Abstract
Purpose
To compare cerebrovascular reactivity (CVR) quantified with pseudo-continuous arterial spin labeling (pCASL) and blood oxygen level dependent (BOLD) fMRI techniques.
Materials and Methods
Sixteen healthy volunteers (age: 37.8±14.3 years; 6 women and 10 men; education attainment: 17+2.1 years) were recruited and completed a 5% CO2 gas-mixture breathing paradigm at 3T field strength. ASL and BOLD images were acquired for CVR determination assuming that mild hypercapnia does not affect the cerebral metabolic rate of oxygen. Both CVR quantifications were derived as the ratio of the fractional cerebral blood flow (CBF) or BOLD signal change over the change in end-tidal CO2 pressure.
Results
The absolute CBF, BOLD and CVR measures were consistent with literature values. CBF derived CVR was 5.11 ± 0.87%/mmHg in gray matter (GM) and 4.64 ± 0.37%/mmHg in parenchyma. BOLD CVR was 0.23±0.04 %/mmHg and 0.22±0.04 %/mmHg for GM and parenchyma respectively. The most significant correlations between BOLD and CBF-based CVRs were also in GM structures, with greater vascular response in occipital cortex than in frontal and parietal lobes (6.8 %/mmHg versus 4.5 %/mmHg, 50% greater). Parenchymal BOLD CVR correlated significantly with the fractional change in CBF in response to hypercapnia (r=0.61, P=0.01), suggesting the BOLD response to be significantly flow driven. GM CBF decreased with age in room air (-5.58 mL/100g/min per decade for GM; r=-0.51, P=0.05), but there was no association of CBF with age during hypercapnia. A trend toward increased pCASL CVR with age was observed, scaling as 0.64 %/mmHg per decade for GM.
Conclusion
Consistent with previously reported CVR values, our results suggest that BOLD and CBF CVR techniques are complementary to each other in evaluating neuronal and vascular underpinning of hemodynamic processes.
Keywords: Cerebrovascular reactivity, BOLD, Arterial spin labeling, Cerebral blood flow, Neurovascular coupling, hypercapnia-based fMRI calibration
1. Introduction
1.1 Cerebrovascular Reactivity
Cerebrovascular reactivity (CVR) describes the compensatory dilatory capacity of cerebral vasculature in upregulating perfusion. An inadequate dilatory response contributes to a higher risk of stroke and other ischemic injuries and is implicated in many pathologic conditions, such as diabetes mellitus and carotid artery disease [1-3]. Historically, CVR has been quantified by measuring the change of cerebral blood flow (CBF) during physiologic stress, using techniques such as MR phase-contrast/angiography, positron emission tomography, and Doppler sonography [4-6]. Some drawbacks of these methods include side effects from contrast agents and poor reproducibility. Alternatively, CVR is calculated as the relative difference between the challenge-induced absolute CBF or blood oxygen level dependent (BOLD) signal change relative to baseline levels using relatively new MRI sequences [7]. Advances in multi-parametric functional neuroimaging have allowed quantification of challenge-induced CBF together with BOLD signal change [8, 9]. Additionally, such quantification allows for measurement of CVR by way of comparing pre- vs. post-stress blood flow changes [10]. By examining the effects of hypercapnia on the physiologic perfusion baseline, a model of CVR assessment can be developed, with or without functional stimulus [8, 11].
