Abstract
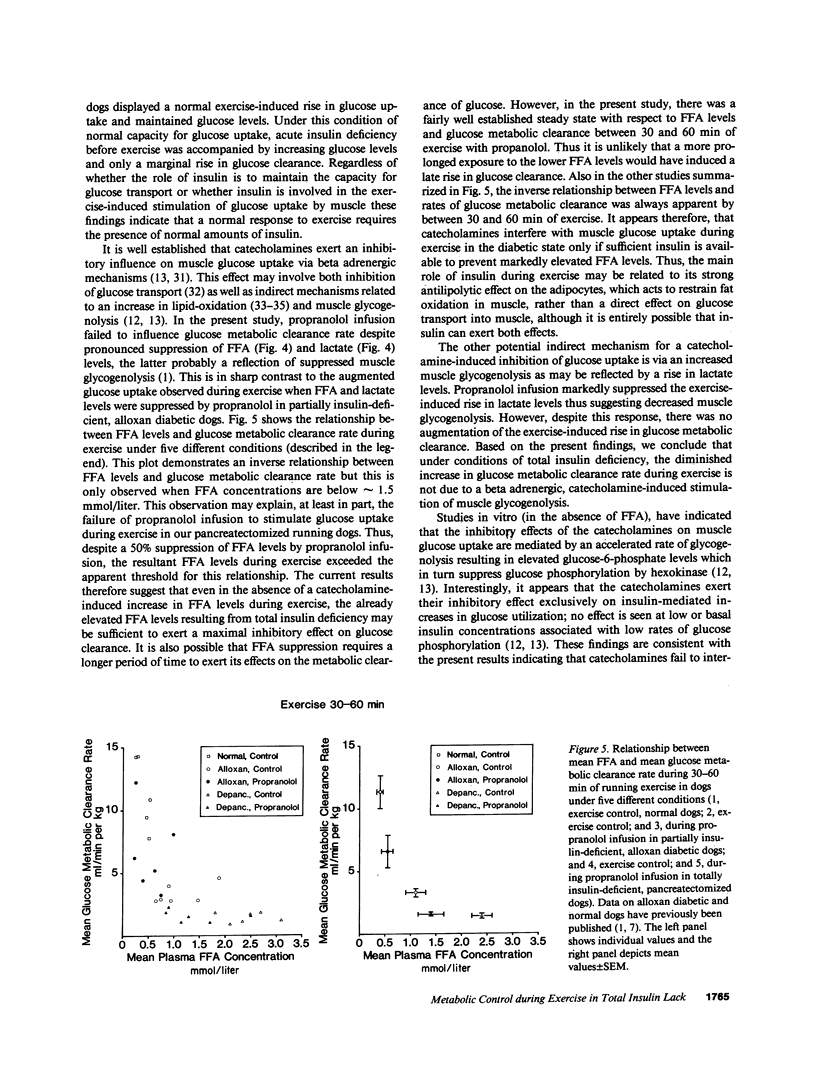
To examine whether glucose metabolic clearance increases and whether catecholamines influence glucose turnover during exercise in total insulin deficiency, 24-h fasted and insulin-deprived pancreatectomized dogs were studied before and during exercise (60 min; 100 m/min; 10% slope) with (n = 8) and without (n = 8) propranolol infusion (PI, 5 micrograms/kg-min). Exercise with or without PI was accompanied by four and fivefold increments in norepinephrine and epinephrine respectively, while glucagon (extrapancreatic) fell slightly. Basal plasma glucose and FFA concentrations and rates of tracer-determined (3[3H]glucose) hepatic glucose production (Ra) and total glucose clearance (including urinary glucose loss) were 459 +/- 24 mg/dl, 1.7 +/- 0.5 mmol/liter, 7.8 +/- 0.9 mg/kg-min and 1.6 +/- 0.1 ml/kg-min, respectively. When corrected for urinary glucose excretion, basal glucose metabolic clearance rate (MCR) was 0.7 +/- 0.1 mg/kg-min and rose twofold (P less than 0.0001) during exercise. Despite lower lactate (3.3 +/- 0.6 vs. 6.6 +/- 1.3 mmol/liter; P less than 0.005) and FFA levels (1.1 +/- 0.2 vs. 2.2 +/- 0.2 mmol/liter; P less than 0.0001) with PI, PI failed to influence MCR during exercise. Ra rose by 3.7 +/- 1.7 mg/kg-min during exercise (P less than 0.02) while with PI the increase was only 1.9 +/- 0.7 mg/kg-min (P less than 0.002). Glucose levels remained unchanged during exercise alone but fell slightly with PI (P less than 0.0001). Therefore, in total insulin deficiency, MCR increases marginally with exercise (13% of normal); the beta adrenergic effects of catecholamines that stimulate both FFA mobilization and muscle glycogenolysis do not regulate muscle glucose uptake. The exercise-induced rise in hepatic glucose production does not require an increase in glucagon levels, but is mediated partially by catecholamines. Present and previous data in normal and alloxan-diabetic dogs, suggest that (a) in total insulin deficiency, control of hepatic glucose production during exercise is shifted from glucagon to catecholamines and that this may involve catecholamine-induced mobilization of peripheral substrates for gluconeogenesis and/or hepatic insensitivity to glucagon, and (b) insulin is not essential for a small exercise-induced increase in muscle glucose uptake, but normal insulin levels are required for the full response. Furthermore, the catecholamines appear to regulate muscle glucose uptake during exercise only when sufficient insulin is available to prevent markedly elevated FFA levels. We speculate that the main role of insulin is not to regulate glucose uptake by the contracting muscle directly, but to restrain lipolysis and thereby also FFA oxidation in the muscle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Best J. D., Taborsky G. J., Jr, Halter J. B., Porte D., Jr Glucose disposal is not proportional to plasma glucose level in man. Diabetes. 1981 Oct;30(10):847–850. doi: 10.2337/diab.30.10.847. [DOI] [PubMed] [Google Scholar]
- Challiss R. A., Lozeman F. J., Leighton B., Newsholme E. A. Effects of the beta-adrenoceptor agonist isoprenaline on insulin-sensitivity in soleus muscle of the rat. Biochem J. 1986 Jan 15;233(2):377–381. doi: 10.1042/bj2330377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington A. D., Lacy W. W., Chiasson J. L. Effect of glucagon on glucose production during insulin deficiency in the dog. J Clin Invest. 1978 Sep;62(3):664–677. doi: 10.1172/JCI109174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson J. L., Shikama H., Chu D. T., Exton J. H. Inhibitory effect of epinephrine on insulin-stimulated glucose uptake by rat skeletal muscle. J Clin Invest. 