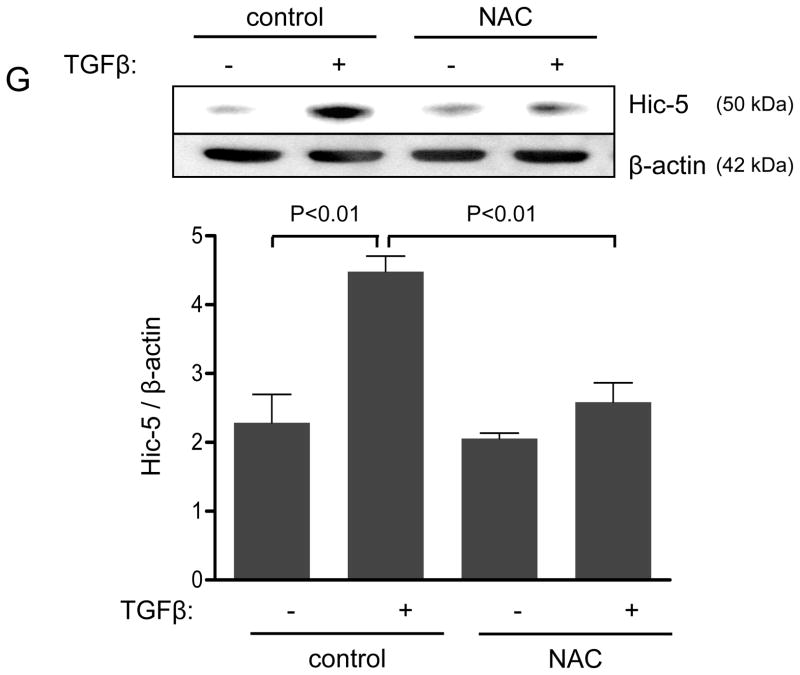
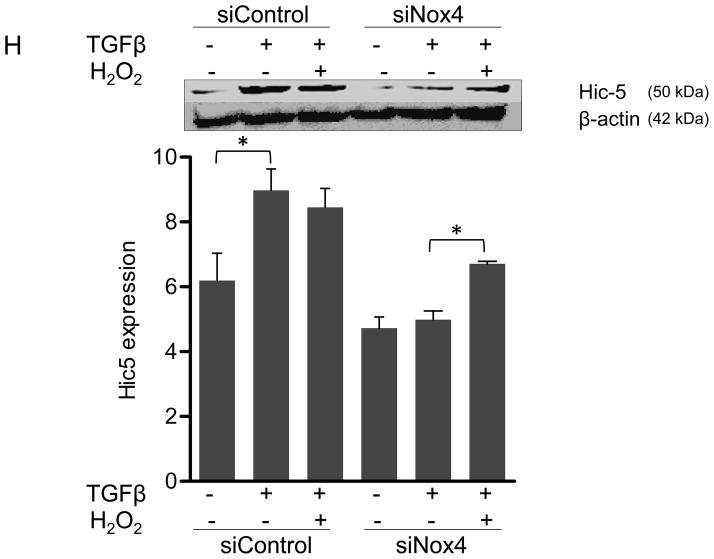
Panels A-B: Hic-5 localization to FAs increases with TGFβ treatment. HASMCs were plated and maintained in serum-free medium for 2 days before treatment with TGFβ (2 ng/ml). Colocalization (red label) between Hic-5 and Vinculin (A), and Hic-5 and pFAK (B) was determined by in situ proximity ligation assay (Duolink). Panel C: Colocalization of Hic5 and Nox4 in HASMCs is increased with TGFβ treatment. HASMCs were plated and serum deprived for 2 days and then treated with TGFβ for 24h. Cells were stained for Hic-5 (red) and Nox4 (green), and nuclei were detected with DAPI (blue). Images were captured using confocal microscopy. Colocalization is evident in yellow in the right panel. Panels D-H: TGFβ increases Hic-5 expression via Nox4-produced H2O2. After 2 days of serum deprivation, HASMCs were treated with TGFβ (2 ng/mL) for indicated times and Hic-5 was detected by WB (D). HASMC were transfected with non-silencing RNA (siNeg) or siNox4 and treated with TGFβ (2 ng/mL) for 24h to analyze Hic-5 protein (E) and RNA (F) by WB and RT-qPCR respectively. (G) Cells incubated with or without N-acetyl cysteine (NAC, 10 mM) for 2 hours prior to addition of TGFβ for 24h were analyzed by western blotting. (H) HASMCs transfected with siNeg or siNox4 were treated with either TGFβ (2 ng/mL) or TGFβ (2 ng/mL) + H2O2 (50 μg/mL) and the expression of Hic-5 was analyzed by WB. All experiments were performed at least three independent times. Data is presented as average±SEM of three independent experiments.
Nox4 is activated by TGFβ to produce hydrogen peroxide.5 Because Hic-5 was first identified as a hydrogen peroxide-inducible clone also activated by TGFβ treatment,13 we hypothesized that TGFβ-mediated upregulation of Hic-5 and that this mechanism may require H2O2 produced by Nox4. Indeed, TGFβ significantly increases Hic-5 expression in HASMCs as early as 6 h after treatment and Hic-5 stays elevated over a period of 24 h (Figure 1D). Importantly, transfection of HASMCs with siNox4 (which successfully downregulates Nox4 mRNA and protein without affecting Nox1 protein levels, Supplemental Figure IA, B and C, respectively) abolished TGFβ-induced Hic-5 protein expression (Figure 1E). This effect seems to be specific for Nox4, because Nox1 deficiency had no effect in the ability of TGFβ to upregulate Hic-5 expression (Supplemental Figure ID). To determine if this effect occurs at the mRNA level, we used RT-qPCR. We found that TGFβ significantly increased Hic-5 mRNA, and that this increase is completely abolished when Nox4 is downregulated (Figure 1F). The regulation of Hic-5 by the TGFβ/Nox4 pathway is quite specific, since neither Nox4 nor TGFβ had any effect on the expression of the close homologue Paxillin at the mRNA or protein level (Supplemental Figure IIA and B). Moreover, this effect is likely to be mediated by H2O2 derived from Nox4 because the upregulation of Hic-5 induced by TGFβ was also blocked by pretreatment with the antioxidant N-acetyl cysteine (NAC) (Figure 1G), and more importantly, exogenously added H2O2 (to a final concentration if 50 μM) was able to partially recover TGFβ-induced Hic-5 expression in Nox4 deficient cells (Figure 1H). Finally, it is important to note that downregulation of Hic-5 had no effect on Nox4 expression (Supplemental Figure IB).
