Abstract
Rationale
Research suggests that nicotine deprivation among smokers is associated with lesser resting cortical activity (i.e., greater power density in theta and alpha-1 EEG bands and lesser power in beta bands). These changes in cortical activity may be indicative of withdrawal-related cognitive deficits, yet the markers of differences in cortical activity are not well-established.
Objective
To clarify the EEG frequency bands affected by nicotine deprivation and assess prospective moderators
Method
124 heavy smokers visited the laboratory on two occasions following overnight smoking/nicotine deprivation. Prior to collecting three minutes of resting EEG data, participants smoked two very low nicotine cigarettes (< .05 mg nicotine yield) at one session and two moderate nicotine cigarettes (.60 mg nicotine yield) at the other.
Results
Theta and alpha-1 band (4–7 & 8–10 Hz) was greater in the very low nicotine (deprivation) relative to higher nicotine (satiation) condition. There were no condition differences in the beta-1 and beta-2 bands (14–20 & 21–30 Hz).
Conclusions
Greater slow wave resting EEG may serve as a reliable marker of decreased cortical activity during smoking deprivation and, in turn, of withdrawal-related deficits in cognitive functioning. This research may inform the development of adjunct strategies for smoking cessation.
Keywords: abstinence, attention, cognitive control, cortical activation, EEG, hemispheric asymmetry, smoking, tobacco, withdrawal
Nicotine-dependent smokers exhibit withdrawal symptoms within 30 minutes of smoking (Hendricks et al. 2006), including deficits in cognitive control relevant to daily functioning (Evans et al. 2013a; Waters, Jarvis, and Sutton, 1998). However, the precise cognitive processes affected by nicotine deprivation are not well-elucidated. The validation of robust and objective cognitive markers of nicotine deprivation is needed in order to facilitate future research investigating the clinical relevance of cognitive effects in relation to nicotine dependence and smoking cessation.
Cognitive control refers to a range of executive functions, including the ability to pay attention, concentrate, reason, plan, and remember information relevant to the task at hand. There are few well-validated neural markers of nicotine deprivation-induced deficits in cognitive control. The best established marker is a reduction in the target P300 (or P3b) event-related potential (ERP) evoked by infrequent target stimuli presented amid a semi-rapid (e.g., every 1000 ms) stream of standard stimuli (Evans et al. 2013a). Other ERP components (e.g., error-related negativity; Schlienz, Hawk, and Rosch 2013), as well as functional magnetic resonance (fMRI) brain imaging findings, have begun to implicate specific brain regions and cognitive functions disrupted during nicotine withdrawal (e.g., Beaver et al. 2011; Lerman et al. 2014; Kozink et al. 2010). However, the extent to which these latter findings are replicable is unclear.
One neurobiological correlate of cognitive control is electroencephalography (EEG) collected while subjects are sitting quietly. A growing literature indicates that individuals with reduced cognitive control show higher power density values within the slower EEG bands (theta; ~4–7 and alpha; ~8–13 Hz) along with reduced fast wave activity (beta, ~14–30 Hz) (Hermens et al. 2005; Lansbergen et al. 2011). For example, it was found that theta-beta ratio correlated negatively with both response inhibition on a go/no-go task and on self-reported attentional control among normal participants (Putman et al. 2010). Stimulant drugs (e.g., methylphenidate) produce reductions of slower wave power and increased faster wave power (Clarke et al. 2002; Loo et al. 1999). Similarly, nicotine appears to have a stimulant-like effect on resting EEG (Fisher et al. 2012; Knott and Fisher 2007; Knott et al. 2005). This may account for nicotine having stimulant-like reductions of attentional deficits (e.g., Potter 2008). The specific frequency bands that are affected by nicotine deprivation vary somewhat across studies. Designs have also varied significantly with respect to sample size and methodology. It was therefore of interest to specifically examine nicotine deprivation in a strong design with a larger sample.
The primary aim of the current study was to clarify the effects of nicotine deprivation relative to satiation on resting EEG in frontal, central, temporal and parietal regions. This was achieved through a within-subject design with counterbalanced conditions and double-blind drug administration. We sought to evaluate previous findings where slow wave power was greater under nicotine deprivation compared to satiation and fast wave power was lower under nicotine deprivation. The large sample size also provided an opportunity to assess potential moderators of condition differences. More specifically, we assessed gender, age, and nicotine dependence.
