Abstract
Purpose
To determine the feasibility of automatic vascular territory region of interest (ROI) construction as a method for standardized quantification of cerebral blood flow (CBF) images.
Materials and Methods
An algorithm for automatic construction of vascular territory ROIs was performed on 10 healthy controls and 25 patients with perfusion abnormalities identified by retrospective chart review. The ROIs were used to quantify perfusion asymmetry for each territory, and perfusion asymmetry was compared in the two cohorts and against blinded neuroradiologist interpretation. The algorithm was additionally applied to a separate cohort of 23 prospectively enrolled patients and perfusion asymmetry was correlated against clinical variables.
Results
There was significantly greater perfusion asymmetry in territories graded by neuroradiologists as hypoperfused compared to those graded as normally perfused (p<.05) and compared to healthy volunteers (p<.01). An ROC analysis showed that perfusion asymmetry was sensitive and specific for identifying hypoperfusion in vascular territories (84.9% sensitivity and 90.5% specificity for a threshold asymmetry index of .829). In the prospective cohort, perfusion asymmetry was correlated with initial NIH stroke scale (NIHSS) (p<.01) and length of stay (p<.05).
Conclusions
Automatic construction of vascular territory ROIs and calculation of perfusion asymmetry is a feasible method for analyzing CBF images. Because the technique is rapid and minimizes bias, it can facilitate analysis of larger scale research studies.
1. Introduction
Robust, reliable and unbiased quantification of perfusion deficits is necessary for the clinical evaluation of cerebral perfusion imaging in cerebrovascular disease. While it is relatively straightforward in a clinical setting to visually inspect perfusion images of cerebral blood flow (CBF) and cerebral blood volume (CBV) to assess local or regional deficits, quantifying these results can be difficult. In addition, given the increasing trend for healthcare institutions to aggregate vast amounts of clinical MRI data, including complex functional MR PWI (perfusion weighted imaging) data, clinical diagnostic and research methods would benefit from an objective approach to sampling this data in a way that is observer-independent, disease-specific and conducive to “Big Data” research.
A common approach to quantification is the comparison of regional values. An operator draws regions of interest (ROIs) and extracts average values from these ROIs. However, this subjective manner of assessment introduces bias into the results and is not quantitatively reproducible or comparative within serial studies of the same patient or between patients in large cohorts. Furthermore, operators are required to possess knowledge of the cerebrovascular anatomy in order for the assessment to be pertinent to the pathology. These issues are particularly problematic in a research setting where large volumes of data render ROI drawing a time-intensive task.
These major shortcomings – inherent bias and lengthy analysis time – could be addressed by a fully automated perfusion sampling algorithm which conforms to the vascular anatomy. Existing automated approaches often rely on parameter thresholds based on absolute quantitative values[1–5]. Because the vast majority of clinical perfusion scans do not measure absolute CBF or CBV, these approaches do not typically analyze CBF or CBV images directly. Instead, they are limited to analyzing only temporal metrics of perfusion such as time to peak bolus arrival (TTP), time to maximum of the residue function (Tmax), or mean transit time (MTT). There is no currently available easily scalable way to directly analyze CBF or CBV images. Additionally, these approaches report only a total lesion size without incorporating any information about underlying cerebrovascular anatomy. Total lesion size would not discriminate, for example, between a lesion localized entirely in the middle cerebral artery (MCA) and a same-sized lesion that spans several territories.
To attempt to address these concerns, we propose a fully automated approach to evaluating regional blood flow based on automated vascular territory ROI selection. This approach combines the advantages of the lesion size and ROI methods in that it is both fully automatic and incorporates information about cerebrovascular anatomy. In this approach, we borrow normalization and coregistration techniques routinely used in neuroimaging research and apply them towards the evaluation of clinical MR PWI data.
2. Materials and Methods
2.1 Subjects and ischemic territory identification
We performed a proof of concept study in a series of 25 patients with perfusion abnormalities identified by retrospective chart review. This study was performed in accordance with protocols approved by the Northwestern University institutional review board. For retrospective chart review, the review board granted a waiver of informed consent. CBF maps were generated offline using automated arterial input function (AIF) selection and singular value decomposition (SVD) deconvolution[6, 7]. Images were then interpreted independently by two blinded neuroradiologists. For each image, each vascular territory was rated as hyperperfused, normal, mildly hypoperfused, or severely hypoperfused. Ischemic regions were defined as those rated mildly or severely hypoperfused. Normal regions were defined as those graded as normal (zero regions were identified as hyperperfused). In this cohort, 17 middle cerebral artery (MCA) (6 inferior division, 1 superior division, 10 both divisions), 5 anterior cerebral artery (ACA), and 2 posterior cerebral artery (PCA) ischemic regions were identified. 20 of these ischemic territories had corresponding vessel occlusions or stenoses identified by magnetic resonance angiography (MRA), computed tomography angiography (CTA), or digital subtraction angiography (DSA). Additionally, 10 healthy volunteers were scanned with identical perfusion scans and CBF maps were reconstructed offline using the same algorithm used to reconstruct the patient CBF maps. All patients and volunteers received anatomical T1 and T2 images for use in coregistration and normalization.