1.2 BOLD fMRI
The BOLD effect is the basis of most fMRI methods, which exploit the increase in T2* during functional activation resulting from a fractional increase in intracellular oxyhemoglobin. The increased metabolic demand induces a greater than required increase in CBF, leading to a transient decrease in the deoxyhemoglobin to oxyhemoglobin ratio, which manifests as the BOLD effect [12]. Measurements of CVR based on breath-holding (BH) and hyperventilation have produced interesting results that could better define the biophysical origins of hemodynamics [13-15]. However, the tasks usually require subjects to be co-operative and cued and results are also prone to intra-subject variability. Further, BH may induce a mixed hypercapnic-hypoxic state during which the CMRO2 level is not constant [16]. Quantification of CVR based on well-validated hypercapnic BOLD measurement alone has been reported recently, with a slight drop of CMRO2 levels observed during hypercapnia [17, 18]. Since the BOLD signal change in response to increased CO2 is relatively small (1-2%), a block design with several repetitions interleaving hypercapnia (similar to task condition) and resting state (baseline) has been used to enhance signal to noise ratio (SNR) [19]. In the present study, BOLD is used for quantifying CVR under the assumption that mild hypercapnia does not change CMRO2 [20].
1.3 Arterial Spin Labeling (ASL) fMRI
Arterial spin labeling (ASL) fMRI is a noninvasive method for measuring CBF by using water in arterial blood as an endogenous tracer [21]. Since its introduction, ASL has played a key role for assessing CBF in that it has several advantages over other methods. For instance, because blood is being used as the tracer, ASL is a direct gauge of CBF. Furthermore, the quick magnetization decay allows for a relatively short scan time. Moreover, ASL expresses arterial perfusion whereas BOLD has been argued to be a largely venous phenomenon [22]. The reliability of ASL-based measurement of CBF has been shown previously [23]. Conventionally, two techniques are used: continuous ASL (cASL), where labeling is achieved with continuous radiofrequency (RF) irradiation, and pulsed ASL (pASL), where short RF pulses are used. Both techniques have limitations, in that cASL has low labeling efficiency in return for high SNR whereas pASL has lower SNR but high labeling efficiency. Pseudo-continuous ASL (pCASL), conceived more recently, uses a train of RF pulses mimicking continuous labeling [24], combining the advantages of both methods. Further, pCASL, unlike CASL, can be implemented on clinical scanners. Moreover, pCASL has been reported to be superior to pASL for measuring CO2 induced CVR due to improved label timing control [24]. Recently, pCASL has been applied for mapping oxygen extraction fraction (OEF), CMRO2, and CVR under hypercapnic and hyperoxic conditions [13, 25]. pCASL-based CBF values have been reported to reach similar precision to standard PET-based CBF values at both baseline and during hypercapnia [26].
1.4 Coupling between BOLD and CBF Signals
While ASL quantifies perfusion (and thus the change in perfusion in response to a stimulus), BOLD measures the combined effect of changes in blood flow, blood volume and metabolism in response to a stimulus. Therefore, the comparison of CBF and BOLD CVR measures could provide insight into the physiologic processes underlying fMRI (e.g. spatiotemporal dynamic brain reserve and plasticity)[27]. Integration of the two techniques into a single scan session is straightforward, and had been implemented under hypercapnia and/or hyperoxia calibration studies [28]. Coupling between direct CBF and the BOLD signal, as well as resulted CBF and BOLD CVR changes under hypercapnia, have been investigated in various animal and human studies [8, 16]. Using a vessel-encoded ASL technique with simultaneous acquisitions of BOLD and CBF data, a close correlation has further been reported during hyperoxia [29], though with lower sensitivity in BOLD reactivity compared to CBF reactivity.