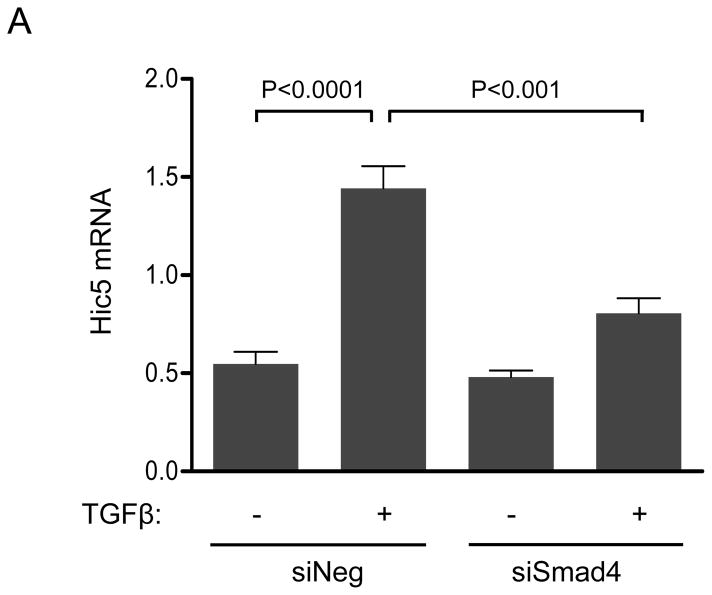
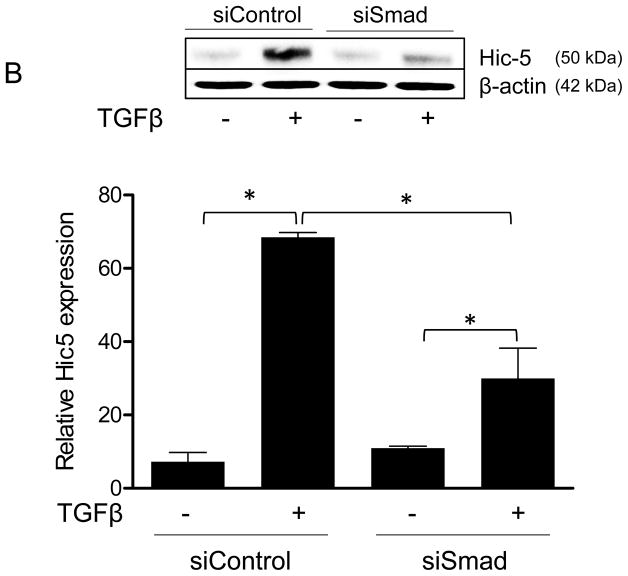
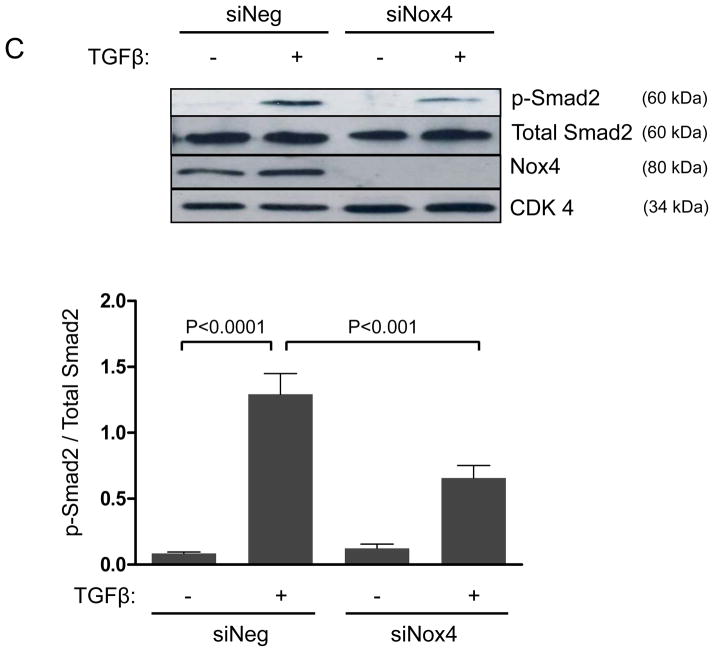
TGFβ superfamily ligands bind to a TGF-type II receptor, which recruits and phosphorylates a TGF-type I receptor.25 The type I receptor then phosphorylates Smads2/3, which bind to Smad4;25 subsequently, the complex is translocated to the nucleus to regulate gene expression.25 Given that Figure 1 clearly shows that Nox4 is required for Hic-5 expression, we sought to determine if the Smad pathway is required for TGFβ-induced upregulation of Hic-5 in HASMCs. Our results (Figure 2A and B) show that Hic-5 mRNA and protein upregulation was significantly blunted in Smad-deficient cells. Moreover, downregulation of Nox4 significantly inhibited TGFβ-induced Smad2 phosphorylation (Figure 2C), suggesting that Smad pathways are downstream of Nox4 in Hic-5 mRNA regulation. This is likely to be a direct effect of Nox4- derived H2O2, because Smad2/3 can be phosphorylated by exogenously added H2O2 (Supplemental Figure IIIA), but the absence of Nox1 does not impair the ability of TGFβ to induce Smad phosphorylation in HASMCs (Supplemental Figure IIIB). Together, these data demonstrate that TGFβ induces Hic-5 mRNA expression by a Nox4- and Smad-dependent mechanism.
Figure 2. Hic-5 upregulation after TGFβ treatment is mediated by Nox4/Smad dependent pathways.