1981 Sep;68(3):706–713. doi: 10.1172/JCI110306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi K., Prentki M., Yip C., Muller W. A., Jeanrenaud B., Vranic M. Identical biological effects of pancreatic glucagon and a purified moiety of canine gastric immunoreactive glucagon. J Clin Invest. 1979 Mar;63(3):525–531. doi: 10.1172/JCI109331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegood D. T., Bergman R. N. Optimal segments: a method for smoothing tracer data to calculate metabolic fluxes. Am J Physiol. 1983 May;244(5):E472–E479. doi: 10.1152/ajpendo.1983.244.5.E472. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 9. Effects of fatty acids and ketone bodies, and of alloxan-diabetes and starvation, on pyruvate metabolism and on lactate-pyruvate and L-glycerol 3-phosphate-dihydroxyacetone phosphate concentration ratios in rat heart and rat diaphragm muscles. Biochem J. 1964 Dec;93(3):665–678. doi: 10.1042/bj0930665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzer D. R., Dalsky G. P., Clutter W. E., Shah S. D., Holloszy J. O., Cryer P. E. Glucoregulation during exercise: hypoglycemia is prevented by redundant glucoregulatory systems, sympathochromaffin activation, and changes in islet hormone secretion. J Clin Invest. 1986 Jan;77(1):212–221. doi: 10.1172/JCI112279. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Issekutz B., Jr, Paul P., Miller H. I. Metabolism in normal and pancreatectomized dogs during steady-state exercise. Am J Physiol. 1967 Oct;213(4):857–862. doi: 10.1152/ajplegacy.1967.213.4.857. [DOI] [PubMed] [Google Scholar]
- Kashiwagi A., Huecksteadt T. P., Foley J. E. The regulation of glucose transport by cAMP stimulators via three different mechanisms in rat and human adipocytes. J Biol Chem. 1983 Nov 25;258(22):13685–13692. [PubMed] [Google Scholar]
- Kemmer F. W., Lickley H. L., Gray D. E., Perez G., Vranic M. State of metabolic control determines role of epinephrine-glucagon interaction in glucoregulation in diabetes. Am J Physiol. 1982 Jun;242(6):E428–E436. doi: 10.1152/ajpendo.1982.242.6.E428. [DOI] [PubMed] [Google Scholar]
- Lickley H. L., Kemmer F. W., Doi K., Vranic M. Glucagon suppression improves glucoregulation in moderate but not chronic severe diabetes. Am J Physiol. 1983 Oct;245(4):E424–E429. doi: 10.1152/ajpendo.1983.245.4.E424. [DOI] [PubMed] [Google Scholar]
- Lickley H. L., Ross G. G., Vranic M. Effects of selective insulin or glucagon deficiency on glucose turnover. Am J Physiol. 1979 Mar;236(3):E255–E262. doi: 10.1152/ajpendo.1979.236.3.E255. [DOI] [PubMed] [Google Scholar]
- Lloyd B., Burrin J., Smythe P., Alberti K. G. Enzymic fluorometric continuous-flow assays for blood glucose, lactate, pyruvate, alanine, glycerol, and 3-hydroxybutyrate. Clin Chem. 1978 Oct;24(10):1724–1729. [PubMed] [Google Scholar]
- Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 7. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, on the concentrations of hexose phosphates, nucleotides and inorganic phosphate in perfused rat heart. Biochem J. 1964 Dec;93(3):641–651. doi: 10.1042/bj0930641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploug T., Galbo H., Richter E. A. Increased muscle glucose uptake during contractions: no need for insulin. Am J Physiol. 1984 Dec;247(6 Pt 1):E726–E731. doi: 10.1152/ajpendo.1984.247.6.E726. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Lickley H. L. The metabolic clearance of glucose: measurement and meaning. Diabetologia. 1985 Jun;28(6):315–322. doi: 10.1007/BF00283136. [DOI] [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Experimental validation of measurements of glucose turnover in nonsteady state. Am J Physiol. 