A secondary aim was to examine trait cognitive control as a moderator. Individuals exhibiting chronic deficits in cognitive control may experience greater cognitive deficits resulting from nicotine deprivation (Evans and Drobes 2009) and/or greater boosts from nicotine (Newhouse et al. 2004). Evans et al. (2013a) found that individuals who scored lower on the Adult Temperament Questionnaire attentional control scale (ATQ-ACS; Evans and Rothbart 2007) showed greater nicotine deprivation-induced reductions in P300 responses to vivid distracting stimuli (P3a). We also found that nicotine administered to nonsmokers suppressed slow wave resting EEG to a greater extent among individuals reporting lower levels of cognitive control (Evans et al. 2013b). Nicotine deprivation versus satiation differences in EEG are expected to be greater among individuals reporting reduced trait cognitive control.
Methods
Participants
Smokers (N = 137) not actively attempting to quit were recruited from the Tampa Bay area. Participants ranged from 18 to 70 years of age, and reported smoking an average of at least 15 cigarettes per day for two years. Smoking status was biochemically verified by an expired air carbon monoxide (CO) sample of 10 or more parts per million (ppm) along with a cotinine level of ≥ 100 ng/mL.
Exclusionary criteria included use of alternative nicotine products (e.g., snus, lozenge) during the past three months, diagnosis of a neurological condition, taking medication that might influence physiological responses (e.g. beta blockers, tricyclic antidepressants), concussion, any other serious medical conditions such as cardiopulmonary problems or respiratory-related illness exacerbated by smoking (e.g. bronchitis, emphysema, asthma), current use of psychoactive substances (e.g. cocaine, amphetamines, barbiturates and benzodiazepines) as determined by urine drug testing, vision problems, pregnancy or breast feeding, or current psychosis, mood (depression, mania), panic (i.e., other anxiety disorders allowed), or non-nicotine substance dependence disorders as determined by administration of the Structured Clinical Interview for DSM disorders (SCID; First et al. 2002).
Procedure
Participants provided informed consent at the beginning of an initial screening session that included establishing eligibility. Demographic information was collected and included gender, ethnicity/race, age, education level, income, current occupation, medical history and current medications. Eligible participants completed the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al. 1991), a self-report measure of smoking/nicotine dependence. In addition, the Cognitive Failures Questionnaire (CFQ; Broadbent et al. 1982) was completed to assess trait cognitive control. The CFQ is a 25 item measure of absent-mindedness that has shown relationships with cognitive control-related performance indices (e.g., Smilek et al. 2010) as well as ERP measures of attentional control (Roche et al. 2005).
Following the initial session, participants completed two experimental sessions each lasting approximately 2.5 hours, following a minimum of 12-hour overnight nicotine/smoking deprivation. Experimental sessions were separated by 3 to 14 days. Deprivation at each session was confirmed biochemically by CO level no greater than 10 ppm, or less than or equal to half the CO level obtained during the screening session. Prior to collecting resting EEG data, participants smoked either two moderate nicotine (.60 mg nicotine yield) or very low nicotine content (VLNC) (< .05 mg nicotine yield) cigarettes (Quest, Vector Tobacco, Inc.) in a double-blind fashion, counterbalanced across sessions. Brody et al. (2009) found that a group smoking the very low and moderate nicotine yield Quest cigarettes used in the current study resulted in 26% and 79% nicotinic receptor occupancy, respectively. Thus, the very low nicotine content Quest cigarettes have pharmacologic impact, but the difference between low and moderate nicotine cigarettes is robust. Participants were prepped with a 64-channel Neuroscan electroencephalogram (EEG) cap after smoking the first cigarette. The next cigarette was smoked 40 minutes after initiation of the first cigarette. Next, participants sat still and quietly with eyes open for three-minutes during collection of resting EEG. Additional cognitive tasks were completed and are reported elsewhere (e.g., Evans et al. 2013a). Participants who completed the entire study were compensated approximately $200.