HASMCs were transfected with siNeg or siSmad4. After 2 days of serum starvation, cells were treated with TGFβ (2 ng/ml) for 24 h. (A) Hic-5 mRNA was measured by RT-qPCR using HPRT-1 as housekeeping gene and (B) Hic-5 protein was detected by WB using a specific antibody. (C) HASMCs were transfected with siNeg or siNox4. After 2 days of serum starvation, cells were treated with TGFβ (2 ng/ml) for 1 h and subjected to western analysis for p-Smad2, total Smad2 and Nox4. CDK4 was used as a loading control. Bars represent the average of four independent experiments ±SEM.
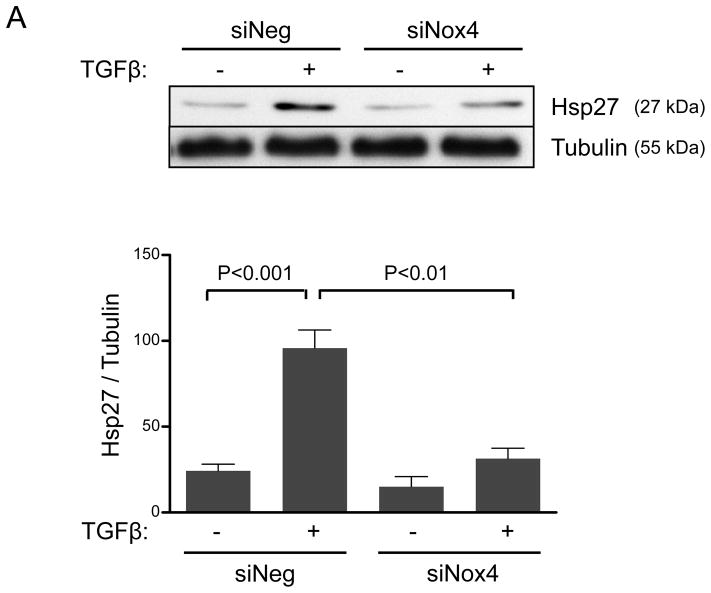
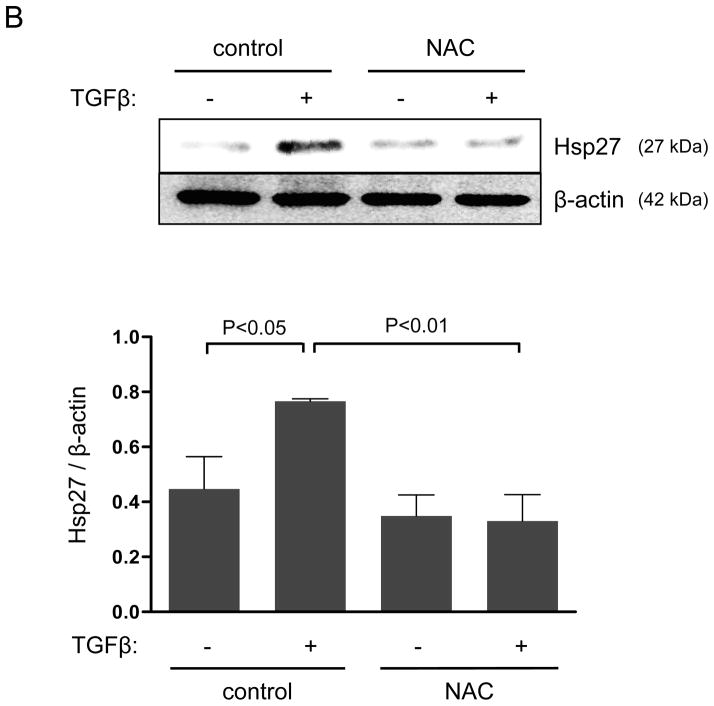
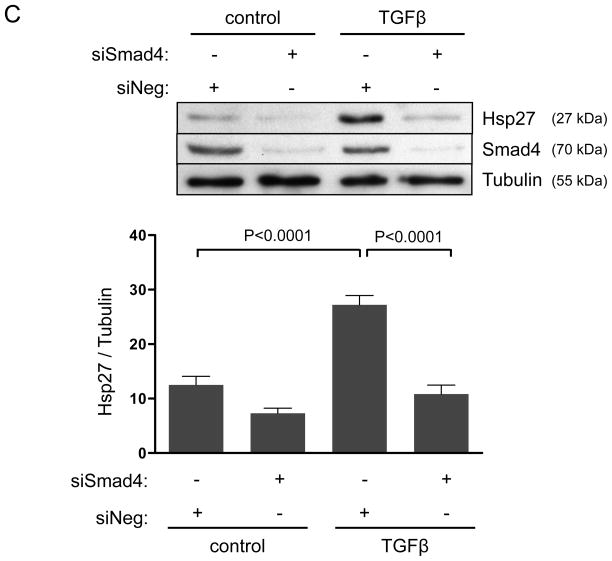
In addition to mRNA regulation, TGFβ may control the subcellular localization of Hic-5. In this regard, more than a decade ago, Jia and colleagues20 identified Hic-5 as an Hsp27 binding protein using two-hybrid screening. Since Hsp27 is a chaperone protein known to modulate FAs, we hypothesized that Hsp27 may participate in the TGFβ/Smad/Nox4 pathway by increasing Hic-5 protein stabilization and aiding its localization to FAs. Because HASMCs have a very low basal expression of Hsp27, we first evaluated the ability of TGFβ to induce Hsp27 expression. As shown in Figure 3A, TGFβ was not only capable of significantly inducing Hsp27 expression, but also it did so in a Nox4-dependent manner that requires H2O2 production, as shown by the ability of siNox4 and NAC to inhibit the Hsp27 upregulation by TGFβ (Figure 3A, B). Similar to what we found for Hic-5, TGFβ-induced Hsp27 expression was not affected by the absence of Nox1 (Supplemental Figure IV). Together, these results prompted us to evaluate the Smad-dependence of Hsp27 expression, and we observed that siRNA against Smad4 completely blocked the increase in Hsp27 observed after TGFβ treatment (Figure 3C). These data indicate that, similar to Hic-5, Hsp27 is upregulated in HASMC after TGFβ treatment by a Nox4-dependent mechanism that requires Smad pathways.
Figure 3. Smads and Nox4 mediate Hsp27 upregulation by TGFβ.
