Abstract
Rationale
Cocaine use has been associated with cognitive impairments that may contribute to poor treatment outcomes. However, the degree to which these deficits extend into periods of abstinence has not been completely elucidated.
Objectives
This study tested whether prior experience self-administering cocaine affected acquisition of two cognitive tasks in 16 adult female cynomolgus monkeys. Seven monkeys had previously self-administered cocaine but had not had access to cocaine for two months at the start of this study.
Methods
After monkeys were trained to respond on a touchscreen, associative learning and behavioral flexibility were assessed using a stimulus discrimination (SD) and reversal (SDR) task from the CANTAB battery. Performance on this task was monitored over the subsequent three months. Additionally, working memory was assessed with a delayed match-to-sample (DMS) task.
Results
Cocaine-naïve monkeys required fewer total trials and made fewer errors and omissions before acquiring the SD and SDR tasks compared to monkeys who had previously self-administered cocaine; two monkeys in the latter group did not acquire the task. However, this cognitive impairment dissipated over several months of exposure to the task. The number of sessions for touch training and delays required to establish a performance-based curve on the DMS task did not differ between groups.
Conclusion
Results suggest that cocaine exposure can impair the ability to learn a novel task requiring behavioral inhibition and flexibility, even after an extended period of abstinence. However, this deficit did not extend to maintenance of the task or to acquisition of a working memory task.
Keywords: abstinence, behavioral inhibition, CANTAB, cocaine, cognition, cognitive flexibility, female, nonhuman primates, working memory
Cocaine abuse continues to be a major public health problem with more than 1.6 million Americans confirming current cocaine use (SAMHSA 2013). Attempts to develop pharmacological treatments have been largely unsuccessful. The success of some non-pharmacological approaches, such as cognitive behavioral therapy, may represent a reversal of detrimental effects of cocaine abuse on executive function, which encapsulates the abilities to weigh multiple options, make complex decisions and organize, implement and control a multitude of cognitive functions (Oscar-Berman and Marinković 2007; van der Plas et al. 2009; Sofuoglu et al. 2013). Although some structural and functional alterations that occur due to cocaine abuse can recover during abstinence (Nader et al. 2006; Moeller et al. 2012; Connolly et al. 2013; Morie et al. 2013), relatively little is known about the extent to which cognitive impairments persist after cessation of cocaine use (De Oliveira et al. 2009). Characterizing the relationship between cocaine abuse and cognitive function during abstinence will aid development of therapies to reverse these deficits and to minimize relapse.
Dysfunction in brain dopamine (DA) systems is a hallmark of cocaine exposure and is believed to drive the associated cognitive impairments (Cools and D’Esposito 2011; Ersche et al. 2011; Moeller et al. 2012; Verrico et al. 2013), although it is impossible to determine whether these deficits precede or result from cocaine exposure in humans. Cocaine users display deficits in many aspects of executive function, including cognitive flexibility and response inhibition (Verdejo-García et al. 2006), which have been attributed specifically to alterations in DA function (Nandam et al. 2013). Cognitive deficits during abstinence have been associated with poorer retention and success in behavioral treatment programs (Volkow et al. 1992; Teichner et al. 2002; Aharonovich et al. 2006; Tomasi et al. 2007; Carroll et al. 2011). However, the time course of recovery of executive function during abstinence is not well characterized. Previous studies reported that memory deficits lessened over periods of abstinence, with cocaine users showing improved cognitive function at 6 months of abstinence when compared to 6 weeks (Di Sclafani et al 2002; Pace-Schott et al. 2008; Hanlon et al. 2011). However, limitations inherent in studies with human subjects make it difficult to track recovery over time. Controlled longitudinal studies in laboratory animals can directly address these questions. Longitudinal studies of cognitive function in monkeys—specifically those that involve repeated drug exposure—have been integral in understanding the development of deficits that accompany chronic drug exposure (e.g. Taffe et al. 2001; Jentsch et al. 2002; Liu et al. 2008; Gould et al. 2012).
Sex differences in sensitivity to cocaine are prevalent in both preclinical and clinical research (O’Brien and Anthony 2005; Anker and Carroll 2011). Because females tend to be underrepresented in clinical and preclinical studies, the extent to which cocaine causes cognitive impairments in females is not well understood. This is concerning because women are particularly vulnerable to cocaine abuse (e.g. Cotto et al. 2010). Women initiate drug use at earlier ages, progress to dependence faster and are more susceptible to the physical, mental and social consequences of abuse (Zilberman et al. 2003; Greenfield et al. 2007b). Furthermore, women are less successful at quit attempts, with lower rates of treatment retention and higher rates of relapse (Siqueland et al. 2002; Hyman et al. 2008). Importantly, previous studies have provided evidence for sex-dependent brain-behavior relationships in the role of the ventromedial prefrontal cortex in mediating emotional processing, decision-making and executive function (Bolla et al. 2004; Tranel et al. 2005; van der Plas et al. 2009). Preclinical evidence also suggests that female subjects are more sensitive to the abuse-related effects of cocaine across a range of behavioral assays including faster acquisition, higher rates and more pronounced reinstatement of self-administration (Lynch and Carroll 1999, 2000; Carroll et al. 2002; Lynch and Taylor 2005). However, nonhuman primate studies of the effects of cocaine on cognitive function have been conducted almost exclusively in male subjects.