An additional separate cohort of 23 patients was used for correlational studies between perfusion deficit and clinical measures. This cohort was recruited as part of an ongoing IRB-approved prospective study (Northwestern University Brain Attack Registry, NUBAR) of patients who received a clinical perfusion scan with suspicion of acute stroke or transient ischemic attack. As part of the ongoing study, various clinical metrics including NIH stroke scale (NIHSS), length of stay (LOS), and modified Rankin Scale (mRS) at 1 month were collected. Here, we include a subset of this cohort who had symptoms indicative of an MCA infarct and were diffusion positive on MRI.
2.2 Image Acquisition
For both patients and volunteers, perfusion images were acquired using bolus tracking with a dynamic susceptibility contrast (DSC) MRI protocol[8] using a 2D gradient recalled echo (GRE) echo planar imaging (EPI) pulse sequence. The DSC-MRI sequence utilized the following parameters: a stack of 13–15, 5mm slices, TR/TE 1500/45 ms, 128 × 128 matrix, 22 cm FOV, 30° flip angle, 50 measurements per slice. A single-dose (0.1 mmol/kg) of Gd-based contrast agent (Magnevist; Berlex, Princeton, NJ, USA) was injected through an antecubital vein at 4 mL/s during the DSC-MRI acquisition. In addition, all patients and volunteers received anatomical imaging for reference. Pre-contrast T1 scans were used when available, otherwise T2 scans or post-contrast T1 scans were used (see Table 1 for typical scan parameters).
Table 1.
Acquisition parameters for perfusion and anatomical reference sequences
| DSC | T1 | T2 | |
|---|---|---|---|
| TR/TE | 1500/45 | 600/14 | 4942.8/102 |
| Slices | 13–15 | 24–25 | 24–25 |
| Voxel size | 1.15×1.15×5mm | .4492×.4492×5 | .4492×.4492×5 |
| FOV | 22cm | 23cm | 23cm |
| Matrix | 192×192 | 384×512 | 456×512 |
| Flip angle | 30 | 90 | 150 |
2.3 Definition of Vascular Territories
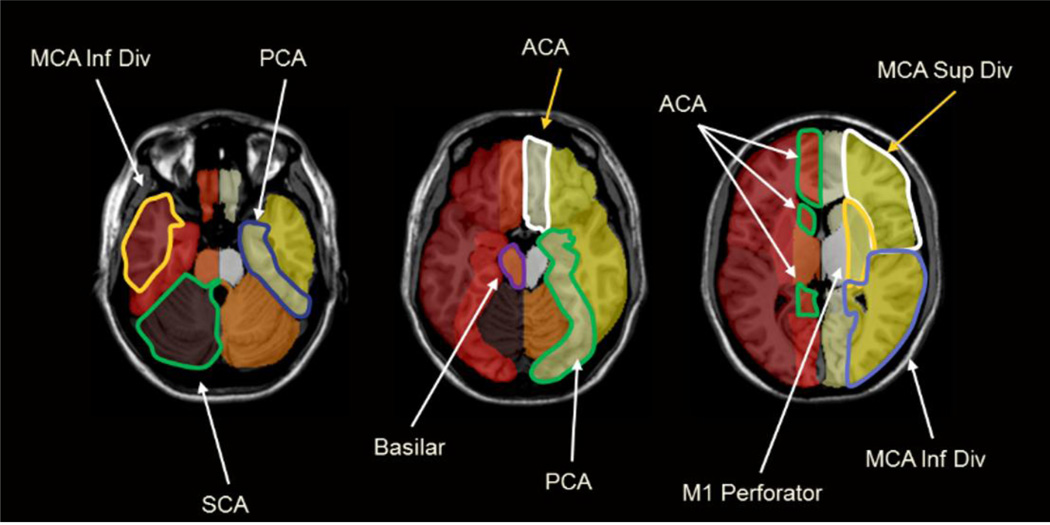
A single vascular territory ROI map was drawn based on anatomic landmarks by an interventional neuroradiologist on a single T1 image in rough Montreal Neurological Institute (MNI) space (ch2bet template included with MRIcron). In this template a vascular territory ROI is a 3D object. Therefore, in the 2D representation of the template, a given vascular territory spans multiple slices and perfusion values are summed over slices. The image was then normalized to MNI space (using the Statistical Parametric Mapping (SPM) software package, see below) to ensure that the vascular territory map was as close to true MNI space as possible. Additionally, to ensure left/right symmetry, territories were drawn on one half of the image only, loaded into Matlab, and then mirrored to the other side by reflecting the territories about the center of the image. The full template is shown in Figure 1. Vascular territories were subdivided into regions perfused by major branches of the Circle of Willis. Major territories included the superior and inferior middle cerebral artery divisions (MCAs, MCAi), M1 perforators, ACA, PCA, superior cerebellar artery (SCA), and anterior and posterior inferior cerebellar arteries (AICA, PICA). This template was then used to automatically generate subject specific vascular territory maps.