Several recent studies have studied the close association between CBF and BOLD changes in normal subjects with aging and in response to carbogen condition [30, 31], as well as in patients with moyamoya disease [32]. Moderate to strong associations have been reported between CVR values obtained using ASL and BOLD in steno-occlusive disease patients [7]. The regional dependence of CVR measured by BOLD and CBF in healthy subjects, however, has not been studied extensively, with inconsistent results using the two techniques reported previously [30, 31]. For example, the uncoupling of pCASL and BOLD signal is suspected to be secondary to the blood volume effect as previously discussed [7]. That is, the increase in CBV is believed to increase the fraction of the imaging voxel occupied by blood, reducing the BOLD signal. This effect is likely minor, however, as Kastrup et al. concluded that in normal subjects, the BOLD response to hypercapnia is more highly dependent on the flow effects and not blood volume effects [33]. On the other hand, Leoni et al. found that BOLD CVR cannot be inferred from resting baseline CBF directly [16]. Earlier work of measuring both BOLD and ASL simultaneously [34] had revealed significant spatiotemporal (e.g. layer specific and dependent) brain physiological processes with the largest CBF changes occurring in layers IV-V but lagging peak BOLD change in layers I-III in rats. Previous CBF studies with 15O PET and BOLD fMRI under hypercapnia found the fMRI BOLD CVR to be strongly correlated with the PET CBF CVR, albeit with different temporal profiles in gray matter (GM) and white matter (WM) [5]. As there is no “gold standard” measure of CVR [35], understanding the sensitivity and relative agreement of pCASL and BOLD CVRs at the voxel-wise, region of interest (ROI) and whole-brain level is needed.
Investigating the hypercapnia-induced CVR characteristics could also provide physiological clue to the underlying neurovascular mechanism in disease conditions. In this study, BOLD and pCASL measurements were made in room air and during hypercapnia (5% CO2) challenge to quantify CVR and to compare the functional parameters derived with the two methods. Other factors such as age and brain volume size were also recorded.
2 Materials and Methods
2.1 Subjects
A total of 16 healthy volunteers (mean age: 37.8 ± 14.3 years and range: 20-62 years; 6 women and 10 men; education attainment: 17 ± 2.1 years) were recruited. None of the subjects had a diagnosis of cardiovascular or cerebrovascular morbidities. There was no history of medication use or occupational exposure to CO2. After the institutional review board approval, the subjects were consented regarding the nature and risks of the procedure. For internal reliability control, three subjects repeated the scans with the same protocol one month after the initial scan.
2.2 Implementation of hypercapnia challenge for vascular reactivity measurements
Subjects were briefed on the hypercapnia protocol before the scan. A 10-minute rest period involving inactivity and restriction of verbal communication was instituted to prevent pre-challenge fatigue. During the experiment, the subjects were asked to breathe through an oral breathing tube with a mouthpiece and fitted with nose clips to prevent nasal breathing. The breathing tube was either open to the room air (normocapnia, breathing freely through tube) or supplied with the hypercapnic gas mixture (5% CO2, 74% N2, and 21% O2). The latter was contained in a Douglas bag and delivered to the subjects through a two-way non-rebreathing valve (Hans Rudolph, Shawnee, KS), with full amount of mixed gas in the bag to ensure constant air delivery with rate of approximately 1 L/min.
The breathing paradigm consisted of a block design of alternating conditions (normocapnia vs. hypercapnia) (Figure 1). The subjects were instructed to lay still and focus on breathing through the tube only, without thinking about any particular topic. End-tidal CO2 pressure (PETCO2, in mmHg) and respiratory rates (beats per minute, Bpm) were monitored and recorded to ensure a steady state/plateau was reached using the Medrad 9500 Multi-gas monitor (Medrad Inc, Indianola, PA). Continuous video recordings were made for quality control. The CVR study started with BOLD sequence, followed by pCASL, for a subtotal of 15 minutes. For the BOLD paradigm, breathing gas was toggled between CO2 and room air alternatively every minute. For pCASL, hypercapnic gas was switched on once after the baseline pCASL finished. A one-minute T1 scan was run between baseline and hypercapnia of pCASL acquisitions to allow the PETCO2 to stabilize.
Figure 1.

Experimental paradigm of sequential BOLD and pCASL acquisitions. The whole experiment, including subject setting and other MRI scans before CVR quantification such as phase-contrast MRI and 3D T1 for global CBF measurements, took about 45 minutes.