In the present experiments, 16 adult female cynomolgus monkeys were studied. Nine monkeys were cocaine-naïve and seven monkeys had previously self-administered cocaine 5 days per week for three months, but had been abstinent for eight weeks at the initiation of this study. To assess effects of prior self-administration experience on cognition, we examined the time it took to train the monkeys to learn to use a touchscreen, then studied the acquisition of a reversal learning task which included a simple discrimination (SD) followed by the reversal of that discrimination (SDR). These behavioral tasks examined the monkeys’ ability to learn a rule to guide behavior (SD) and to inhibit responding under that rule while learning a new rule (SDR). We used a 3-choice visual discrimination to permit examination of the patterns of errors made to determine if they are perseverative (Arnsten et al. 1997; Jentsch et al. 2002). To assess whether cognitive deficits persisted following acquisition, we assessed performance for three months with re-exposure to the reversal-learning task using novel stimuli. We also evaluated performance on a delayed match-to-sample (DMS) task to measure working memory. In this task, subjects are trained to identify previously presented stimuli after various delay intervals. Because all monkeys had a similar previous experience of lever-pressing reinforced by delivery of a food pellet, we did not expect to observe group differences in touchscreen training. However, we hypothesized that cocaine-experienced monkeys would take longer to acquire the SD/SDR task based on previous results in male rhesus monkeys (Gould et al. 2012). Moreover, we expected to observe that these impairments would be task-specific. Because impairments on the DMS task were shown to resolve within 30 days of abstinence in male rhesus monkeys (Gould et al. 2012), we hypothesized that the delay lengths used to generate performance-based curves would be similar between groups.
Materials and Methods
Subjects
Sixteen adult female cynomolgus macaques (Macaca fascicularis) served as subjects. Seven monkeys had been surgically implanted with an indwelling intravenous catheter as described previously (Czoty et al. 2005) and had experience self-administering cocaine (approximately 150 mg/kg cocaine over 6 months). Eight weeks prior to the start of the present experiments, access to cocaine was discontinued for the cocaine-experienced monkeys and they and 9 additional cocaine-naïve monkeys, who had been self-administering food pellets for three months, began self-administering sucrose pellets 3–5 days per week during under a fixed-ratio schedule. There were no differences between the groups in average age or weight at the start of these experiments (Table 1); weights did not change significantly during the course of the experiments. All monkeys were fitted with an aluminum collar (Primate Products, Redwood City, California) and trained to sit in a standard primate chair (Primate Products). Monkeys were weighed weekly and feed enough fresh fruit and food (Nestle Purina PetCare Company, St. Louis, Missouri) to maintain healthy body weights (2.6–3.2 kg) as determined by physical appearance and periodic veterinary exams; water was available ad libitum in the home cage which measured 0.71 × 1.68 × 0.84 m (Allentown Caging Inc., Allentown, New Jersey). Environmental enrichment was provided as outlined in the Institutional Animal Care and Use Committee’s Non-Human Primate Environmental Enrichment Plan. All experimental procedures were performed in accordance with the 2003 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research and were approved by the Wake Forest University Institutional Animal Care and Use Committee.
Table 1.
Subjects’ ages (years), weights (kg), lifetime cocaine intakes (mg/kg) and the individual delay times in the DMS task (seconds).
| Subject | Age | Weight | Intake | Short | Medium | Long |
|---|---|---|---|---|---|---|
| Cocaine-naïve | ||||||
| C-7905 | 5 | 4.0 | 0.0 | 3 | 25 | 45 |
| C-7902 | 5 | 2.8 | 0.0 | 3 | 25 | 50 |
| C-7664 | 6 | 2.9 | 0.0 | 2 | 45 | 70 |
| C-7591 | 6 | 3.1 | 0.0 | 2 | 15 | 40 |
| C-7558 | 5 | 3.0 | 0.0 | 0 | 10 | 30 |
| C-7870 | 11 | 2.8 | 0.0 | 1 | 15 | 35 |
| C-7964 | 5 | 2.6 | 0.0 | 3 | 15 | 25 |
| C-8202 | 6 | 2.4 | 0.0 | did not become stable | ||
| C-7460 | 10 | 4.0 | 0.0 | 0 | 10 | 30 |
| Cocaine-experienced | ||||||
| C-7438 | 7 | 2.6 | 207.8 | 1 | 15 | 45 |
| C-7458 | 7 | 3.4 | 170.9 | 0 | 10 | 30 |
| C-7437 | 7 | 3.5 | 1979.8 | 0 | 30 | 60 |
| C-7431 | 7 | 3.2 | 290.0 | 0 | 10 | 30 |
| C-7457 | 10 | 2.7 | 986.0 | 1 | 10 | 30 |
| C-7434 | 10 | 2.8 | 962.0 | did not become stable | ||
| C-7441 | 10 | 3.3 | 680.0 | not tested | ||
Cognitive assessments
Cognitive testing was conducted 5 to 7 days per week between 9:00 am and 12:00 pm using the Cambridge Neuropsychological Test Automated Battery apparatus (CANTAB; Lafayette Instruments, Lafayette, Indiana) as described previously (Gould et al. 2012). Animals completed a maximum of 200 trials in the stimulus discrimination and reversal (SD/SDR) task and 80 trials in the DMS task. Only one task was assessed in each behavioral session. Total session length of the reversal learning task depended on task performance, as session terminated once reversal criteria were met or a maximum of 200 trials were completed (see below). In the DMS task, each animal completed 80 total trials with three delay lengths (short, medium and long, see below), therefore the total session length varied between animals but lasted one hour on average.
Experiment 1. Effects of prior cocaine self-administration on acquisition of touchscreen training, a reversal-learning task and a DMS task
(1a) Training
Using a touch-sensitive computer screen (Lafayette Instruments; Lafayette, IN) monkeys were trained to touch a square that became progressively smaller across trials. Specifically, there were six sizes of the square, which became smaller after every fourth consecutive touch. Each touch was reinforced with a 190-mg food pellet; touching any other part of the screen resulted in a 10-second timeout (Weed et al. 1999). The primary dependent variable for touchscreen training was the number of daily sessions to reach criteria (two daily sessions in which the monkey reached the smallest square). Data from cocaine-experienced (n=7) and cocaine-naïve (n=9) monkeys were statistically compared using a t-test. Differences were considered statistically significant when p<0.05. Once the touch-training criterion was met monkeys were exposed to the SD and SDR tasks.