Figure 1. Vascular territory template.
Vascular Territory template (in MNI space) used for automatic generation of subject-specific vascular territory maps.
2.4 Subject Specific Vascular Territory Maps and Asymmetry
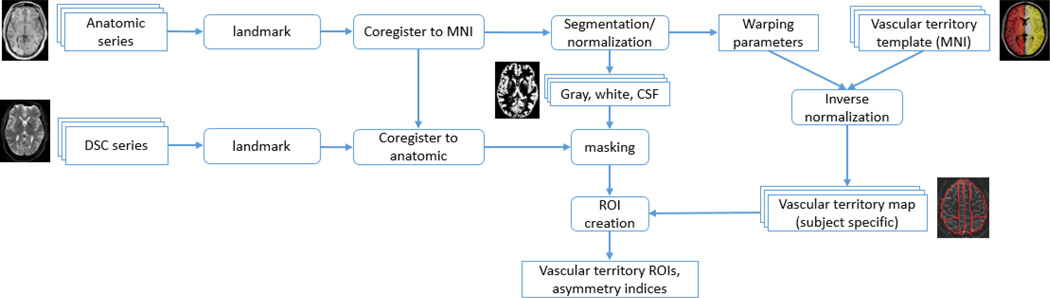
For each subject, the anatomical reference image was first coregistered with that subject’s perfusion image, and the anatomical image was then normalized to MNI space. Using the parameters derived from the normalization, the vascular template was inverted from MNI space to subject specific space, resulting in a subject specific map of vascular territory ROIs. During the normalization process, each anatomical image was additionally segmented into images of gray matter, white matter, and cerebrospinal fluid (CSF) which were later utilized for masking purposes (perfusion images were masked to exclude any voxels with less than 80% probability of being gray or white matter). All normalization and segmentation was performed using the unified segmentation model [9] as implemented in the statistical parametric mapping software package (SPM8; Wellcome Department of Cognitive Neurology, London, England), and all coregistration and inverse-normalization were done using standard SPMfunctions (spm_coreg.m, spm_write_sn.m). The workflow is completely unsupervised, coregistration and segmentation were done using default parameters, and no parameters are changed on a subject to subject basis. Individual territory maps were visually inspected for each subject to ensure the algorithm completed successfully without gross errors in segmentation or coregistration, but no adjustments were made to any territory ROIs. To minimize segmentation & coregistration errors, the image series are first re-landmarked if the image center is far from the origin (ie the x, y, and/or z coordinates of the center voxel are set to 0 if the magnitude of x, y, or z is large) and coregistered to a T1 MNI template (included with SPM). These steps serve to put the image series in rough alignment with MNI space pre-segmentation, which reduces the likelihood that segmentation fails. The entire process is summed up schematically in Figure 2. Total processing time (including CBF map calculation) was roughly 5 minutes per subject on a single desktop computer (3.4 GHz i7 Intel processor, 16GB RAM).
Figure 2. Schematic representation of workflow.
The only user inputs are the anatomic and DSC series for analysis.
From this subject specific territory map, the average perfusion was automatically computed in each vascular territory ROI. Cerebellar regions with insufficient coverage or the presence of significant artifact from the arterial blush of contrast were excluded. For each ROI, an asymmetry index was then calculated by dividing by average perfusion in the contralateral ROI (for healthy volunteers, asymmetry for an ROI was instead defined as left/right). The theoretical normal asymmetry index in an ROI is 1, which indicates equal perfusion on each side of the brain. Conversely, an asymmetry index <1 indicates a relative perfusion deficit in a region compared to the same region in the opposite hemisphere. The lower the asymmetry index, the greater the relative perfusion deficit. All between group asymmetry index comparisons were performed using t-tests.