Electroencephalogram (EEG) recording
The Neuroscan Synamps 2 system with SCAN 4.3 software was used to record EEG data from a 64-channel electrode cap montage, including the 10–20 system along with additional electrodes. Impedance values were monitored and kept below 50kΩ. EEG was sampled at 250 Hz. An online low-pass filter of 100 Hz was applied. The EEG was referenced online to a midline vertex electrode. Electro-oculogram (EOG) electrodes were placed above and below the left eye and lateral to each eye for measuring vertical and horizontal eye movements, respectively.
EEG data processing
EEG was visually examined for artifact (e.g., eye blinks and movements, excessive EMG). Artifact containing segments were removed at all electrode sites. 1000 ms epochs were extracted through a Hanning window with 50% overlap (maximum of 360 epochs per 3-minute session). Two sessions with less than 72 valid epochs (20%) were excluded from analysis. Eleven other sessions were lost due to EEG acquisition errors (e.g., poor reference electrode). Therefore, 13 participants were excluded from analyses by failing to provide sufficient EEG for both sessions (i.e., condition), resulting in final data analyses based on 124 participants. Missing data from individual electrode sites averaged 4.5%.
Power densities (µV2/Hz) for theta (4–7 Hz), alpha-1 (8–10 Hz), alpha-2 (11–13 Hz), Beta-1 (14–20 Hz) and Beta-2 (21–30 Hz) were computed at 17 electrode sites, with 15 sites from the 10–20 montage plus two additional frontal sites (AF3 and AF4). This included seven homologous pairs from the left and right hemisphere (Fp1/Fp2, AF3/AF4, F3/F4, F7/F8, C3/C4, T7/T8, and P3/P4), as well as three midline (Fz, Cz, and Pz) sites. A natural log transform was used to de-skew the distribution of power density values for each band at each electrode.
Data Analyses
Condition differences (nicotine deprivation vs. satiation) were assessed using mixed models on each EEG band for the seven homologous pairs and for the midline set. The mixed modeling approach has a number of advantages over traditional analysis of variance, including more optimal handling of covariates and allowance for randomly missing data without exclusion of participants (Tabachnick and Fidell 2007). In addition to condition, models included session (first/second), hemisphere or region (left/right or frontal/central/parietal), the three 2-way interactions and the one 3-way interaction. Subject was the only random effect in the models. All statistical tests were performed with alpha set at .01 given the number of analyses on outcome measures that are (highly) correlated for nearby EEG sites and adjacent EEG bands.
Additional models assessed the four prospective moderators: sex, age, nicotine dependence (FTND), and trait cognitive control (CFQ). Each model added the prospective moderator and its interaction with condition to a base model with condition, session, hemisphere/region, and any significant interaction terms from the model assessing condition. As above, all tests of prospective moderators were two-sided with α = .01.
Results
95 men and 29 women provided EEG data from both sessions. The age range was 19 to 63 years (M = 38.3, SD = 11.7). 115 were predominantly right-handed. Years of education ranged from 6 to 18 (M = 12.7, SD =1.9). The racial makeup of the sample included 100 White, 21 Black, 1 Pacific Islander, 1 Native American, and 1 participant who did not report race. 105 participants reported non-Hispanic ethnicity, 14 reported Hispanic ethnicity, and 5 did not report ethnicity. Mean score on the FTND was 5.8 (SD = 1.8), which reflects a moderate to high level of nicotine dependence. Mean CO levels were 29.5 (SD = 13.1) ppm at the preliminary session, 9.6 (SD = 4.3) ppm prior to nicotine session, and 9.8 (SD = 4.7) ppm prior to the satiation session. CO from both the nicotine deprivation and nicotine satiation sessions were significantly decreased from baseline (t[123]’s > 12.6, p’s < .0001), but did not differ significantly from each other (t[123] = −0.6, p=.54).