HASMCs were transfected with siNeg, siNox4 (A) or siSmad4 (C) or treated with NAC (B) and maintained in serum-free medium for 2 days prior to TGFβ treatment (2 ng/mL) for 24 h. Total proteins were extracted and subjected to western blot analysis for Hsp27 and Smad4. Tubulin was used as a loading control. Bars represent the average of four independent experiments ± SEM.
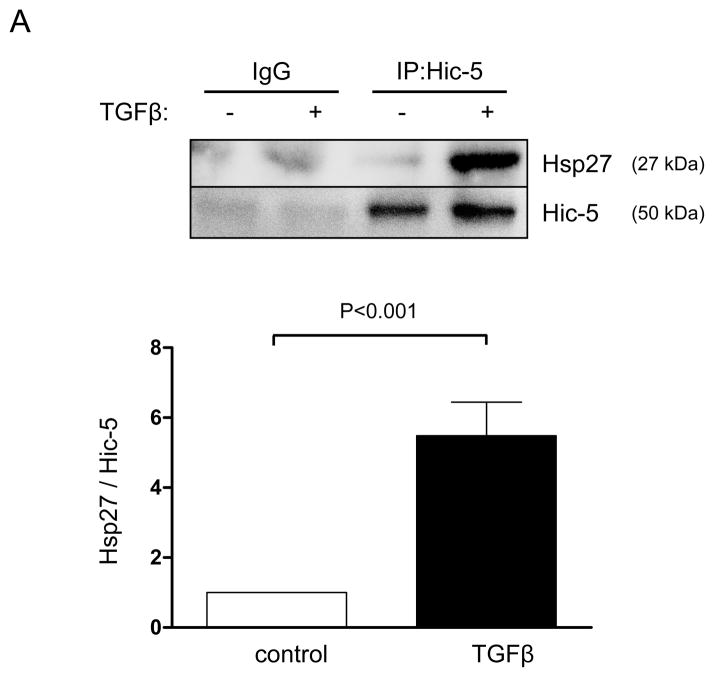
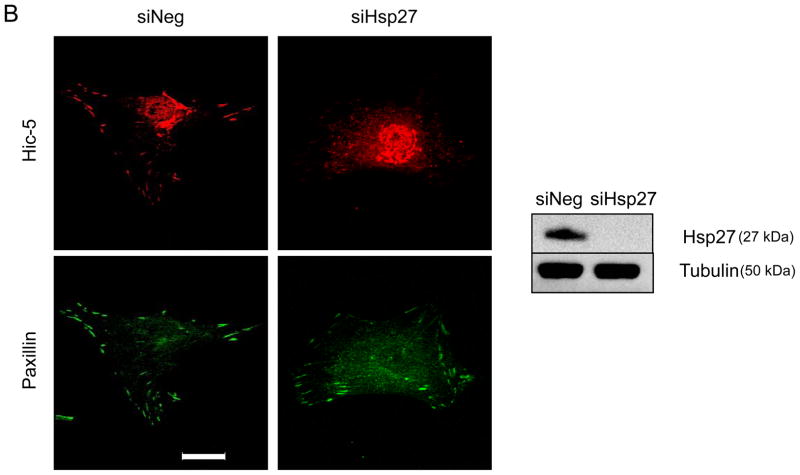
As a chaperone protein, Hsp27 is likely to affect Hic-5 in two possible ways: increasing its protein stability and/or affecting its subcellular localization. In agreement with previous reports, 20, 26 we found that Hic-5 and Hsp27 physically interact after TGFβ treatment as they coimmunoprecipitate (Figure 4A). However, their interaction has no consequence on Hic-5 protein stability or expression since downregulation of Hsp27 does not affect Hic-5 mRNA or protein levels (Supplemental Figure VA and B, respectively). Significantly, we found that Hsp27 was required for proper localization of Hic-5 to FAs after TGFβ treatment (Figure 4B). Interestingly, this effect seems to be specific for Hic-5 since the other closely related member of the family, Paxillin, localizes to FAs even in Hsp27-deficient cells (Figure 4B).
Figure 4. Hsp27 associates with Hic-5 and aids its localization to FA.
HASMCs were serum deprived for 48 h before treatment with or without TGFβ (2 ng/mL) for 24 hrs. (A) Co-immunoprecipitation was performed using an unrelated IgG or primary antibody against Hic-5. The membrane was immunoblotted using anti-Hsp27 and Hic-5 antibodies. Bars represent the average of three independent experiments ± SEM. (B) HASMCs were transfected with siRNA against Hsp27 (siHsp27) and then serum deprived for 48 h before treatment with TGFβ (2 ng/ml) for 24 h. Cells were fixed and stained for Paxillin (green), Hic-5 (red). Scale Bar = 20 μm. The images are representative four independent experiments.