2.5 Correlation with clinical measures
After evaluating the correspondence between the automated vascular territory algorithm and neuroradiologist interpretation in the cohort of retrospectively identified acute strokes, we then applied the algorithm to the prospectively recruited cohort to investigate the relationship between asymmetry index and various clinical measures. MCA asymmetry was correlated against initial NIHSS, LOS, and 1 month mRS. As a control, ACA and PCA asymmetry were also correlated against NIHSS, LOS, and mRS.
3. Results
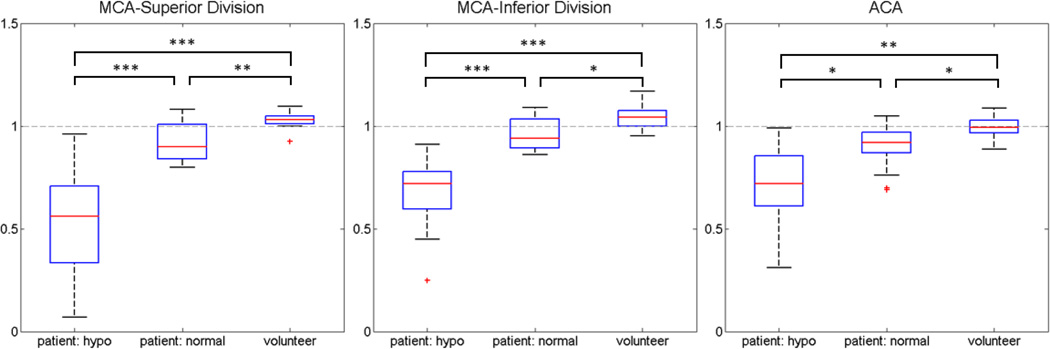
Since there were not enough patients identified with PCA ischemia, the PCA territory was excluded from analysis. In the remaining cases, the segmentation was successfully performed in all subjects producing asymmetry indices for ACA and MCA regions in all patients and volunteers. Mean asymmetry indices for normal and hypoperfused regions in patients as well as from volunteers are shown in Table 2. Boxplots for each territory are shown in Figure 3. As expected, asymmetry indices were lowest (indicating greater perfusion asymmetry) in hypoperfused regions in patients and highest in healthy volunteers. In the ACA and both MCA territories in patients, asymmetry index was significantly lower in regions graded as hypoperfused than in regions graded as normal (p<.001 for MCAi and MCAs, p<.05 for ACA). When compared to volunteer data, asymmetry indices for patients were significantly lower for both hypoperfused (p<.001 MCAi, MCAs, p<.01 ACA) and normal regions (p<.01 MCAs, p<.05 MCAi, ACA). For all territories in volunteers, there was no significant perfusion asymmetry, and the asymmetry index approached the theoretical normal value of 1. However, both divisions of the MCA territory trended towards having significantly greater perfusion in the left hemisphere than right hemisphere (p=.057 for MCAi, p=.098 for MCAs, p=.676 for ACA).
Table 2.
Mean perfusion asymmetry indices in the inferior and superior divisions of the middle cerebral artery (MCA) and the anterior cerebral artery (ACA)
| MCA inferior | MCA superior | ACA | |
|---|---|---|---|
| Stroke: hypoperfused | .68 +/− .17 | .56 +/− .25 | .71 +/− .25 |
| Stroke: normal perfusion | .96 +/− .09 | .92 +/− .10 | .90 +/− .10 |
| Healthy volunteer | 1.04 +/− .06 | 1.03 +/− .05 | .99 +/− .05 |
Figure 3. Asymmetry indices in patients and volunteers.