(1b) SD/SDR task
In the SD task, three shapes (A, B, C) appeared in a horizontal row across the center of the screen. The same three shapes were used for each monkey during this stage of the experiment. A response on one shape (A+) resulted in delivery of a 190-mg food pellet and initiation of a 7-second inter-trial interval (ITI) while responding on either of the other two shapes (B−, C−) resulted in a 10-second timeout, followed by a 7-second ITI. Shapes were randomly distributed throughout the three possible positions on the screen with a maximum of 200 trials per day. Acquisition of the SD was defined as 18 correct responses out of the previous 20 completed trials. Once the acquisition criterion was met, contingencies were altered in the SDR phase so that responding on the previously correct shape was now incorrect (i.e. A−) while a response on one of the previous incorrect shapes now counted as a correct response (i.e. B+). The third shape, which was incorrect in the SD phase, remained incorrect in the SDR phase (i.e. C−). The same consequences of responding on a correct or incorrect stimulus and the same criterion for acquisition used in the SD phase were used in the SDR phase.
Dependent variables included the total number of trials, the number of errors and the number of omissions to acquisition during the SD and SDR tasks. Total trials included correct trials, errors and omissions. Data for errors to criterion underwent a square-root transformation prior to analysis to normalize their distribution (see Wright Jr et al. 2013). Response and pellet retrieval latencies were also recorded. For the SDR task, perseverative errors were determined, defined as responses on the stimulus that had been reinforced in the SD phase (A−). Incorrect responses on the stimulus that had not been reinforced in the SD phase (C−) were termed seeking errors. Two-way analyses of variance (ANOVAs) were conducted using group (cocaine-experienced, cocaine-naïve) and phase of task (SD, SDR) as factors. A two-way ANOVA was also conducted to compare the distribution of errors across the two incorrect stimuli in the SD and SDR phases between groups. Significant main effects were followed by post hoc comparisons using Fisher’s LSD tests. For each dependent variable, there were four comparisons of interest (i.e. the comparison of cocaine-naïve vs. cocaine-experience groups for both tests, and the comparison for each group across tests). To maintain a family-wise error rate less than 0.05, a Bonferroni correction was applied following Fisher’s LSD analysis. The resulting critical value of p was equal to 0.0125. A similar adjustment was made for analysis of error distribution.
(1c) DMS task
Collection of DMS data began after all animals finished Experiment 1b. In the DMS task, a target image appeared on the screen and when a response was made (i.e. after a 0-second delay), three images appeared. The three images were taken from a limited stimulus set of six images (i.e. stimuli were not trial-unique). A response on the previously displayed image resulted in delivery of a 190-mg food pellet. A response on either of the two novel images resulted in a 10-second time out and no pellet delivery. Once percent accuracy exceeded 80% for 3 consecutive days with a short (0-sec) delay, delays were gradually increased. Baseline performance was established by increasing delay lengths until similar reductions in percent accuracy were reached in all monkeys. There were three target levels of accuracy in each monkey: >78% accuracy (the corresponding delay was considered the “short” delay), 55%–78% accuracy (“medium” delay) and <55% accuracy (“long” delay). Delays were randomly distributed throughout each session so that there were ~27 trials per delay. Once accuracy remained within these ranges for 5 consecutive days, performance was deemed stable for each monkey and the average short, medium and long delay lengths were calculated. A two-way ANOVA was conducted with group (cocaine-naïve, cocaine-experienced) and delay (short, medium, long) as factors. Significant main effects were followed by post hoc comparisons using Fisher’s LSD tests.
Experiment 2. Effects of prior cocaine self-administration on maintenance of cognitive performance
Once all monkeys had acquired the SD/SDR task, they were tested once each week on the SD/SDR task for three months; data from the last week of each month were averaged across monkeys for analysis. On the other four days per week, monkeys responded on the DMS task (Experiment 1c). For the SD/SDR task, shapes were selected randomly from the “CAMCOG 0” list associated with the CANTAB system and presented in non-overlapping sets of three. Sets of shapes were randomized across monkeys and time points, with the stipulation that no monkey saw the same set twice; the set used for Experiment 1b was not re-used during this experiment. Task completion criteria were identical to those described above. The same dependent variables were analyzed as in Experiment 1b. Data were analyzed using a 3-way ANOVA with group (cocaine-experienced, cocaine-naïve), phase of task (SD, SDR) and month (1, 2, 3) as factors, followed by post hoc Fisher’s LSD tests.
Results
Experiment 1. Effects of prior cocaine self-administration on acquisition of touchscreen training, the reversal-learning task and the DMS task
Training
There were no differences between the 7 cocaine-experienced and 9 cocaine-naïve monkeys in the number of days necessary to complete touchscreen training (7.2 ± 2.6 and 5.9 ± 1.2 days, respectively).
(1a) SD/SDR task
There were no significant differences between groups in response latencies or pellet retrieval latencies during the SD/SDR task (Table 2). Two monkeys in the cocaine- experienced group did not reach a criterion level of performance and were excluded from data analysis and presentation. One monkey (C-7431) acquired the SD task after 419 total trials (146 errors and 102 emissions) but developed a side bias once the SDR was implemented. The other monkey (C-7441) took 703 trials to acquire the SD (293 errors and 148 omissions) and after switching to the SDR task, soon began to omit nearly all trials.
Table 2.
Mean (± SEM) response latencies and pellet retrieval latencies (in sec) in cocaine-naïve (coc-naïve) and cocaine-experienced (coc-exp) monkeys.