For the BOLD protocol, a study team member stayed in the magnet room throughout the experiment to switch the valve. Gas mixture breathing started at 16 seconds, with six alternating blocks (3 experimental and 3 baseline, 7 minutes and 8 seconds total) (Figure 1). For the last block, the subject breathed room air until the conclusion of the scan. For the pCASL protocol, each block lasted 3 minutes and 35 seconds. Two blocks (one baseline and one experimental) were run for a total of 7 minutes and 10 seconds (Figure 1). After completion of the first block a member of the research team entered the scan room to manually switch the valve to enable breathing the gas mixture.
2.3 Imaging Protocol
The imaging protocols consisted of pCASL, BOLD, high-resolution 3D T1, and MR angiography (MRA) sequences. All images were performed on a 3T whole-body MR scanner (Siemens Magnetom Tim Trio; Siemens Healthcare, Erlangen, Germany) using a body transmit coil and 12-channel SENSE head receiver coil.
BOLD CVR data were acquired with a gradient echo EPI sequence (TR/TE: 2 sec/25 msec, FA: 70°, FOV: 220 × 220 mm2, matrix size: 74 × 74). A total of 35 axial slices (3 mm thick with 0.6 mm gap) were collected parallel to the anterior-posterior commissure, covering the entire brain. T1-weighted high-resolution spin-echo anatomic images with matching geometrical parameters were acquired for the measurement of brain volume (TR/TE: 349 msec/2.42 msec, FA: 70°, voxel size: 0.43 × 0.43 × 3.6 mm3, acquisition time: 89 seconds) for purposes of registration and normalization of global blood flow to brain volume.
For pCASL, a balanced labeling method was implemented with mean gradient strength of 0.6 mT/m and a total of 1640 RF pulses (560μsec duration and 360μsec gap with 920 μsec inter-pulse interval, phase between two RF adjusted), resulting in a total labeling duration of 1.5 seconds. Labeling was performed 97 mm below the center of the imaging region. A post-labeling delay of 1.23 seconds was chosen to maintain sufficient temporal resolution and to allow the labeled blood to enter the imaging section. A multi-slice gradient-echo EPI readout was used (TR/TE: 3.9 sec/17 msec, FA: 90°, FOV: 220 × 220 mm2, matrix size: 64 × 64). 24 slices (5 mm thickness) were collected with the same orientation as for the BOLD scan, covering the entire brain. Each pCASL exam consisted of 26 tag/control pairs and took 215 seconds. Between two pCASL sequences, a 2D T1-weighted high-resolution spin-echo anatomic image data set with matching geometrical parameters was acquired for registration and segmentation (TR/TE: 320 msec/2.42 msec, FA: 70°, voxel size: 0.9 × 0.9 × 1 mm3, acquisition time: 78 sec).
In addition to pCASL, global CBF was measured during baseline, with a single-slice phase-contrast (PC) sequence (TR/TE: 35.2 msec/5.18 msec, FA: 15°, voxel size: 0.45 × 0.45 × 5 mm3, maximum velocity encoding = 80 cm/sec) to provide an alternative global CBF measurement for labeling efficiency calculation. Ten averages were acquired, resulting in scan duration of 1.5 min. The PC slice was placed 2mm distal to the left carotid artery bifurcation and oriented perpendicular to the internal carotid and vertebral arteries, allowing simultaneous assessment of the four feeding vessels [36]. The slice was positioned based on a time-of-flight MRA sequence (TR/TE: 17 msec/4.12 msec, FA: 18°, voxel size: 1 × 1 × 2.5 mm3, 40 slices, one saturation band placed above the imaging slab, acquisition time: 2.9 min). A posterior neck coil was employed to improve spatial coverage and SNR. The entire protocol, including positioning and hypercapnia paradigm, took approximately 45 minutes.