For the ACA, inferior MCA division, and superior MCA division vascular territories, the asymmetry index (1 = no asymmetry) was significantly lower in regions graded as hypoperfused than in region graded as normally perfused or in healthy volunteers. *** p<.001, ** p<.01, * p<.05
3.1 Receiver Operating Characteristic (ROC) Analysis
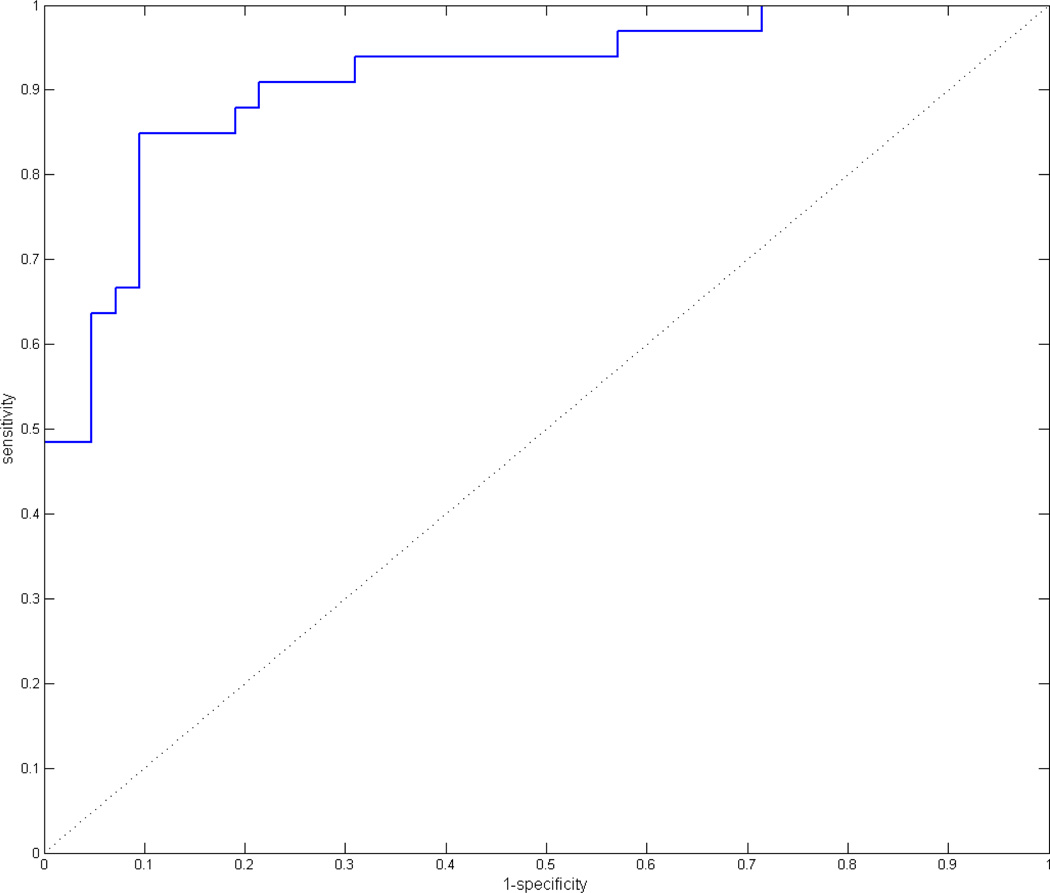
For the ROC analysis, both MCA territories and the ACA territory were analyzed together. True positives for ischemia were defined as regions graded by the neuroradiologists as hypoperfused, and true negatives were defined as regions graded as normal (volunteers were excluded from the ROC analysis). Test positive for ischemia was defined as any region under a threshold asymmetry index, and test negative was defined as any region above this threshold. The threshold was then varied to generate the full ROC curve. The resulting curve is shown in Figure 4 and has an area under the curve (AUC) of .91. At a threshold asymmetry index of .829, the sensitivity was 84.9% and the specificity was 90.5%.
Figure 4. Patient ROC analysis.
ROC analysis for combined data from ACA, inferior MCA, and superior MCA territories in stroke patients. An asymmetry index threshold was used to discriminate normally perfused from hypoperfused regions, and the threshold was varied from 0 to 1 to generate the ROC curve.