| Response latency, SD | Response latency, SDR | Retrieval latency, SD | Retrieval latency, SDR | |
|---|---|---|---|---|
| SD/SDR ACQUISITION | ||||
| Coc-naïve (n=9) | 2.9 (0.4) | 3.4 (0.7) | 1.6 (0.5) | 1.2 (0.2) |
| Coc-exp (n=5) | 2.1 (0.4) | 2.8 (0.4) | 1.5 (0.3) | 1.3 (0.4) |
| MONTH 1 | ||||
| Coc-naïve | 2.4 (0.2) | 2.8 (0.3) | 1.2 (0.2) | 1.3 (0.2) |
| Coc-exp | 2.8 (0.5) | 3.4 (0.8) | 1.0 (0.2) | 1.0 (0.2) |
| MONTH 2 | ||||
| Coc-naïve | 2.0 (0.2) | 2.2 (0.1) | 1.3 (0.3) | 1.2 (0.2) |
| Coc-exp | 2.8 (0.8) | 2.7 (0.8) | 1.4 (0.3) | 1.1 (0.2) |
| MONTH 3 | ||||
| Coc-naïve | 2.9 (0.4) | 3.4 (0.7) | 1.6 (0.5) | 1.2 (0.2) |
| Coc-exp | 2.1 (0.4) | 2.8 (0.4) | 1.5 (0.3) | 1.3 (0.4) |
| Response latency, sample | Response latency, match | Retrieval latency | |
|---|---|---|---|
| DMS | |||
| Coc-naïve (n=8) | 1.4 (0.2) | 3.0 (0.4) | 1.5 (0.3) |
| Coc-exp (n=5) | 2.1 (0.4) | 2.4 (0.3) | 1.0 (0.2) |
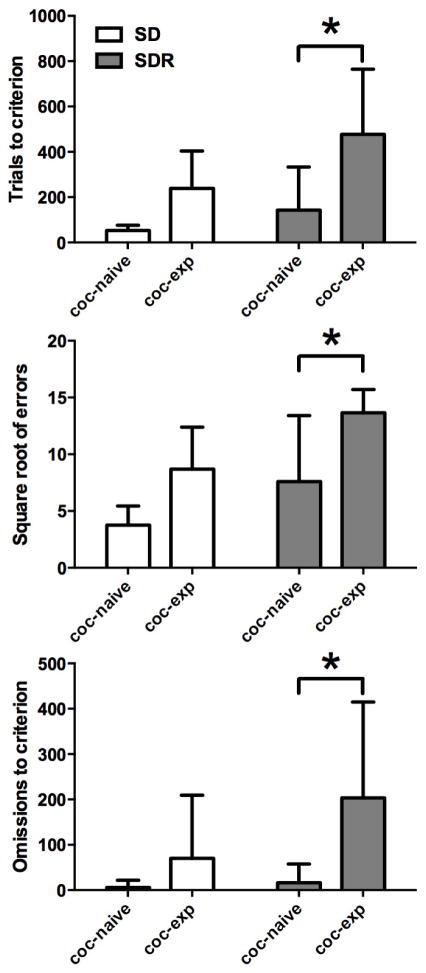
Results of analysis of total trials to criterion (Fig. 1, top) revealed main effects of group (cocaine-naïve, n=9 vs. cocaine-experienced, n=5; F1,12= 16.91, p<0.001) and test (SD vs. SDR; F1,12=4.93, p<0.05), and no significant interaction. Post-hoc tests confirmed the difference between the SD and SDR task collapsed across groups (p<0.05), as well as the effect of group, collapsed across test (p<0.001). Cocaine-naïve and -experienced groups were not significantly different on the SD task but differed significantly on the SDR (p<0.0125). In errors to criterion (Fig. 1, middle) there were also significant main effects of group (F1,12= 11.86, p<0.01) and test (F1,12= 8.85, p<0.05) and no interaction. As with trials to criterion, post-hoc testing confirmed the difference between the SD and SDR task collapsed across groups (p<0.05), as well as the effect of group collapsed across test (p<0.01). Post-hoc comparisons revealed a significant group difference only in the SDR phase (p<0.0125). Finally, there was a main effect of group (F1,12= 8.79, p<0.05) but not test in the number of omissions that occurred prior to reaching criterion for acquisition (Fig. 1, bottom). Post-hoc tests confirmed that the groups differed significantly in omissions made during acquisition of the SDR test (p<0.0125) but not the SD test.
Figure 1.
Performance during acquisition (total trials, errors and omissions to criterion) of the simple discrimination (SD) and reversal (SDR) phases in cocaine-naïve (coc-naïve, n=9) and cocaine-experienced (coc-exp, n=5) monkeys. Data for errors was square-root transformed prior to analysis. Bars depict mean (± SD) values. *, p < 0.0125.
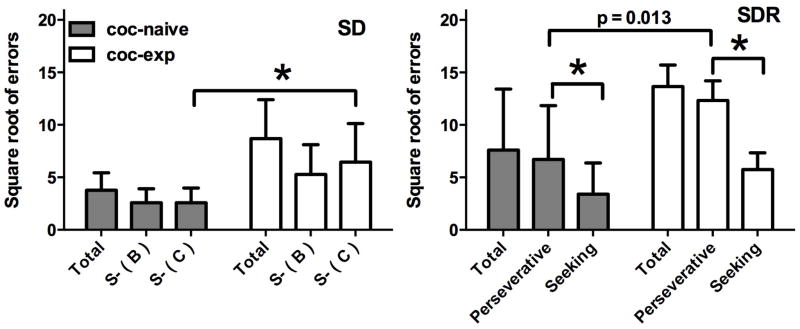
Using three stimuli in the SD/SDR task permitted the determination of whether errors made in the SDR phase were perseverative. During the SD phase (Fig. 2, left), cocaine-experienced monkeys made significantly more errors (main effect of group, F1,12=11.42, p<0.01 and post-hoc difference in errors on the C stimulus, p<0.01), but both groups made a similar number of errors on each incorrect stimulus (no main effect of stimulus B vs. C and no interaction), indicating that, prior to reversal, no bias existed. During the SDR, however (Fig 2, right), a main effect of error type (F1,12=157.20, p<0.001) and a significant interaction (F1,12=5.61, p<0.05) was found, and the main effect of group approached significance (F1,12=4.56, p=0.054). Although both groups made significantly more perseverative than seeking errors, the difference was larger in the cocaine-experienced monkeys. Moreover, the difference in number of perseverative errors between cocaine-naïve and –experienced monkeys approached significance (p=0.013).
Figure 2.
Error distribution during acquisition of the reversal-learning task in cocaine-naïve (n=9) and cocaine-experienced (n=5) monkeys. Bars represent mean (± SD) number of errors (square-root transformed) on each non-reinforced stimulus during the SD phase (left) and SDR phase (right). *, p < 0.0125.