Main effect: Nicotine deprivation versus satiation
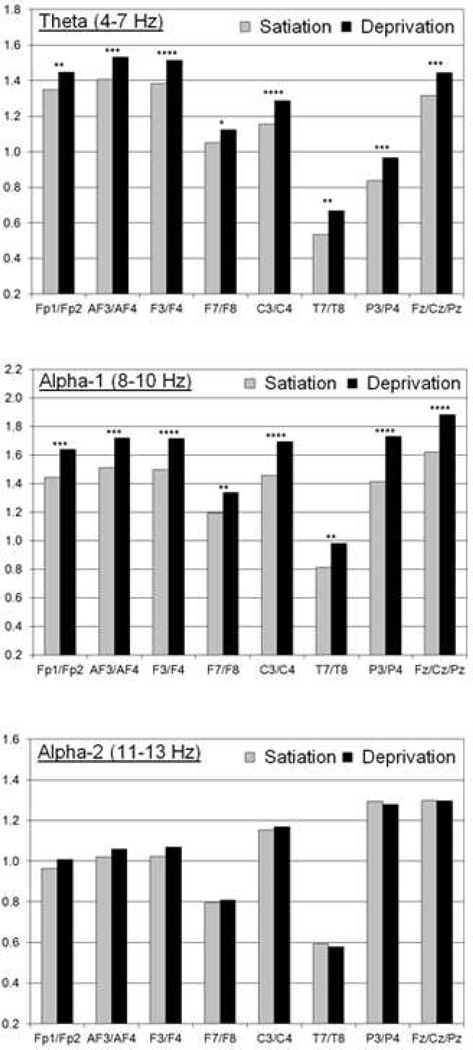
Figure 1 shows adjusted means for the two conditions in the theta, alpha-1, and alpha-2 frequency bands, along with p-values for condition. All power density values were significantly greater for the theta and alpha-1 bands in the nicotine deprivation condition (i.e., less cortical activation), except for the marginally significant difference in the theta band for the lateral frontal pair (F7/F8). Effect sizes based on those with complete data were estimated to be small to medium in the theta and alpha-1 bands as measured by Cohen’s d (.17 to .43). In contrast, there were no significant condition differences for alpha-2 (p’s > .20), even though some small effects (d < .12) were in the same direction as for alpha-1 and theta. Furthermore, there were no significant condition differences for beta-1 and beta-2 (p’s > .14).
Figure 1.
Adjusted means for the moderate nicotine/satiation (left) and very low nicotine/deprivation (right) condition are presented for the theta (top), alpha-1 (middle), and alpha-2 (bottom) bands. The adjusted means are the average ln-transformed power density values across the homologous pair (e.g., Fp1/Fp2 for the frontal pole) controlling for the influence of session, hemisphere/region, and all interaction terms as presented in the primary mixed models.
P-values for the condition effect are noted as follows: * for p < .05 (marginally significant), ** for p < .01, *** for p < .001, and **** for p < .0001.
Session and hemisphere: main effects and interactions with condition
Across all analyses of the 8 site pairs/sets and the 5 EEG bands, there was only one instance where Session was a significant predictor. For the P3/P4 pair, alpha-1 power density was lower during the first session (p=.008). There were no significant Session × Condition interactions.
There was a significant main effect for Hemisphere (1) at the AF3/AF4, F3/F4, C3/C4, and T7/T8 pairs in the theta, alpha-1, alpha-2, and beta-1 bands; (2) at the Fp1/Fp2 pair in the theta, alpha-1, and alpha-2 bands; and (3) at the P3/P4 pair in the theta band. Furthermore, there were three significant Hemisphere × Condition interactions for the AF3/AF4 pair with greater deprivation-satiation differences on the left side (AF3) in the theta, alpha-1, and alpha-2 bands.
Finally, for the midline set (Fz/Cz/Pz), there was a significant main effect for Region in the theta, alpha-2, beta-1 and beta-2 bands. The magnitude of power density values was greatest at Fz, in the middle at Cz, and lowest Pz in the theta, beta-1, and beta-2 bands. The opposite pattern was observed in the alpha-2 band. Although there was no significant main effect in the alpha-1 band, the Region × Condition interaction was significant. The deprivation-satiation difference was greater at Pz than Fz.
Sex, age, nicotine dependence, and trait cognitive control as moderators
Across all analyses of the 8 site pairs/sets and the 5 EEG bands, there were no significant interactions between Condition and sex, age, nicotine dependence (FTND), or trait cognitive control (CFQ).