3.2 Correlation with clinical measures
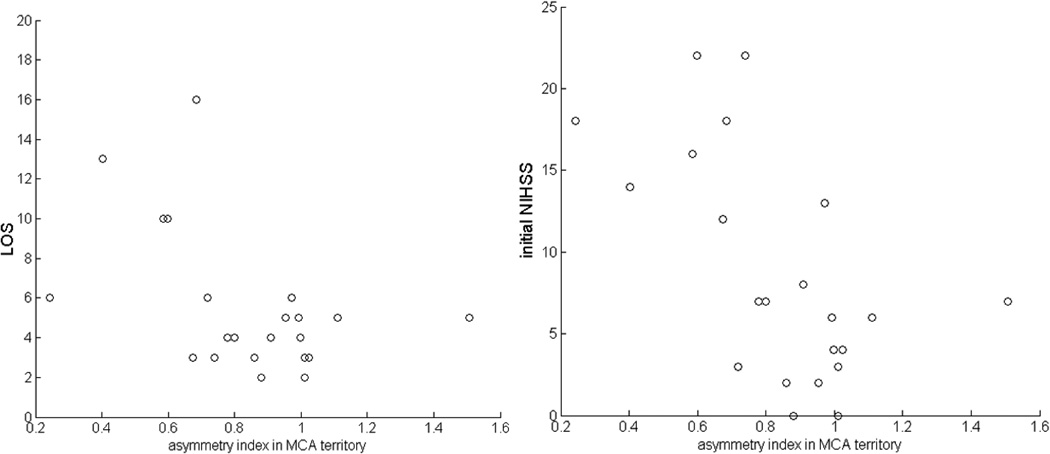
MCA perfusion asymmetry index was significantly correlated with initial NIHSS (r=−.59, p<.005) and LOS (r=−.46, p<.05) (Figure 5) but not with 1 month mRS, although it trended towards significance (r=−.46, p=.06). The control ACA territories did not significantly correlate with any measure (NIHSS: r=−.22, p=.32; LOS: r=.004, p=.97; mRS: r=−.01, p=.97). However, perfusion asymmetry index in the PCA territories did correlate with NIHSS (r=−.44, p<.05) but not LOS (r=−.08, p=.71) or mRS (r=−.03, p=.92).
Figure 5. Correlations with clinical measures.
Perfusion asymmetry in the MCA was significantly correlated with both initial NIH stroke scale (p < .01) and LOS (p<.05) in a cohort of stroke patients with MCA localized symptoms.
4. Discussion
In this study, we evaluated the performance of automatic vascular territory ROI construction as a method for standardizing the analysis of MR perfusion images. In a cohort of acute stroke patients, we found that perfusion asymmetry in vascular territory ROIs was significantly greater in regions that neuroradiologists interpreted as hypoperfused compared to regions interpreted as normally perfused. Additionally, in a control group of healthy volunteers asymmetry indices approached the theoretical normal limit of 1, and there was significantly less perfusion asymmetry in controls when compared to the stroke group. An ROC analysis showed that our standardized region analysis was both sensitive and specific when compared to neuroradiologist interpretation. An important requirement for perfusion image analysis in large scale research is a method of analysis that is fast and minimizes bias, and an automated algorithm would address both of these needs. These results indicate that regional perfusion asymmetry as calculated by an automated vascular territory analysis is an accurate measure of perfusion deficit. In a separate cohort of MCA stroke patients obtained as part of an ongoing study, we found that perfusion asymmetry in the MCA territory was significantly correlated with both NIHSS and LOS.
It has been shown that neurovascular insufficiency exists in over half the population over the age of 60 years[10] and that systematic changes in cerebral perfusion are predictors of the severity of stroke[11], but no large scale studies of cerebral perfusion exist due in part to the difficulty and cost of manual interpretation of cerebral perfusion images. Automated construction and comparison of vascular territory ROIs could provide a method of analysis that is fast, observer independent, and easily scalable to large scale studies, but currently there are no widely available means of automatically segmenting cerebral perfusion images into parenchymal territories. There are currently available approaches to segmentation (SPM, AIR, etc) and templates that follow functional definitions (e.g. AAL), but none that reflect specific vascular territories. Vessel selective ASL has been used to image individual vascular territories[12–15] but requires additional scanning and could not be applied retrospectively to existing data. Additionally, existing vessel selective ASL techniques have been mostly applied to the internal carotid arteries (ICA), and it is unclear whether these methods would be successful in selecting smaller vessels such as those that supply the inferior and superior MCA territories. A recent study investigating multiparametric classification of MR PWI data included pre-drawn vascular territory ROIs[16] but no measurements from ROIs were validated against observer interpretations. Vascular territories have been shown to greatly vary both in the general population[17] and in patients with stroke[18], and to our knowledge there are no current studies that have investigated whether pre-drawn ROIs are sufficiently accurate in spite of this observed heterogeneity.
In our analysis, comparison of perfusion asymmetry indices identified clear differences between hypoperfused and normally perfused regions. As expected, perfusion asymmetry was greatest in regions graded as hypoperfused, however, asymmetry indices of “normally” perfused regions in acute stroke patients were still significantly smaller than in the control group of healthy volunteers. This could reflect some error in the ROI boundaries, and is one limitation of the automatic ROI drawing used in this study. These values could also reflect real hypoperfusion as well. Adjacent regions with severe ischemia could affect perfusion via collateralization and vascular steal or ischemia in watershed zones. It may also be that not all of the true perfusion deficit was identified by the neuroradiologists. For example, in one patient graded with only MCAi and MCAs hypoperfusion, the vascular territory analysis generated asymmetry indices of .61 for the MCAi, .59 for the MCAs, .70 for the ACA, and .68 for the PCA. Angiographic data (which was not available to the neuroradiologists during perfusion grading) showed occlusion in the ipsilateral ICA. Lack of flow from the ICA and MCA steal could contribute to the additional ACA and PCA deficits seen in the automated vascular territory analysis.