(1b) DMS task
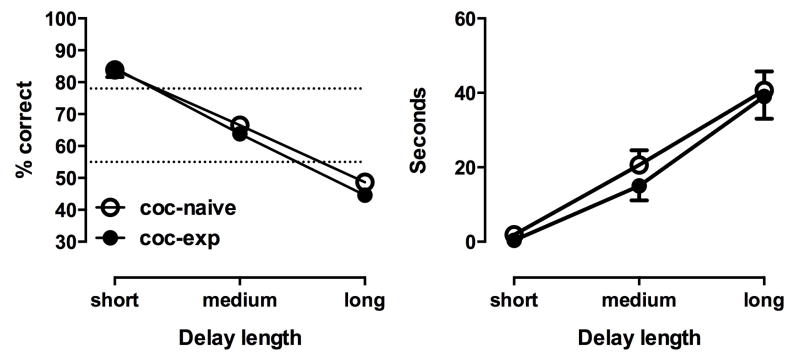
One cocaine-naïve monkey (C-8202) and one cocaine-experienced monkey (C-7434) did not achieve stable DMS task performance with relatively short delays (<10 seconds) within 50 daily sessions; their data were excluded from analysis and presentation. For the remaining monkeys, increasing the delay between the disappearance of the sample stimulus and the presentation of the match and comparison stimuli resulted in delay-dependent decreases in accuracy from near 100% to chance levels (F2,20=88.68, p<0.001; Fig. 3, left). There was no main effect of group and no interaction. The specific delay lengths for individual monkeys are shown in Table 1. Analysis of the delay lengths that made up each accuracy level (short, >78% accuracy, medium, 55%–78% accuracy, long <55% accuracy) for cocaine-naïve (n=8) and cocaine-experienced (n=5) monkeys showed no significant main effect of group and no interaction (Fig. 3. right). There was, as expected, a significant main effect of delay length (F2,20=73.36, p<0.001); post-hoc analysis confirmed that short, medium and long delays were all significantly different from each other. There no significant differences between groups in response latencies or pellet retrieval latencies during the DMS task (Table 2).
Figure 3.
Accuracy at (left), and absolute lengths of (right), the delays deemed short, medium and long in cocaine-naïve (n=8) and cocaine-experienced (n=5) monkeys. Points represent mean (± SEM).
Experiment 2. Effects of prior cocaine self-administration on maintenance of cognitive performance
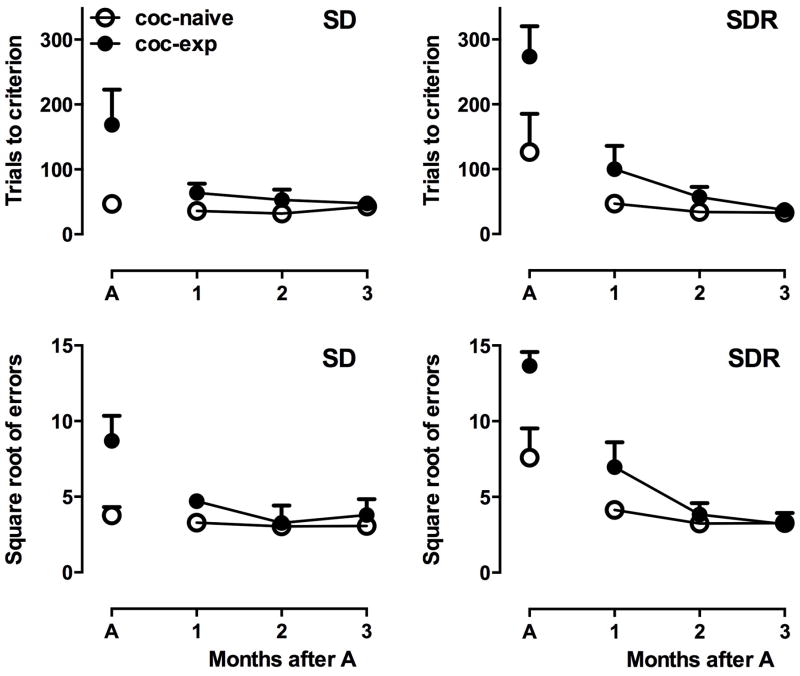
The SD/SDR test was repeated weekly over three months after initial acquisition. In analyzing total trials to criterion (Fig 4, top row), a three-way ANOVA revealed main effects of month (F2,72=3.82, p<0.05) and group (F1,72=9.75, p<0.01) but not test; there were no significant interactions. Post-hoc analyses indicated that the main effects are explained by the increased trials needed by the cocaine-experienced monkeys to complete the SDR task during month 1. For example, post-hoc tests indicated that cocaine-naïve and cocaine-experienced monkeys only differed in month 1 and only on the SDR task, and that overall month 1 was different from months 2 and 3. Moreover, there was no difference across months for the cocaine-naïve group, but month 1 was significantly different from months 2 and 3 for the cocaine-experienced group. The same pattern of statistical test results was observed for errors to criterion (Fig 4, bottom row); there were main effects of month (F2,72=5.97, p<0.01) and group (F1,72=5.93, p>0.05) but not test. The pattern of results of post-hoc tests was identical to those for trials to criterion, indicating that the significant main effects are explained by the higher number of errors in month 1 in the cocaine-experienced monkeys on the SDR task. Note that acquisition data are included in Fig. 4 for comparison but were not included in statistical analysis. Omissions were very low during the SD task (1.0 ± 0.4 omissions per month across all monkeys) and during the SDR task (1.2 ± 0.5 omissions per month when one cocaine-experienced monkey who averaged 11 omissions per month is excluded).
Figure 4.
Trials to criterion (top row) and errors to criterion (square-root transformed, bottom row) during the simple discrimination (SD, left column) and reversal (SDR, right column) phase for the three months following acquisition (A). Points depict mean (± SEM) values in cocaine-naïve (n=9) and cocaine-experienced (n=5) monkeys. Data from acquisition (A) are included for comparison.
Discussion
Understanding the extent to which cognitive deficits observed in cocaine abusers persist into protracted abstinence will help guide treatment decisions. Although some research has shown improvements in cognitive tasks in abstinence (Gould et al. 2012; Bell et al. 2013; Morie et al. 2013), other studies demonstrate that deficits in inhibition, cognitive flexibility and verbal memory can persist into at least short term abstinence (for review see Verdejo-Garcia 2004; van Holst and Schilt 2011). These experiments in cynomolgus monkeys were designed to assess the effects of prior cocaine exposure and subsequent abstinence on acquisition and maintainance of two cognitive tasks in female monkeys.