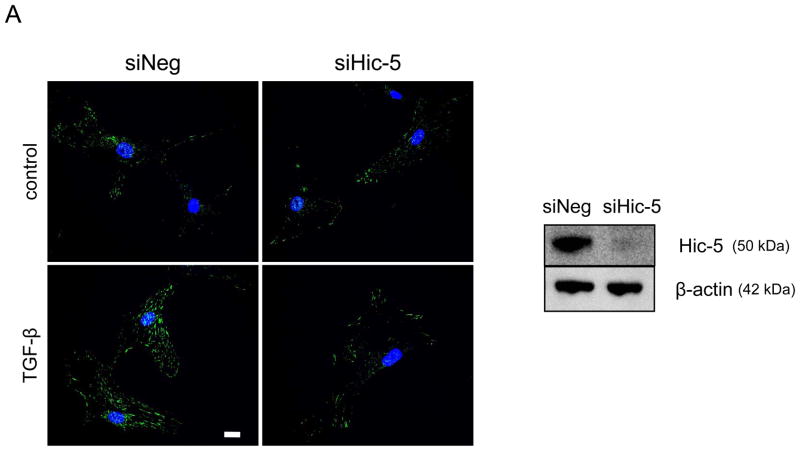
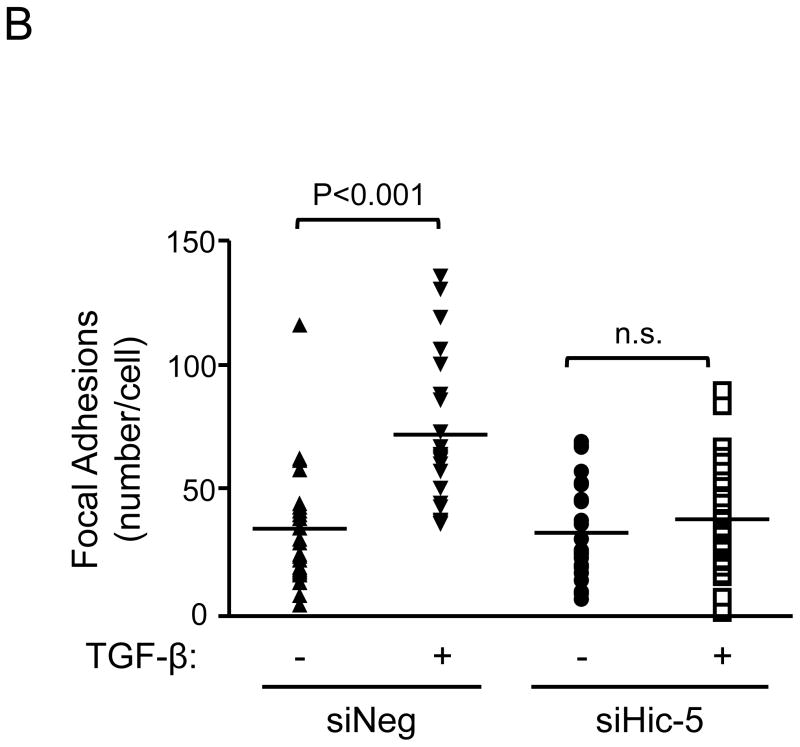
Our results demonstrate that Hic-5 is regulated by a Nox4-dependent mechanism and that association with Hsp27 is required for its proper localization to the FAs. Once we established the upstream signaling pathway that regulates Hic-5, we focused on examining the downstream consequence of Hic-5 expression on FA formation and cell adhesion in HASMC. We found that, as measured by Vinculin staining, downregulation of Hic-5 resulted in significantly reduced numbers of FAs (Figures 5A and B), resembling the loss of FAs observed when either Nox47 or Hsp27 is downregulated (Figure 4B). This effect was more evident when the cells were stimulated with TGFβ, and was not due to Hic-5-mediated downregulation of Vinculin expression (Supplemental Figure VI).
Figure 5. Hic-5 mediates TGFβ-induced focal adhesions.
Quiescent HAMSCs were treated with non-silencing RNA (siNeg) or siRNA against Hic-5 (siHic-5) for 2 days. After treatment with TGFβ (2 ng/mL) for 24 h, cells were fixed and stained for Vinculin (red), and nuclei were detected with DAPI (blue). Scale Bar = 20 μm. (A). The image represents the average of the FA number per cell, n=25. Four independent experiments were performed with consistent results (B).
Finally, we investigated the physiological relevance of this pathway. We reasoned that the role of this pathway to modulate FA composition and size may affect the ability of the cell to engage extracellular matrix components, and accordingly, it is likely to affect cell adhesion and as a consequence of impaired adhesion, cell migration.
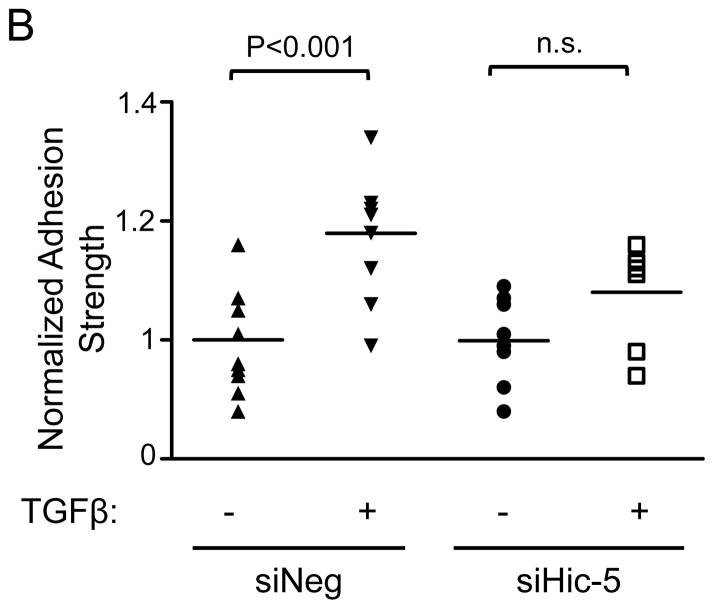
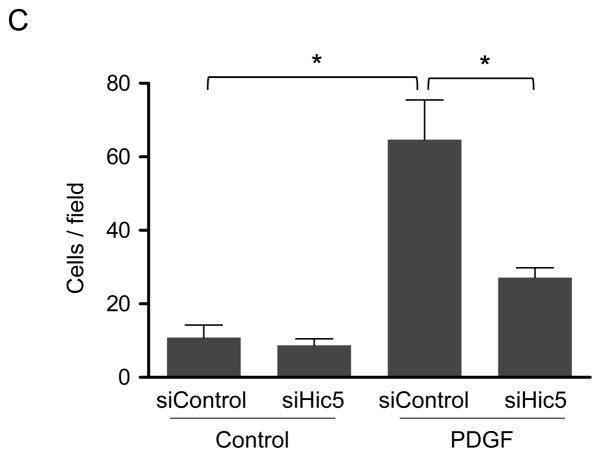
To the role of Hic-5 in the regulation of TGFβ-mediated HASMC adhesion strength, We measured the force required to detach the cell from the extracellular matrix, which correlates with FA function, number, and size.27 To measure cell adhesion strength, we used the spinning disk system, which exposes an adherent cell population to a range of hydrodynamic shear forces that increase linearly with radial distance from the disk center. Subsequently, the number of adherent cells was counted at different radial positions, corresponding to known shear stress values. The adherent cell fraction decreases nonlinearly with respect to fluid shear stress, and cell adhesion strength is defined as the fluid shear stress that produces 50% cell detachment. We observed that TGFβ treatment for 24 h significantly increases HASMC adhesion strength, and that this effect is abrogated by Hic-5 downregulation (Figure 6A and B). Cell adhesion, together with motor-clutches-mediated force transmission, is necessary to generate traction that moves the cell forward during cell motility.26 Therefore, we evaluated if Hic-5 deficient cells exhibit impaired cell migration. Indeed, cells where Hic-5 has been downregulated using siRNA display significantly lower motility towards a chemoattractant stimulus than control cells (Figure 6C), similar to results previously obtained with knockdown of Nox4.7
Figure 6. Hic-5 mediates TGFβ-induced cell adhesion strength and PDGF-induced migration in HASMCs.