Discussion
Nicotine deprivation effects
This study provides the strongest evidence to date regarding the effects of nicotine deprivation on resting EEG. Power density values in the 4–10 Hz range that include theta and alpha-1 were enhanced by nicotine deprivation (or equivalently reduced by smoking that reversed nicotine withdrawal). These small to medium effects were highly significant. In contrast, no effects for alpha-2, beta-1 and beta-2 frequencies (i.e., > 10 Hz.) were observed. Other studies have reported that smoking enhances these faster wave activities (e.g., Newton et al. 1998), but we found no evidence to support this position. Our findings are consistent with studies of differences in cognitive control as a function of theta-beta ratios, except that the effects appear to be completely driven by nicotine deprivation enhancing slow wave power, while having no influence on faster wave power. As noted, it has been suggested that the contribution of greater slow wave power and reduced fast wave power to attentional deficits may be independent. Accordingly, we believe that separate consideration of slow and fast wave activity as employed herein provides greater specificity. That the theta and alpha-1 frequencies are affected by nicotine deprivation, whereas alpha-2 is not affected might be informative with respect to the types of cognitive processes affected. Alpha-1 effects are diffuse across the scalp, and it has been suggested that this frequency band reflects general attentional processing, whereas the alpha-2 frequency band is believed to involve semantic processing (Klimesch et al. 2007).
The current findings suggest that indices of greater slow wave (theta and alpha-1) resting EEG power during nicotine deprivation serve as robust markers of withdrawal-related deficits in cognitive functioning. Some preliminary findings suggest that nicotine withdrawal-related cognitive disruption may predict actual smoking behavior (e.g., Powell et al. 2004). For example, Krishnan-Sarin et al. (2007) reported that a significantly greater number of commission errors on a continuous performance task predicted relapse to smoking. Further research should determine whether slow wave EEG markers of cognitive disruption predict the capacity to resist smoking. This approach may inform treatment strategies (e.g., cognitive training, cognitive pharmacotherapy) that target these cognitive deficits in an effort to enhance smoking cessation outcomes. Additionally, identification smokers who are the most affected by nicotine withdrawal-related cognitive disruption may assist in understanding who may profit the most from cognitive treatment approaches.
There were two results involving an interaction of condition with brain region. First, there was a condition × hemisphere interaction in theta, alpha-1, and alpha-2 in the anterior frontal region. The deprivation-satiation difference was greater on the left than on the right. This finding in the context of left-right frontal asymmetry for the appetitive and aversive motivation systems (e.g., Sutton and Davidson 1997) suggests that nicotine deprivation has a greater effect on the cortical components on the appetitive motivation system, which may make goal-directed quitting more difficult.
Other nicotine resting EEG studies have not reported laterality effects involving slow wave power. However, fMRI nicotine withdrawal studies have found hemisphere specific effects during working memory tasks. Xu et al. (2005) found that left dorsolateral prefrontal cortex activation was maximal during low working memory load and did not change across higher loads in the nicotine deprivation condition, whereas activation in this brain area continued to increase with higher working memory load in the nicotine satiation condition. In contrast, Ernst et al. (2001) found that abstinent smokers showed prefrontal cortical activation exclusively in the right hemisphere during performance of a working memory challenge. However, the extent to which our findings may contrast with and/or relate to the hemisphere specific findings of Xu et al. and Ernst et al. is unclear, as we cannot directly compare activation during rest (i.e., resting EEG) with activation during performance of a cognitive task as measured with fMRI.
Second, there was an interaction of condition with frontal-central-parietal cortical region (midline). The deprivation-satiation difference was greater at Pz than at Fz. This may simply be a measurement artifact. Another possibility is that the smaller decrease indicates greater compensatory use of cognitive resources involving frontal relative to posterior neural areas amid general cognitive disruption.
