Abstract
In atherosclerotic rabbits (SCLER), decreases in vascular resistance in response to acetylcholine (ACH), an endothelium-dependent agent, are suppressed, whereas those to nitroprusside (NP), an endothelium-independent vasodilator, are preserved. To determine whether defective vasodilation in SCLER is related to altered reactivity of resistance vessels, we visualized arterioles of rabbit cremaster muscle by videomicroscopy. Arteriolar diameter was monitored during topical (superfusional) delivery of ACh and NO, interventions that did not affect systemic hemodynamics. Diameter changes in response to NP (0.01-100.0 microM) did not differ between SCLER and controls; maximal dilations amounted to 110 +/- 10% (mean +/- SE). In contrast, responses to ACH (0.001-100 microM) differed; maximal dilations averaged 54 +/- 4% in SCLER and 124 +/- 9% in controls (P less than 0.001). These differences persisted after blockade with phentolamine, propranolol, and indomethacin. Phenidone and hydroquinone blockers of endothelium-dependent vasodilation, inhibited arteriolar dilation to ACH without affecting that to NP. Microvascular responses to intra-arterial drug were similar to those elicited by topical drug. Thus, hypercholesterolemia and atherosclerosis in the rabbit appear to produce a microvascular defect characterized by an impaired endothelium-dependent dilation and a preserved endothelium-independent dilation. This defect could play a role in limiting vasodilator reserve in atherosclerosis.
Full text
PDF

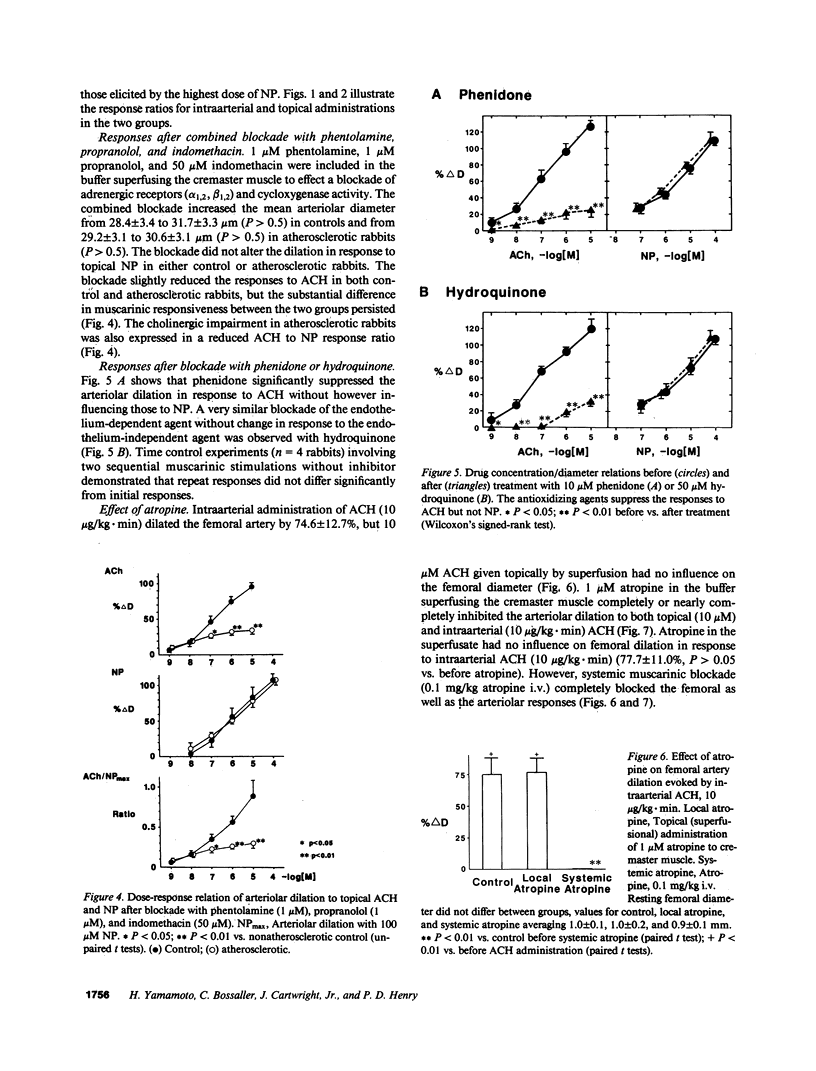
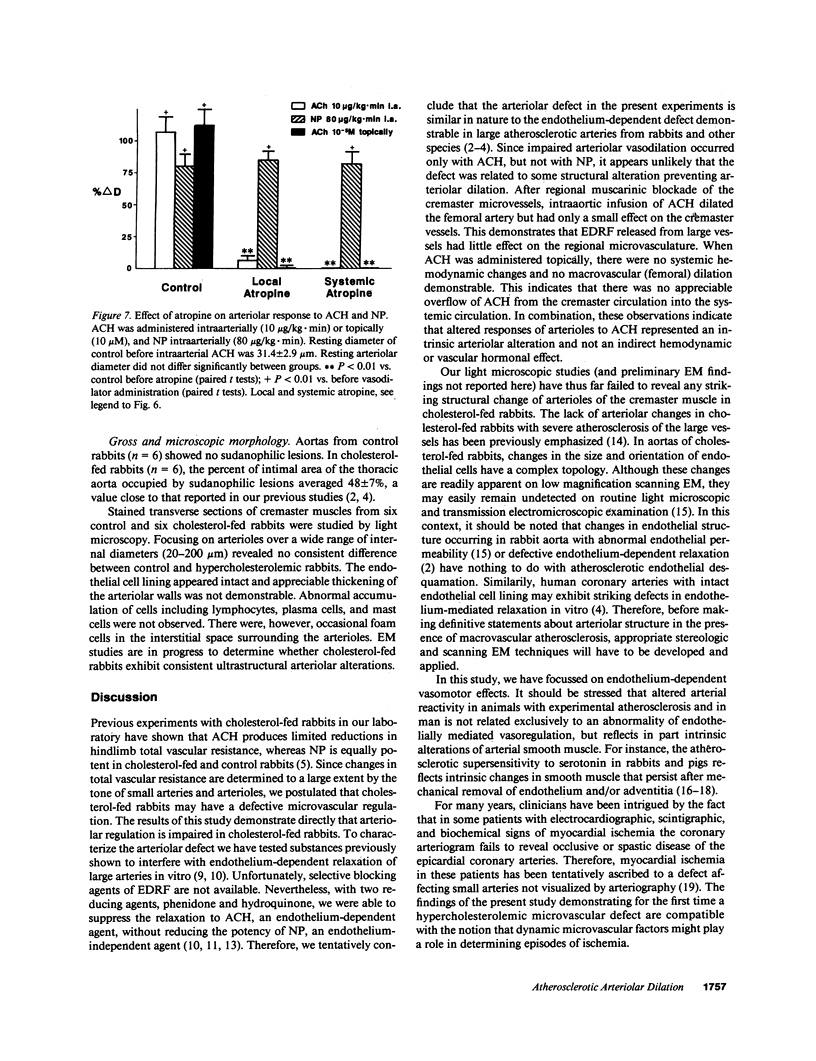

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baez S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc Res. 1973 May;5(3):384–394. doi: 10.1016/0026-2862(73)90054-x. [DOI] [PubMed] [Google Scholar]