In the separate cohort of patients with MCA localized symptoms, we expected only MCA perfusion asymmetry to correlate with NIHSS, LOS, and mRS. As expected, greater MCA asymmetry did in fact significantly correlate with increased NIHSS and LOS and trended towards significance with increased mRS, and ACA asymmetry did not correlate with any measure. However we did find, contrary to expectations, that PCA asymmetry was correlated with NIHSS but not LOS or mRS. This could indicate that although increased initial perfusion deficit in regions adjacent to the infarct initially impacts function, it has less effect on longer term outcomes. This further highlights the importance of interpreting images using anatomical information as deficits in the principally affected region may have a different interpretation than those in adjacent regions. While these results might suggest a relationship between perfusion deficits and clinical outcomes, it would be premature to come to any conclusions about perfusion deficits and NIHSS, LOS, or mRS based off our small cohort. Especially for longer term measures of LOS and 1 month mRS, there are a multitude of factors (eg demographics, comorbidities, treatment type/success, follow up, etc) that must be accounted for. This is exactly the role for larger cohort “Big Data” studies that this approach is targeted towards, which are better suited towards controlling for a multitude of confounding variables.
We believe that an automated vascular territory approach offers many important advantages in analyzing MRI PWI data, but there also important limitations that must be considered. First and foremost, while the vascular territory analysis reflected manual radiologist interpretation across our cohort as a whole, it did not agree in all cases. Moreover, it lacks the versatility of manual reading: it cannot appreciate the image in a wider clinical context, it is limited to certain regions, and it would likely be suboptimal for assessment of borderzone watershed regions and small punctate infarcts located in large vascular beds. As such, this tool is limited to larger research studies and is not intended to replace diagnostic neuroradiology reading in a clinical setting. However, it could prove to be a valuable adjunct allowing a neuroradiologist to confirm their own interpretation, re-examine the MR PWI data in cases of disagreement, or focus on regional asymmetry maps from the automated analysis. Another limitation is the amount of volumetric overage and vascular territories covered. In this experiment, we only evaluated vascular territory performance in the ACA, inferior MCA, and superior MCA. Importantly, we omitted the PCA and any cerebellar regions. The PCA was omitted because in our small cohort we did not identify a sufficient number of patients with PCA ischemia to be able to critically evaluate the tool’s performance. In the cerebellum, we were limited by inadequate spatial coverage and/or blooming artifacts in most patients. Other artifacts could affect analysis as well. Significant artifacts (eg from an aneurysm clip) or significant anatomical abnormalities (eg a large tumor) could cause the coregistration and/or segmentation steps of the algorithm to fail, producing erroneous territory ROIs and asymmetry indices. These problems could possibly be alleviated with some minimal intervention, for example by masking out any artifacts prior to segmentation, and further investigation is needed to determine how robust the algorithm is in these situations.
While the vascular territory approach has its limitations, it may offer substantial benefits as a research tool. It is becoming increasingly common for institutions to aggregate their data in large databases, and with contrast-free technologies such as ASL making it possible to acquire MRI perfusion images with minimal invasiveness, the number of stored perfusion datasets may dramatically increase in the next several years. With an increasing research population, the traditional manual interpretation by a physician becomes time consuming, costly, and eventually infeasible. Blinding must be managed carefully, and unless a single physician is able to personally review all the cases, more complex models and randomization must be considered to minimize bias when using multiple readers to cover the entire dataset. When data collection spans multiple years, this could be a major problem if there is significant personnel turnover. An automated vascular territory approach largely addresses most of these issues. There is no bias or blinding required by eliminating human interpretation, and a single reasonably powerful computer could analyze several hundred datasets per day reliably and accurately. Unlike automated lesion volume approaches, the vascular territory approach can work directly with non-quantitative CBF images and incorporate information about underlying cerebrovascular anatomy.