Moderators
There was no evidence that satiation-deprivation differences in EEG activity differed by demographic, smoking-related, or baseline cognitive characteristics. We also did not find evidence of trait cognitive control moderation of deprivation-induced influences on resting EEG regarding any of the above effects. The lack of moderation by trait cognitive control moderation is in contrast to Evans et al. (2013b) who found that nonsmokers reporting greater cognitive failures related to daily functioning exhibited greater nicotine-induced reduction of resting EEG alpha-1 slow wave activity. The nonsmoker study showed some similarity in slow wave suppression following administration of nicotine, but the effects were not as robust as in the current smoker nicotine withdrawal study. It is possible the effects of deprivation on the smokers in the present study were sufficiently strong to overwhelm any potential moderating effects, or similarly, that pre-existing deficits in cognitive control in this group limited our ability to detect a relationship between these two variables.
Limitations
The nicotine deprivation-induced changes in the resting EEG reported in this study are intriguing, but a number of limitations deserve attention. First, although we have suggested the substantially larger sample size of the current study may clarify a number of previous conflicting findings, differences in populations and measurement strategies may also contribute to these differences. For example, Fisher et al. (2012) found that fast wave activity was increased by nicotine, but this was among nonsmokers and did not involve nicotine withdrawal. Additionally, open and closed eye conditions are often integrated in the study of resting EEG, but the current study only included a three-minute eyes open condition. Second, the unexpected nicotine deprivation-induced hemispheric and region effects on slow wave power are interesting, but effects were not consistent across frontal electrodes and effect sizes were small. These effects should therefore be interpreted cautiously.
Conclusion
The main finding of this study is that nicotine deprivation increased slow wave power in the theta and alpha-1 frequency bands, whereas fast wave power was unaffected. Changes in slow wave power may serve as markers of withdrawal-related cognitive disruption in future studies examining cognitive factors as predictors of smoking cessation outcomes.
Acknowledgements
This study was funded by NIH grants R21 DA027001 (DE) and R21 DA024226 (DD). Additional support was provided by grant 13PRE14660076 from the American Heart Association (JO). The authors would like to thank Renee Ornduff, Natasha Garcia, and Lauren Meltzer for their work on the project.
Footnotes
Conflict of Interest
None of the authors have potential conflicts of interest (financial or other) regarding information reported herein. David Drobes has served as an expert witness in litigation against tobacco companies.
Works Cited
- Beaver JD, Long CJ, Cole DM, Durcan MJ, Bannon LC, Mishra RG, Matthews PM. The effects of nicotine replacement on cognitive brain activity during smoking withdrawal studied with simultaneous fMRI/EEG. Neuropsychopharmacol. 2011;36:1792–1800. doi: 10.1038/npp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21:1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J, London ED, Olmstead RE, Rose JE, Mukhin AG. Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. Int J Neuropsychopharmacol. 2009;12:305–316. doi: 10.1017/S146114570800922X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, Bond D, McCarthy R, Selikowitz M. Effects of stimulant medications on the EEG of children with attention-deficit/hyperactivity disorder. Psychopharmacology. 2002;164:277–284. doi: 10.1007/s00213-002-1205-0. [DOI] [PubMed] [Google Scholar]
- Ernst M, Matochik JA, Heishman SJ, Van Horn JD, Jons PH, Henningfield JE, London DE. Effect of nicotine on brain activation during performance of a working memory task. Proc Natl Acad Sci. 2001;98:4728–4733. doi: 10.1073/pnas.061369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Drobes DJ. Nicotine self-medication of cognitive-attentional processing. Addict Biol. 2009;14:32–42. doi: 10.1111/j.1369-1600.2008.00130.x. [DOI] [PubMed] [Google Scholar]
- Evans DE, Maxfield ND, Van Rensburg KJ, Oliver JA, Jentink KG, Drobes DJ. Nicotine deprivation influences P300 markers of cognitive control. Neuropsychopharmacol. 2013a;38:2525–2531. doi: 10.1038/npp.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Rothbart MK. Developing a model for adult temperament. J Res Pers. 2007;41:868–888. [Google Scholar]