In the simple discrimination (SD) task, although cocaine-experienced females required more trials to criterion, committed more errors and made more omissions than cocaine-naïve monkeys on average, these effects did not reach statistical significance. Any differences observed on this first phase of the task were not due to general impairments in ability to respond using the touchscreen since there were no differences between groups in initial reinforcement training with the CANTAB system or in response or pellet retrieval latency. A previous study in male rhesus monkeys (Gould et al. 2012), also showed no significant differences between cocaine-naïve and cocaine-experienced monkeys in acquiring the SD task. Although the difference between groups appears to be larger in the present study compared to Gould et al. (2012), convincing evidence for an effect of cocaine experience on SD performance is lacking. Moreover, results of the present experiments do not provide evidence of sex differences, although firm conclusions are limited by the relatively small sample size.
More trials were required to reach criterion and more errors were made in the SDR phase compared to the SD phase, indicative of a higher cognitive demand in this stage of the task. This supports previous findings utilizing reversal-learning tasks (Jentsch et al, 2002; Gould et al. 2012; Kangas and Bergman, 2014), which require inhibition of a previously established response while learning a new contingency without explicit signals (for review see Bari and Robbins, 2013). Unlike the SD phase, during acquisition of the SDR task cocaine-experienced monkeys required significantly more trials to criterion compared to cocaine-naïve monkeys. Cocaine-experienced monkeys also made more than twice as many errors on average. These group differences are even more striking in light of the fact that two cocaine-experienced monkeys never learned the reversal task and were thus not included in the data analysis.
The design of the SDR phase of the task permitted analysis of the distribution of errors across the two incorrect shapes, which provides insight into the mechanisms mediating poor cognitive performance following cocaine experience. In the SD portion of the task both groups of monkeys made a similar number of error responses on each non-reinforced shape, indicating that no inherent bias existed. In the SDR phase, however, both groups made significantly more perseverative errors—choices of the stimulus that had been reinforced in the SD task that was no longer correct—than errors on the third stimulus that was never correct. Some responding on the previously reinforced stimulus is to be expected since monkeys must learn that the contingency has changed. On average, cocaine-experienced monkeys made more perseverative errors (but not more seeking errors) compared to cocaine-naïve monkeys, a difference (p=0.013) that approached significance (p<0.0125) after Bonerroni adjustments were made for multiple comparisons. Although it is important to note that perseverative responding was observed in both groups of monkeys, the observation of more perseverative responding in cocaine-experienced monkeys would support the interpretation that cocaine use causes deficits that may be driven by an inability to inhibit previously formed associations in order to decipher the new contingencies. This conclusion would be consistent with an earlier report demonstrating that perseverative errors increased following 14 days of cocaine treatment in vervet monkeys (Jentsch et al. 2002), and suggest that that these impairments can be present months after drug taking ceases.
In addition to group differences in trials to criterion and perseverative errors, the number of omissions was also significantly different in the SDR phase. An increase in number of omissions resulting from dopaminergic manipulations has been documented in other cognitive tasks, including Go/No-Go tasks and the 5-choice serial reaction time test (Nakamura et al. 1998; Czernecki et al. 2002; Fletcher et al. 2007; Verdejo-Garcia et al. 2007). Increases in rate of omissions can result from an inability to perform the task due to motor deficits or because monkeys did not find the pellets delivered after correct responses to be reinforcing. The lack of group differences in response latencies or pellet retrieval latencies argue against these explanations. Increased omissions may also result from impairments in attention, an aspect of cognitive function that is markedly impaired by cocaine use (Jovanovski et al. 2005; Spronk et al. 2013; Wood et al. 2013). However, impairments of attention would likely be accompanied by increased latencies to respond, which were not observed. A definitive assessment of deficits in attention would require cognitive tasks that directly measure attention, but these were not performed in the present study. Because omissions tended to occur after several incorrect responses were made, it is also possible that higher omissions in cocaine-exposed monkeys are a manifestation of a lack of motivation to engage in the task; cocaine-exposed monkeys appeared to be more likely to stop initiating trials after several incorrect responses. Ultimately, it is difficult to determine what factors drive increased omissions in animal models. Whatever the mechanism, there was a clear effect of prior cocaine experience on the rate of omissions. Taken together, these results suggest that cocaine self-administration produced cognitive impairments in these female monkeys during abstinence consistent with those observed during and following cocaine exposure in male rats, monkeys and humans (Jentsch et al. 2002; Schoenbaum et al. 2004; Fillmore and Rush 2006; Calu et al. 2007; Ersche et al. 2008; Liu et al. 2008; Krueger et al. 2009; Camchong et al. 2011; Porter et al. 2011; Gould et al. 2012).
The second cognitive domain that was addressed in this study was working memory, which we evaluated using a DMS task. A delay-performance curve was established in the monkeys by introducing varying delay periods between the disappearance of the sample stimulus and the presentation of the match and comparison stimuli. This increasing delay length raised the difficulty of the task, which was reflected in delay-dependent reductions in accuracy. Because the short, medium and long delay lengths required to produce the respective target performance accuracies did not differ between groups, we conclude that any deficits in working memory caused by cocaine self-administration did not persist to this point in abstinence. It is not known whether deficits existed during or shortly after cocaine self-administration. It is possible that cocaine intake was too low to produce deficits. It is also possible that cognitive impairment was present, but alterations in brain function compensated over time (e.g. Porter et al. 2014). The specificity of these cognitive impairments in abstinence (i.e., group differences in discrimination/reversal but not working memory tasks) mirrors findings in male rhesus monkeys (Gould et al. 2012). It is also possible that the lack of effect in the present study could be due in part to the extensive training necessary to establish stable delay curves.