5. Conclusion
We have demonstrated a method for automated analysis of MRI PWI data in the context of incorporating the underlying cerebrovascular anatomy. By automatically generating subject specific vascular territory ROIs, we are able to quantitatively compare perfusion values in the ACA and MCA regions. High sensitivity and specificity was observed in comparison to manual interpretations by blinded neuroradiologists. In a preliminary analysis of an ongoing collection of clinical perfusion data, we found significant correlation with clinical variables. Because the process is fully automatic, it greatly reduces bias, time, and cost needed to analyze perfusion data. While our method could be utilized as an adjunct in the clinical setting, these features make it particularly well suited for larger scale MR PWI research screening and analysis projects.
Acknowledgements
This work was supported by grants from the NINDS (T32 EB005170, NS 049395) and NHLBI (F31 HL117618)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest
No disclosures or conflicts of interest.
References
- 1.Davis SM, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7(4):299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 2.Inoue M, et al. Clinical outcomes strongly associated with the degree of reperfusion achieved in target mismatch patients: pooled data from the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution studies. Stroke. 2013;44(7):1885–1890. doi: 10.1161/STROKEAHA.111.000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma H, et al. A multicentre, randomized, double-blinded, placebo-controlled Phase III study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND) Int J Stroke. 2012;7(1):74–80. doi: 10.1111/j.1747-4949.2011.00730.x. [DOI] [PubMed] [Google Scholar]
- 4.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32(5):1024–1037. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wheeler HM, et al. Early diffusion-weighted imaging and perfusion-weighted imaging lesion volumes forecast final infarct size in DEFUSE 2. Stroke. 2013;44(3):681–685. doi: 10.1161/STROKEAHA.111.000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carroll TJ, Rowley HA, Haughton VM. Automatic calculation of the arterial input function for cerebral perfusion imaging with MR imaging. Radiology. 2003;227(2):593–600. doi: 10.1148/radiol.2272020092. [DOI] [PubMed] [Google Scholar]
- 7.Ostergaard L, et al. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36(5):715–725. doi: 10.1002/mrm.1910360510. [DOI] [PubMed] [Google Scholar]
- 8.Rosen BR, et al. Susceptibility contrast imaging of cerebral blood volume: human experience. Magn Reson Med. 1991;22(2):293–299. doi: 10.1002/mrm.1910220227. discussion 300-3. [DOI] [PubMed] [Google Scholar]
- 9.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Baker AB, Resch JA, Loewenson RB. Hypertension and cerebral atherosclerosis. Circulation. 1969;39(5):701–710. doi: 10.1161/01.cir.39.5.701. [DOI] [PubMed] [Google Scholar]
- 11.Nemoto EM, Yonas H, Chang Y. Stages and thresholds of hemodynamic failure. Stroke. 2003;34(1):2–3. doi: 10.1161/01.str.0000041048.33908.18. [DOI] [PubMed] [Google Scholar]
- 12.Alfke K, et al. Magnetic resonance imaging of individual cerebral perfusion territories improves the diagnosis of embolic stroke. J Comput Assist Tomogr. 2007;31(6):894–895. doi: 10.1097/rct.0b013e31804b2159. [DOI] [PubMed] [Google Scholar]
- 13.Kansagra AP, Wong EC. Mapping of vertebral artery perfusion territories using arterial spin labeling MRI. J Magn Reson Imaging. 2008;28(3):762–766. doi: 10.1002/jmri.21462. [DOI] [PubMed] [Google Scholar]
- 14.van Laar PJ, van der Grond J, Hendrikse J. Brain perfusion territory imaging: methods and clinical applications of selective arterial spin-labeling MR imaging. Radiology. 2008;246(2):354–364. doi: 10.1148/radiol.2462061775. [DOI] [PubMed] [Google Scholar]
- 15.Dang Y, et al. Quantitative assessment of external carotid artery territory supply with modified vessel-encoded arterial spin-labeling. AJNR Am J Neuroradiol. 2012;33(7):1380–1386. doi: 10.3174/ajnr.A2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Artzi M, et al. Unsupervised multiparametric classification of dynamic susceptibility contrast imaging: study of the healthy brain. Neuroimage. 2011;56(3):858–864. doi: 10.1016/j.neuroimage.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 17.van der Zwan A, et al. A quantitative investigation of the variability of the major cerebral arterial territories. Stroke. 1993;24(12):1951–1959. doi: 10.1161/01.str.24.12.1951. [DOI] [PubMed] [Google Scholar]
- 18.Lang EW, et al. Variability of vascular territory in stroke. Pitfalls and failure of stroke pattern interpretation. Stroke. 1995;26(6):942–945. doi: 10.1161/01.str.26.6.942. [DOI] [PubMed] [Google Scholar]