1978 Jan;234(1):E84–E93. doi: 10.1152/ajpendo.1978.234.1.E84. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Haymond M. W., Gerich J. E. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest. 1980 Mar;65(3):682–689. doi: 10.1172/JCI109714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G., Lickley L., Vranic M. Extrapancreatic glucagon in control of glucose turnover in depancreatized dogs. Am J Physiol. 1978 Feb;234(2):E213–E219. doi: 10.1152/ajpendo.1978.234.2.E213. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Sole M. J., Hussain M. N. A simple specific radioenzymatic assay for the simultaneous measurement of picogram quantities of norepinephrine, epinephrine, and dopamine and in plasma and tissues. Biochem Med. 1977 Dec;18(3):301–307. doi: 10.1016/0006-2944(77)90064-3. [DOI] [PubMed] [Google Scholar]
- Verdonk C. A., Rizza R. A., Gerich J. E. Effects of plasma glucose concentration on glucose utilization and glucose clearance in normal man. Diabetes. 1981 Jun;30(6):535–537. doi: 10.2337/diab.30.6.535. [DOI] [PubMed] [Google Scholar]
- Vranic M., Kawamori R., Pek S., Kovacevic N., Wrenshall G. A. The essentiality of insulin and the role of glucagon in regulating glucose utilization and production during strenuous exercise in dogs. J Clin Invest. 1976 Feb;57(2):245–255. doi: 10.1172/JCI108275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranic M., Pek S., Kawamori R. Increased "glucagon immunoreactivity" in plasma of totally depancreatized dogs. Diabetes. 1974 Nov;23(11):905–912. doi: 10.2337/diab.23.11.905. [DOI] [PubMed] [Google Scholar]
- Vranic M., Wrenshall G. A. Exercise, insulin and glucose turnover in dogs. Endocrinology. 1969 Jul;85(1):165–171. doi: 10.1210/endo-85-1-165. [DOI] [PubMed] [Google Scholar]
- Wahren J., Felig P., Ahlborg G., Jorfeldt L. Glucose metabolism during leg exercise in man. J Clin Invest. 1971 Dec;50(12):2715–2725. doi: 10.1172/JCI106772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Hagenfeldt L., Felig P. Splanchnic and leg exchange of glucose, amino acids, and free fatty acids during exercise in diabetes mellitus. J Clin Invest. 1975 Jun;55(6):1303–1314. doi: 10.1172/JCI108050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallberg-Henriksson H., Holloszy J. O. Activation of glucose transport in diabetic muscle: responses to contraction and insulin. Am J Physiol. 1985 Sep;249(3 Pt 1):C233–C237. doi: 10.1152/ajpcell.1985.249.3.C233. [DOI] [PubMed] [Google Scholar]
- Wallberg-Henriksson H., Holloszy J. O. Contractile activity increases glucose uptake by muscle in severely diabetic rats. J Appl Physiol Respir Environ Exerc Physiol. 1984 Oct;57(4):1045–1049. doi: 10.1152/jappl.1984.57.4.1045. [DOI] [PubMed] [Google Scholar]
- Wasserman D. H., Lickley H. L., Vranic M. Important role of glucagon during exercise in diabetic dogs. J Appl Physiol (1985) 1985 Oct;59(4):1272–1281. doi: 10.1152/jappl.1985.59.4.1272. [DOI] [PubMed] [Google Scholar]
- Wasserman D. H., Lickley H. L., Vranic M. Interactions between glucagon and other counterregulatory hormones during normoglycemic and hypoglycemic exercise in dogs. J Clin Invest. 1984 Oct;74(4):1404–1413. doi: 10.1172/JCI111551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman D. H., Lickley H. L., Vranic M. Role of beta-adrenergic mechanisms during exercise in poorly controlled diabetes. J Appl Physiol (1985) 1985 Oct;59(4):1282–1289. doi: 10.1152/jappl.1985.59.4.1282. [DOI] [PubMed] [Google Scholar]
- Wolfe R. R., Nadel E. R., Shaw J. H., Stephenson L. A., Wolfe M. H. Role of changes in insulin and glucagon in glucose homeostasis in exercise. J Clin Invest. 1986 Mar;77(3):900–907. doi: 10.1172/JCI112388. [DOI] [PMC free article] [PubMed] [Google Scholar]