Group differences in performance on the SD/SDR task dissipated when monkeys performed the SD/SDR task weekly. Although some group differences were still apparent at the end of the first month of testing, cocaine-experienced monkeys had shown improvement by then. Performance of the previously cocaine-naïve and cocaine-exposed monkeys was not different, and was near perfect, by the third month of weekly exposure to the task. These data are consistent with a study in male rhesus monkeys (Porter et al. 2013) in which no differences were observed in SD, SDR or DMS tasks after three months of abstinence. Importantly, Porter et al. had previously reported the presence of cognitive deficits in these same subjects (Porter et al. 2011). Taken together, these results suggest that although cognitive deficits may be present in cocaine users and acquisition of certain novel tasks may be more difficult for abstinent cocaine users, performance can improve with practice and/or over time. From a translational point of view these studies provide the encouraging clinical message that cocaine-induced cognitive impairments are reversible if abstinence can be maintained.
Acknowledgments
This study was supported by Public Health Service grants DA 007246, DA 29178, DA 17763 and DA 10584.
Footnotes
Conflicts of interest: none
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81:313–22. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–62. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Bari A, Robbins TW. Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Bell RP, Foxe JJ, Ross LA, Garavan H. Intact inhibitory control processes in abstinent drug abusers (I): A functional neuroimaging study in former cocaine addicts. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.02.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–64. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn Mem. 2007;14:325–328. doi: 10.1101/lm.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, MacDonald AW, 3rd, Nelson B, Bell C, Mueller BA, Specker S, Lim KO. Frontal hyperconnectivity related to discounting and reversal learning in cocaine subjects. Biol Psychiatry. 2011;69:1117–1123. doi: 10.1016/j.biopsych.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Kiluk BD, Nich C, Babuscio TA, Brewer JA, Potenza MN, Ball SA, Martino S, Rounsaville BJ, Lejuez CW. Cognitive function and treatment response in a randomized clinical trial of computer-based training in cognitive-behavioral therapy. Subst Use Misuse. 2011;46:23–34. doi: 10.3109/10826084.2011.521069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Bell RP, Foxe JJ, Garavan H. Dissociated grey matter changes with prolonged addiction and extrnded abstinence in cocaine users. PLoS One. 2013;8:359645. doi: 10.1371/journal.pone.0059645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. Inverted U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotto JH, Davis E, Dowling GJ, Elcano JC, Staton AB, Weiss SR. Gender effects of drug use, abuse and dependence: a special analysis of results form the National Survey on Drug Use and Health. Gend Med. 2010;7:402–413. doi: 10.1016/j.genm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Czernecki V, Pillon B, Houeto JL, Pochon JB, Levy R, Dubois B. Motivation, reward, and Parkinson’s disease: influence of dopatherapy. Neuropsychologia. 2002;40:2257–2267. doi: 10.1016/s0028-3932(02)00108-2. [DOI] [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. Assessment of the relative reinforcing strength of cocaine in socially housed monkeys using a choice procedure. J Pharmacol Exp Ther. 2005;312:96–102. doi: 10.1124/jpet.104.073411. [DOI] [PubMed] [Google Scholar]
- De Oliveira LG, Barroso LP, Silveira CM, Sanchez ZV, De Carvalho Ponce J, Vaz LJ, Nappo SA. Neuropsychological assessment of current and past crack cocaine users. Subst Use Misuse. 2009;44:1941–1957. doi: 10.3109/10826080902848897. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug Alcohol Depend. 2002;66:161–171. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Abbott S, Craig KJ, Müller U, Suckling J, Ooi C, Shabbir SS, Clark L, Sahakian BJ, Fineberg NA, Merlo-Pich EV, Robbins TW, Bullmore ET. Response perseveration in stimulant dependence is associated with striatal dysfunction and can be ameliorated by a D(2/3) receptor agonist. Biol Psychiatry. 2011;70:754–762. doi: 10.1016/j.biopsych.2011.06.033. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology. 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR. Polydrug abusers display impaired discrimination-reversal learning in a model of behavioural control. J Psychopharmacol. 2006;20:24–32. doi: 10.1177/0269881105057000. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tenn CC, Sinyard J, Rizos Z, Kapur S. A sensitizing regimen of amphetamine impairs visual attention in the 5-choice serial reaction time test: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Neuropsychopharmacology. 2007;32:1122–1132. doi: 10.1038/sj.npp.1301221. [DOI] [PubMed] [Google Scholar]
- Gould RW, Gage HD, Nader MA. Effects of chronic cocaine self-administration on cognition and cerebral glucose utilization in rhesus monkeys. Biol Psychiatry. 2012;72:856–863. doi: 10.1016/j.biopsych.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CA. Gender and the use of substance abuse treatment services. Alcohol Res Health. 2006;29:55–62. [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, Lincoln M, Hien D, Miele GM. Substance abuse treatment entry, retention and outcome in women: a review of the literature. Drug Alcohol Depend. 2007a;86:1–21. doi: 10.1016/j.drugalcdep.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Trucco EM, McHugh RK, Lincoln M, Gallop RJ. The Women’s Recovery Group Study: a Stage I trial of women-focused group therapy for substance use disorders versus mixed-gender group drug counseling. Drug Alcohol Depend. 2007b;90:39–47. doi: 10.1016/j.drugalcdep.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology. 2011;218:681–692. doi: 10.1007/s00213-011-2360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SM, Paliwal P, Chaplin TM, Mazure CM, Rounsaville BJ, Sinha R. Severity of childhood trauma is predictive on cocaine relapse outcomes in women but not men. Drug Alcohol Depend. 2008;92:208–216. doi: 10.1016/j.drugalcdep.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, De La Garza R, 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- Kangas BD, Bergman J. Repeated acquisition and discrimination reversal in the squirrel monkey (Saimiri sciureus) Anim Cogn. 2014;17:221–8. doi: 10.1007/s10071-013-0654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger DD, Howell JL, Oo H, Olausson P, Taylor JR, Nairn AC. Prior chronic cocaine exposure in mice induces persistent alterations in cognitive function. Behav Pharmacol. 