Immediately after siRNA electroporation, HASMCs were seeded onto fibronectin-coated glass coverslips. One day post-seeding, the cells were serum-starved for 48 h, stimulated with 2 ng/mL of TGF-β for 24 h, and spun inside the spinning disk device to quantify adhesion strength, which is defined as the shear stress at which 50% of cell detachment occurs. Representative adhesion profiles for each of the each condition (A) and bar graph is the average of n=7–10 (B). Hic-5 deficient cells have impaired cells migration (C). After transfections, HASMCs were starved for 48 h, trypsinized, and reseeded in a collagen-coated Transwell insert. After stimulation for 3 h with PDGF (10 ng/mL), the cells migrating through the membrane were stained with DAPI and counted. The graph represents mean±SEM of migrated cells per field from 3 independent experiments. *P<0.01.
Discussion
Our previous work showed that Nox4 is a critical component of FAs.3, 7 Indeed, not only is Nox4 a FA resident protein, 3 but also its absence significantly reduces the number of FAs in the cell.7 However, and despite the striking nature of this effect, very little is known about the means by which Nox4 affects FA. In the present work, we identify two important downstream effectors of Nox4: Hic-5 and the chaperone protein Hsp27. We describe a mechanism by which Nox4 increases Hsp27 and Hic-5 expression and promotes their physical interaction, which is necessary for the proper localization of Hic-5 to FA, especially after TGFβ treatment (Figure 6C). Most importantly, the localization of Hic-5 to the FAs is functionally relevant since it mediates the TGFβ-induced increase in adhesion strength (Figure 6A and B) and cell migration (Figure 6C).
Manipulation of Nox4 and Hsp27 had no effect on Paxillin expression (Supplemental Figures IIA and B) or localization (Figure 4B). Because Paxillin and Hic-5, both members of the Paxillin family of proteins, share highly conserved structural domains and exhibit extensive crosstalk and synergy,12 it is particularly interesting that the TGFβ/Nox4 pathway uses Hic-5 to strengthen FAs. This specificity seems to lie in two factors: the ability of the TGFβ/Nox4 pathway to induce Hic-5 expression (Figures 1D-H) and the unique association of Hic-5 with Hsp27 (Figure 4A).20
The fact that Hic-5 is positively regulated by a Nox4-dependent mechanism is not surprising considering that Hic-5 has been identified as a marker for smooth muscle contractile phenotype28 and that differentiation in this cell type is a Nox4-dependent process.6 On the other hand, it was recently reported that in a lung myofibroblast cell line, Hic-5 functions as a negative regulator of myofibroblast differentiation by promoting Nox4 degradation,29 raising the interesting possibility that the role of Hic-5 is cell type dependent.
Furthermore, our data clearly indicate that it is Nox4-derived H2O2 that is responsible for the TGFβ-mediated increased of Hic-5 expression. The ability of exogenous H2O2 to reverse the effect of the siNox4 (Figure 1H) demonstrates that Nox4-produced H2O2 participates in the signaling pathways initiated by TGFβ in VSMC. This conclusion agrees with previous reports showing that in VSMCs, TGFβ induces Nox4-derived H2O2 starting 4 h after treatment and continues over a period of 24 h.5 Although the specific targets of H2O2 are unclear, we speculate that inactivation of Smad phosphatases is one likely possibility. Furthermore, even though, the colocalization of Hic-5 and Nox4 is suggestive of a role for Nox4 in the FA, the local production of H2O2 is unlikely to participate in the Smad-mediated transcriptional mechanism described in this work and additional studies are required to test a possible role of local ROS production in focal adhesion or adhesive forces.
The lack of suitable commercial Nox4 antibodies has been a limiting factor for the field of Nox biology. In this study, we used a custom made antibody (kindly provided by Dr. David Lambeth) originally characterized in the supplemental material of Hilenski, et al.,3. This study was performed with the last remaining vial of the antibody, which is unfortunately no longer available. However, the additional experiments shown in Supplemental Figure IA clearly demonstrate the specificity of this antibody, since the immunofluorescence signal is attenuated by siRNA against Nox4 (Supplemental Figure IA i), and the 65 and 80 kD bands are no longer detected after preincubation of the antibody with blocking peptide (Supplemental Figure IA ii). The 80 kD band is the predominant form that we detect in VSMC as well as in tissues of wild type mice, but not their Nox4 KO littermates (Supplemental Figure IA iii). The reason for the apparent increase in molecular weight is unclear, but may be related to glycosylation (unpublished observations). .
With regard to Hsp27/Hic-5 complex formation, it has been determined that the entire αB-crystallin domain in the C-terminal region of Hsp27 and the fourth LIM domain of Hic-5 are both necessary and sufficient for their interaction.20 Since Hic-5 and Paxillin share significant homology in that region, the specific structural determinant that facilitates Hic-5 specific interaction of Hsp27 remains to be identified.
Concerning the regulation of Hic-5, our data suggest that this occurs mainly at the mRNA level and requires both Nox4-produced ROS (Figure 1) and Smads (Figure 2A and B). The fact that NAC completely reverses TGF-β induced Hic-5 expression (Figure 1G) supports the idea that Hic-5 expression is downstream of Nox4-induced Smad activation (Figure 2C). It is also possible that Smad activation may be necessary, but not sufficient, to drive Hic-5 expression. It was recently demonstrated that the serum response factor (SRF) is essential for TGF-β mediated induction of Hic-5 expression in VSMC.28 Our own and other studies have linked SRF expression to TGFβ-induced H2O25, 30 and directly to Nox4-derived H2O2.6, 8 Based on these observations, it is possible that the activation of SRF by a Nox4-produced H2O2 mediated pathway may also contribute to Hic-5 upregulation after TGFβ treatment.