- Evans DE, Sutton SK, Janse Van Rensburg K, Jentink KG, Drobes DJ. Cognitive control trait moderation of nicotine induced cortical activation among Nonsmokers. Paper presented at the Annual Meeting of the Society for Research on Nicotine and Tobacco; Boston, Massachusetts. 2013b. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams, Janet BW. Structured Clinical Interview for SM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fisher DJ, Daniels R, Jaworska N, Knobelsdorf A, Knott VJ. Effects of acute nicotine administration on resting EEG in nonsmokers. Exp Clin Psychopharm. 2012;20:71. doi: 10.1037/a0025221. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hendricks P, Ditre J, Drobes D, Brandon T. The early time course of smoking withdrawal effects. Psychopharmacology (Berl) 2006;187:385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Hermens DF, Soei EX, Clarke SD, Kohn MR, Gordon E, Williams LM. Resting EEG theta activity predicts cognitive performance in attention-deficit hyperactivity disorder. Pediatr Neurol. 2005;32:248–256. doi: 10.1016/j.pediatrneurol.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition–timing hypothesis. Br Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, Potenza MN. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depen. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott VJ, Fisher DJ. Naltrexone alteration of the nicotine-induced EEG and mood activation response in tobacco-deprived cigarette smokers. Exp Clin Psychopharm. 2007;15:368. doi: 10.1037/1064-1297.15.4.368. [DOI] [PubMed] [Google Scholar]
- Knott VJ, Raegele M, Fisher D, Robertson N, Millar A, McIntosh J, Ilivitsky V. Clonidine pre-treatment fails to block acute smoking-induced EEG arousal/mood in cigarette smokers. Pharm Biochem Be. 2005;80:161–171. doi: 10.1016/j.pbb.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Kozink RV, Kollins SH, McClernon FJ. Smoking withdrawal modulates right inferior frontal cortex but not presupplementary motor area activation during inhibitory control. Neuropsychopharmacol. 2010;35:2600–2606. doi: 10.1038/npp.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbergen MM, Arns M, van Dongen-Boomsma M, Spronk D, Buitelaar JK. The increase in theta/beta ratio on resting-state EEG in boys with attention-deficit/hyperactivity disorder is mediated by slow alpha peak frequency. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35:47–52. doi: 10.1016/j.pnpbp.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiat. 2014;71:523–530. doi: 10.1001/jamapsychiatry.2013.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo SK, Teale PD, Reite ML. EEG correlates of methylphenidate response among children with ADHD: a preliminary report. Biol Psychiat. 1999;45:1657–1660. doi: 10.1016/s0006-3223(98)00250-9. [DOI] [PubMed] [Google Scholar]
- Newton TF, Cook I, Holschneider D, Rosenblatt M, Lindholm J, Jarvik M. Quantitative EEG Effects of Nicotine Replacement by Cigarette Smoking1. Neuropsychobiology. 1998;37:112–116. doi: 10.1159/000026488. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharm Biochem Be. 2008;88:407–417. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Powell JH, Pickering A, Dawkins L, West R, Powell JF. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addict Behav. 2004;29:1407–1426. doi: 10.1016/j.addbeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Putman P, van Peer J, Maimari I, van der Werff S. EEG theta/beta ratio in relation to fear-modulated response-inhibition, attentional control, and affective traits. Biol Psychol. 2010;83:73–78. doi: 10.1016/j.biopsycho.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Roche RAP, Garavan H, Foxe JJ, O'Mara SM. Individual differences discriminate event-related potentials but not performance during response inhibition. Exp Br Res. 2005;160:60–70. doi: 10.1007/s00221-004-1985-z. [DOI] [PubMed] [Google Scholar]
- Schlienz NJ, Hawk LW, Jr, Rosch KS. The effects of acute abstinence from smoking and performance-based rewards on performance monitoring. Psychopharmacology. 2013;229:701–711. doi: 10.1007/s00213-013-3131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilek D, Carriere JS, Cheyne JA. Failures of sustained attention in life, lab, and brain: ecological validity of the SART. Neuropsychologia. 2010;48:2564–2570. doi: 10.1016/j.neuropsychologia.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychol Sci. 1997;8:204–210. [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. Fifth Edition. Boston: Allyn and Bacon; 2007. [Google Scholar]
- Waters AJ, Jarvis MJ, Sutton SR. Nicotine withdrawal and accident rates. Nature. 1998;394:137–137. doi: 10.1038/28076. [DOI] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, Brody A, Jarvik M, Domier CP, Olmstead R, Ernst M, London E. Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biol Psychiat. 2005;58:143–150. doi: 10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]