2009;20:695–704. doi: 10.1097/FBP.0b013e328333a2bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Heitz RP, Sampson AR, Zhang W, Bradberry CW. Evidence of temporal cortical dysfunction in rhesus monkeys following chronic cocaine self-administration. Cereb Cortex. 2008;18:2109–2116. doi: 10.1093/cercor/bhm236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology. 2000;148:196–2000. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Decreased motivation following cocaine self-administration under extended access conditions: effects of sex and ovarian hormones. Neuropsychopharmacology. 2005;30:927–935. doi: 10.1038/sj.npp.1300656. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Tomasi D, Honorio J, Volkow ND, Goldstein RZ. Dopaminergic involvement during mental fatigue in health and cocaine addiction. Transl Psychiatry. 2012;2:e176. doi: 10.1038/tp.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morie KP, Garavan H, Bell RP, De Sanctis P, Krakowski MI, Foxe JJ. Intact inhibitory control processes in abstinent drug abusers (II): A high-density electrical mapping study in former cocaine and heroin addicts. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.02.023. in press. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Bucjhheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kurasawa M, Tanaka Y. Apomorphine-induced hypo-attention in rats and reversal of the choice performance impairment by aniracetam. Eur J Pharmacol. 1998;342:127–38. doi: 10.1016/s0014-2999(97)01457-x. [DOI] [PubMed] [Google Scholar]
- Nandam LS, Hester R, Wagner J, Dean AJ, Messer C, Honeysett A, Nathan PJ, Bellgrove MA. Dopamine D2 receptor modulation of human response inhibition and error awareness. J Cogn Neurosci. 2013;25:649–656. doi: 10.1162/jocn_a_00327. [DOI] [PubMed] [Google Scholar]
- O’Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology. 2005;30:1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinković K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Morgan PT, Malison RT, Hart CL, Edgar C, Walker M, Stickgold R. Cocaine users differ from normals on cognitive tasks which show poorer performance during drug abstinence. Am J Drug Alcohol Abuse. 2008;34:109–121. doi: 10.1080/00952990701764821. [DOI] [PubMed] [Google Scholar]
- Porter JN, Minhas D, Lopresti BJ, Price JC, Bradberry CW. Altered cerebellar and prefrontal cortex function in rhesus monkeys that previously self-administered cocaine. Psychopharmacol. 2014;231:4211–4218. doi: 10.1007/s00213-014-3560-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW. Chronic cocaine self-administration in rhesus monkeys: Impact on associative learning, cognitive control, and working memory. J Neurosci. 2011;31:4926–4934. doi: 10.1523/JNEUROSCI.5426-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Siqueland L, Crits-Christoph P, Gallop R, Berber JP, Griffin ML, Thase ME, Daley D, Frank A, Gastfriend DR, Blaine J, Connolly MB, Gladis M. Retention in psychosocial treatment of cocaine dependence: predictors and impact on outcome. Am J Addict. 2002;11:24–40. doi: 10.1080/10550490252801611. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk DB, van Wel JH, Ramaekers JG, Verkes RJ. Characterizing the cognitive effects of cocaine: a comprehensive review. Neurosci Biobehav Rev. 2013;37:1838–59. doi: 10.1016/j.neubiorev.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-44, HHS Publication No. (SMA) 12–4713. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. Results from the 2011 National Survey on Drug Use and Health: Volume I. Summary of National Findings. [Google Scholar]
- Taffe MA, Weed MR, Davis S, Huitron-Resendiz S, Schroeder R, Parson sLH, Henriksen SJ, Gold LH. Functional consequences of repeated (+/−)3,4-methylenedioxymethamphetamine (MDMA) treatment in rhesus monkeys. Neuropsychopharmacology. 2001;24:230–239. doi: 10.1016/S0893-133X(00)00185-8. [DOI] [PubMed] [Google Scholar]
- Teichner G, Horner MD, Roitzsch JC, Herron J, Thevos A. Substance abuse treatment outcomes for cognitively impaired and intact outpatients. Addict Behav. 2002;27:751–763. doi: 10.1016/s0306-4603(01)00207-6. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Res. 2007;1171:83–92. doi: 10.1016/j.brainres.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine in men and women. J Clin Exp Neuropsychol. 2009;31:706–719. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst RJ, Schilt T. Drug-related decrease in neuropsychological functions of abstinent drug users. Curr Drug Abuse Rev. 2011;4:42–56. doi: 10.2174/1874473711104010042. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Bechara A, Recknor EC, Pérez-García M. Executive dysfunction in substance dependent individuals during drug use and abstinence: an examination of the behavioral, cognitive and emotional correlates of addiction. J Int Neuropsychol Soc. 2006;12:405–415. doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, López-Torrecillas F, Giménez CO, Pérez-García M. Clinical implications and methodological challenges in the study of the neuropsychological correlates of cannabis, stimulant, and opioid abuse. Neuropsychol Rev. 2004;14:1–41. doi: 10.1023/b:nerv.0000026647.71528.83. [DOI] [PubMed] [Google Scholar]
- Verdejo-García AJ, Perales JC, Pérez-García M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007;32:950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Verrico CD, Haile CN, Newton TF, Kosten TR, De La Garza R. Pharmacotherapeutics for substance-use disorders: a focus on dopaminergic medications. Expert Opin Investig Drugs. 2013;22:1549–1568. doi: 10.1517/13543784.2013.836488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico CD, Liu S, Asafu-Adjei JK, Sampson AR, Bradberry CW, Lewis DA. Acquisition and baseline performance of working memory tasks by adolescent rhesus monkeys. Brain Res. 2011;1378:91–104. doi: 10.1016/j.brainres.2010.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-Term Frontal Brain Metabolic Changes in Cocaine Abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, Bloom FE, Gold LH. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Brain Res Cogn Brain Res. 1999;8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Wood S, Sage JR, Shuman T, Anagnostaras Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol Rev. 2013;66:193–221. doi: 10.1124/pr.112.007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Jr, Glavis-Bloom C, Taffe MA. Acute ethanol reduces reversal cost in discrimination learning by reducing perseverance in adolescent rhesus macaques. Alcohol Clin Exp Res. 2013;37:952–960. doi: 10.1111/acer.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman ML, Hochgraf PB, Andrade AG. Gender differences in treatment-seeking Brazilian drug-dependent individuals. Subst Abus. 2003;24:17–25. doi: 10.1080/08897070309511530. [DOI] [PubMed] [Google Scholar]