The small GTPase RhoA seems to be required for the cytoskeletal effects of Nox4. In fact, a constitutively active form of RhoA rescues the loss of focal adhesions induced by Poldip2/Nox4 deficiency.7 It is noteworthy that inhibition of Hic-5 reduces RhoA activation in TGFβ-stimulated epithelial cells31 and that overexpression of Hic-5 in these cells leads to increased formation of stress fibers in a Rho kinase-dependent manner. These observations thus raise the possibility that Hic-5 might modulate Nox4-mediated Rho activity, perhaps in a scaffolding capacity. Independently of the mechanism, the presence of Hic-5 in the FA has profound effects on the function of FAs since the knockdown of this protein was sufficient to completely abrogate the increase in adhesion strength generated by TGFβ treatment in HASMC (Figure 6A and B), which exactly resembles the reduction in the number of FAs observed when Hic-5 is downregulated (Figure 5A and B).
After vascular injury or during advanced stages of atherosclerosis, medial VSMCs proliferate and then migrate into the intimal layer.32 The process of VSMC migration involves a series of well-orchestrated steps where ROS play a central role.8 Indeed, we and others have demonstrated that migration of VSMC is blocked in vivo and in vitro by antioxidants or after reducing the expression of Nox-based enzymatic complexes.33–36 Our data further support this notion and offer new targets for H2O2-mediated signaling pathways during migration (Figure 6C).
In summary, we have shown that Hic-5 is a novel effector of Nox4 that is upregulated in VSMCs after TGFβ treatment by a Smad-dependent mechanism that requires H2O2 produced by Nox4. Additionally, we found that the correct subcellular localization of Hic-5 within FAs requires the chaperone protein Hsp27, also a target of Nox4-mediated gene transcription. Our results provide a molecular explanation for the striking effects of Nox4 on FA dynamics and identify two important mediators that act jointly. This pathway seems to be true not only in smooth muscle cells derived from humans, but also from rat (Figure 1C), and mouse (Supplemental figure I, III and IV). These findings shed light on the redox-sensitive pathways that lead to cell attachment and may be potentially important in understanding those functions of VSMCs associated with actin reorganization, such as migration and contractility.
Supplementary Material
Significance.
This work unveils downstream effectors of Nox4, a protein that is known to play an essential role in smooth muscle biology and differentiation by still unknown mechanisms. Additionally, our data indicate a novel role for Hic-5 as a key mediator of TGFβ-induced vascular smooth muscle cell adhesion, which not only impairs cell migration but is likely to impact vascular wall mechanical properties.
Acknowledgments
We thanks Dr. David Lambeth for providing us with the antibody against Nox4.
SOURCES OF FUNDING
The National Institutes of Health, through awards R01HL095070, R01HL38206, R01HL113167, R01GM065918 and R01HL58863, supported this work. We acknowledge additional support from the National Science Foundation as a Graduate Research Fellowship award to D.W.Z.
ABBREVIATIONS
- TGFβ
transforming growth factor beta
- Hic-5
the hydrogen peroxide (H2O2)-inducible clone-5 (Hic-5
- Smad
(Small body size & Mothers Against Decapentaplegic
- Hsp27
heat shock protein 27
- FA
focal adhesion
Footnotes
DISCLOSURES
None.
References
- 1.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of nadph oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells : Nox1 mediates angiotensin ii-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 3.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of nox1 and nox4 in vascular smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 4.Boudreau HE, Casterline BW, Rada B, Korzeniowska A, Leto TL. Nox4 involvement in tgf-beta and smad3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic Biol Med. 2012;53:1489–1499. doi: 10.1016/j.freeradbiomed.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin-Garrido A, Brown DI, Lyle AN, Dikalova A, Seidel-Rogol B, Lassegue B, San Martin A, Griendling KK. Nadph oxidase 4 mediates tgf-beta-induced smooth muscle alpha-actin via p38mapk and serum response factor. Free Radic Biol Med. 2011;50:354–362. doi: 10.1016/j.freeradbiomed.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:42–48. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.San Martin A, Griendling KK. Redox control of vascular smooth muscle migration. Antioxid Redox Signal. 2010;12:625–640. doi: 10.1089/ars.2009.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Z, Martinez-Lemus LA, Hill MA, Meininger GA. Extracellular matrix-specific focal adhesions in vascular smooth muscle produce mechanically active adhesion sites. Am J Physiol Cell Physiol. 2008;295:C268–278. doi: 10.1152/ajpcell.00516.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner CE, Glenney JR, Jr, Burridge K. Paxillin: A new vinculin-binding protein present in focal adhesions. The Journal of cell biology. 1990;111:1059–1068. doi: 10.1083/jcb.111.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas SM, Hagel M, Turner CE. Characterization of a focal adhesion protein, hic-5, that shares extensive homology with paxillin. Journal of cell science. 1999;112 ( Pt 2):181–190. doi: 10.1242/jcs.112.2.181. [DOI] [PubMed] [Google Scholar]
- 12.Deakin NO, Ballestrem C, Turner CE. Paxillin and hic-5 interaction with vinculin is differentially regulated by rac1 and rhoa. PloS one. 2012;7:e37990. doi: 10.1371/journal.pone.0037990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibanuma M, Mashimo J, Kuroki T, Nose K. Characterization of the tgf beta 1-inducible hic-5 gene that encodes a putative novel zinc finger protein and its possible involvement in cellular senescence. The Journal of biological chemistry. 1994;269:26767–26774. [PubMed] [Google Scholar]