- Bossaller C., Habib G. B., Yamamoto H., Williams C., Wells S., Henry P. D. Impaired muscarinic endothelium-dependent relaxation and cyclic guanosine 5'-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. J Clin Invest. 1987 Jan;79(1):170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaller C., Yamamoto H., Lichtlen P. R., Henry P. D. Impaired cholinergic vasodilation in the cholesterol-fed rabbit in vivo. Basic Res Cardiol. 1987 Jul-Aug;82(4):396–404. doi: 10.1007/BF01907027. [DOI] [PubMed] [Google Scholar]
- Cannon R. O., 3rd, Schenke W. H., Leon M. B., Rosing D. R., Urqhart J., Epstein S. E. Limited coronary flow reserve after dipyridamole in patients with ergonovine-induced coronary vasoconstriction. Circulation. 1987 Jan;75(1):163–174. doi: 10.1161/01.cir.75.1.163. [DOI] [PubMed] [Google Scholar]
- Chan C. T., Brecher P., Haudenschild C., Chobanian A. V. The effect of cholesterol feeding on the metabolism of rabbit cerebral microvessels. Microvasc Res. 1979 Nov;18(3):353–369. doi: 10.1016/0026-2862(79)90043-8. [DOI] [PubMed] [Google Scholar]
- Colantuoni A., Bertuglia S., Intaglietta M. Quantitation of rhythmic diameter changes in arterial microcirculation. Am J Physiol. 1984 Apr;246(4 Pt 2):H508–H517. doi: 10.1152/ajpheart.1984.246.4.H508. [DOI] [PubMed] [Google Scholar]
- Freiman P. C., Mitchell G. G., Heistad D. D., Armstrong M. L., Harrison D. G. Atherosclerosis impairs endothelium-dependent vascular relaxation to acetylcholine and thrombin in primates. Circ Res. 1986 Jun;58(6):783–789. doi: 10.1161/01.res.58.6.783. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Cherry P. D., Zawadzki J. V., Jothianandan D. Endothelial cells as mediators of vasodilation of arteries. J Cardiovasc Pharmacol. 1984;6 (Suppl 2):S336–S343. doi: 10.1097/00005344-198406002-00008. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Henderson A. H., Edwards D. H., Lewis M. J. Isolated perfused rabbit coronary artery and aortic strip preparations: the role of endothelium-derived relaxant factor. J Physiol. 1984 Jun;351:13–24. doi: 10.1113/jphysiol.1984.sp015228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib J. B., Bossaller C., Wells S., Williams C., Morrisett J. D., Henry P. D. Preservation of endothelium-dependent vascular relaxation in cholesterol-fed rabbit by treatment with the calcium blocker PN 200110. Circ Res. 1986 Feb;58(2):305–309. doi: 10.1161/01.res.58.2.305. [DOI] [PubMed] [Google Scholar]
- Henry P. D., Bentley K. I. Suppression of atherogenesis in cholesterol-fed rabbit treated with nifedipine. J Clin Invest. 1981 Nov;68(5):1366–1369. doi: 10.1172/JCI110384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry P. D., Yokoyama M. Supersensitivity of atherosclerotic rabbit aorta to ergonovine. Mediation by a serotonergic mechanism. J Clin Invest. 1980 Aug;66(2):306–313. doi: 10.1172/JCI109858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor K. G., Damon D. N., Duling B. R. Inaccuracies in blood flow estimates in microvessels during arteriolar vasoconstriction. Microvasc Res. 1984 Jul;28(1):23–36. doi: 10.1016/0026-2862(84)90026-8. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B. Effects of moderate hypercholesterolemia on rabbit endothelium. Arteriosclerosis. 1981 Jan-Feb;1(1):25–32. doi: 10.1161/01.atv.1.1.25. [DOI] [PubMed] [Google Scholar]
- Verbeuren T. J., Jordaens F. H., Zonnekeyn L. L., Van Hove C. E., Coene M. C., Herman A. G. Effect of hypercholesterolemia on vascular reactivity in the rabbit. I. Endothelium-dependent and endothelium-independent contractions and relaxations in isolated arteries of control and hypercholesterolemic rabbits. Circ Res. 1986 Apr;58(4):552–564. doi: 10.1161/01.res.58.4.552. [DOI] [PubMed] [Google Scholar]