- 14.Kim-Kaneyama JR, Wachi N, Sata M, Enomoto S, Fukabori K, Koh K, Shibanuma M, Nose K. Hic-5, an adaptor protein expressed in vascular smooth muscle cells, modulates the arterial response to injury in vivo. Biochemical and biophysical research communications. 2008;376:682–687. doi: 10.1016/j.bbrc.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 15.Kim-Kaneyama JR, Suzuki W, Ichikawa K, Ohki T, Kohno Y, Sata M, Nose K, Shibanuma M. Uni-axial stretching regulates intracellular localization of hic-5 expressed in smooth-muscle cells in vivo. Journal of cell science. 2005;118:937–949. doi: 10.1242/jcs.01683. [DOI] [PubMed] [Google Scholar]
- 16.Kim-Kaneyama JR, Takeda N, Sasai A, Miyazaki A, Sata M, Hirabayashi T, Shibanuma M, Yamada G, Nose K. Hic-5 deficiency enhances mechanosensitive apoptosis and modulates vascular remodeling. Journal of molecular and cellular cardiology. 2011;50:77–86. doi: 10.1016/j.yjmcc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 17.Salinthone S, Tyagi M, Gerthoffer WT. Small heat shock proteins in smooth muscle. Pharmacology & therapeutics. 2008;119:44–54. doi: 10.1016/j.pharmthera.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu AL, Fuchshofer R, Birke M, Kampik A, Bloemendal H, Welge-Lussen U. Oxidative stress and tgf-beta2 increase heat shock protein 27 expression in human optic nerve head astrocytes. Investigative ophthalmology & visual science. 2008;49:5403–5411. doi: 10.1167/iovs.07-1478. [DOI] [PubMed] [Google Scholar]
- 19.Schneider GB, Hamano H, Cooper LF. In vivo evaluation of hsp27 as an inhibitor of actin polymerization: Hsp27 limits actin stress fiber and focal adhesion formation after heat shock. J Cell Physiol. 1998;177:575–584. doi: 10.1002/(SICI)1097-4652(199812)177:4<575::AID-JCP8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Jia Y, Ransom RF, Shibanuma M, Liu C, Welsh MJ, Smoyer WE. Identification and characterization of hic-5/ara55 as an hsp27 binding protein. The Journal of biological chemistry. 2001;276:39911–39918. doi: 10.1074/jbc.M103510200. [DOI] [PubMed] [Google Scholar]
- 21.Dabiri G, Tumbarello DA, Turner CE, Van de Water L. Hic-5 promotes the hypertrophic scar myofibroblast phenotype by regulating the tgf-beta1 autocrine loop. The Journal of investigative dermatology. 2008;128:2518–2525. doi: 10.1038/jid.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croke JM, Pike LR, MacPhee DJ. The focal adhesion protein hic-5 is highly expressed in the rat myometrium during late pregnancy and labour and co-localizes with fak. Reproductive biology and endocrinology : RB&E. 2007;5:22. doi: 10.1186/1477-7827-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-beta1 induces nox4 nad(p)h oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. American journal of physiology Lung cellular and molecular physiology. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 24.Riedy MC, Brown MC, Molloy CJ, Turner CE. Activin a and tgf-beta stimulate phosphorylation of focal adhesion proteins and cytoskeletal reorganization in rat aortic smooth muscle cells. Experimental cell research. 1999;251:194–202. doi: 10.1006/excr.1999.4573. [DOI] [PubMed] [Google Scholar]
- 25.Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, Gassmann M, Messing A, Klein R, Schwab MH, Lloyd KC, Lai C. Receptor tyrosine kinase erbb4 modulates neuroblast migration and placement in the adult forebrain. Nature neuroscience. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- 26.Giannone G, Mege RM, Thoumine O. Multi-level molecular clutches in motile cell processes. Trends in cell biology. 2009;19:475–486. doi: 10.1016/j.tcb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Bulin C, Albrecht U, Bode JG, Weber AA, Schror K, Levkau B, Fischer JW. Differential effects of vasodilatory prostaglandins on focal adhesions, cytoskeletal architecture, and migration in human aortic smooth muscle cells. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:84–89. doi: 10.1161/01.ATV.0000146814.81581.68. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Hu G, Betts C, Harmon EY, Keller RS, Van De Water L, Zhou J. Transforming growth factor-beta1-induced transcript 1 protein, a novel marker for smooth muscle contractile phenotype, is regulated by serum response factor/myocardin protein. The Journal of biological chemistry. 2011;286:41589–41599. doi: 10.1074/jbc.M111.250878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weissenbach M, Clahsen T, Weber C, Spitzer D, Wirth D, Vestweber D, Heinrich PC, Schaper F. Interleukin-6 is a direct mediator of t cell migration. European journal of immunology. 2004;34:2895–2906. doi: 10.1002/eji.200425237. [DOI] [PubMed] [Google Scholar]
- 30.Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, Griendling KK. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res. 2004;94:1219–1226. doi: 10.1161/01.RES.0000126848.54740.4A. [DOI] [PubMed] [Google Scholar]
- 31.Tumbarello DA, Turner CE. Hic-5 contributes to epithelial-mesenchymal transformation through a rhoa/rock-dependent pathway. J Cell Physiol. 2007;211:736–747. doi: 10.1002/jcp.20991. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz SM. Perspectives series: Cell adhesion in vascular biology. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest. 1997;99:2814–2816. doi: 10.1172/JCI119472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nunes GL, Sgoutas DS, Redden RA, Sigman SR, Gravanis MB, King SB, III, Berk BC. Combination of vitamins c and e alters the response to coronary balloon injury in the pig. Arterioscler Thromb and Vasc Biol. 1995;15:156–165. doi: 10.1161/01.atv.15.1.156. [DOI] [PubMed] [Google Scholar]
- 34.Nunes GL, Robinson K, Kalynych A, King SB, III, Sgoutas DS, Berk BC. Vitamins c and e inhibit o2- production in the pig coronary artery. Circulation. 1997;96:3593–3601. doi: 10.1161/01.cir.96.10.3593. [DOI] [PubMed] [Google Scholar]
- 35.Konneh MK, Rutherford C, Li SR, Anggard EE, Ferns GA. Vitamin e inhibits the intimal response to balloon catheter injury in the carotid artery of the cholesterol-fed rat. Atherosclerosis. 1995;113:29–39. doi: 10.1016/0021-9150(94)05423-g. [DOI] [PubMed] [Google Scholar]
- 36.Kappert K, Sparwel J, Sandin A, Seiler A, Siebolts U, Leppanen O, Rosenkranz S, Ostman A. Antioxidants relieve phosphatase inhibition and reduce pdgf signaling in cultured vsmcs and in restenosis. Arterioscler Thromb Vasc Biol. 2006;26:2644–2651. doi: 10.1161/01.ATV.0000246777.30819.85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.