2.4 Data Processing and Statistical Analysis
2.4.1 ASL CVR Computation
After performing motion correction for the label and control image series separately, CBF was calculated on the basis of the two-compartment perfusion model by Alsop and Detre [37], using equation [1] [23, 38].
| [1] |
where ΔM is the signal difference between control and labeling states,
λ = 0.9 g/mL is the blood/tissue water partition coefficient,
α = 0.85 is the inversion tagging efficiency of pCASL at 3T [39, 40],
M0 is the equilibrium magnetization,
T1b = 1.493 sec, is the commonly-used T1 constant of blood at 3T [39],
ω = 1.23 sec, is the post labeling delay in addition to the slice acquisition delay, which is different for each individual slice, and
τ = 1.5 sec is the labeling duration.
The equilibrium magnetization, M0, was determined from a whole brain region of interest (ROI) using control condition images with equation [2]:
| [2] |
where T1 (tissue) = 0.93 sec [41].
The final mean CBF was derived by averaging across all 26 tag and control image pairs. The percent change of CBF was then scaled relatively to the change in end-tidal CO2 pressure (ΔPETCO2) for each subject as in Equation [3] [25]. PETCO2 values at baseline and hypercapnia were obtained by averaging measurements sampled every 30 seconds, and CVR was calculated as
| [3] |
An individualized tagging efficiency was also calculated as the ratio of the average brain parenchymal CBF measured at baseline to the global CBF value based on the PC sequence.
Preprocessing of pCASL data included re-alignment of the EPI series for control and labeling series, smoothing with 6mm full width at half maximum (FWHM) Gaussian kernel and masking of brain-only regions using minimum threshold of 10% of maximum of the whole brain
For global and regional CVR estimation, the T1-weighted 2D image was first segmented into GM, WM, and cerebrospinal fluid (CSF), and then co-registered to mean pCASL EPI image with the Statistical Parametric Mapping package (SPM8, University of College London, UK; www.fil.ion.ucl.ac.uk/spm). Average CBF in the GM as well as parenchyma (combined GM and WM) were obtained based on the 3 tissue-type masks generated from co-registered T1 image. The global CVR of the GM and parenchyma were then calculated from equation [3]. WM CBF and CVR were not reported due to low sensitivity of CBF signal in WM.
The averaged CBF image of each subject was warped to the Montreal Neurological Institute (MNI) template space in SPM8 based on T1-weighted image. A CVR map in the template space was generated by calculating CVR as the ratio of the fractional change in CBF relative to ΔPETCO2 for each voxel. Group-wise one-sample paired t-test was performed to compare CBF at baseline and hypercapnic conditions with ΔPETCO2 as co-variant. The CVR T significance distribution map across subjects at cluster-based ROI levels was generated, with statistical adjustment for multiple comparisons to control for family-wise error. Clustering results based on voxel-wise comparison from paired t-test were reported in regions identified from the automated anatomical labeling template [42]. The regional and lobar mean CVRs were calculated with scripts developed based on the FSL toolbox (http://fsl.fmrib.ox.ac.uk/fsl, version 4.1.2) and Matlab-based SPM8 software, with well-established Brodmann Area (BA) parcellations [43].
2.4.2 BOLD CVR Computation
Similar preprocessing was done in SPM, including realignment of EPI series to remove movement artifacts and 6mm FWHM Gaussian smoothing. A representative time course was derived from the whole brain regions over three contiguous slices which centered at the thalamus, to obtain optimal temporal matching of averaged BOLD time course and measured PETCO2. The thalamic region was chosen because BOLD signal arrival time in the center brain slices is expected to be representative of the whole brain regions. In conventional BOLD-based fMRI studies, an optimal match between the BOLD signal and the reference function that models the hemodynamic function is found by temporally shifting the reference functions [44] to obtain the largest BOLD signal change. In this study, similar shifting was done to account for the physiologic delay between gas mixture breathing initiation and BOLD signal change induction. A model with PETCO2 as a physiological parameter was adapted from Yezhuvath et al. [45]. The optimal delay was calculated by shifting the re-sampled PETCO2 to best correlate with the BOLD signal. The shifted PETCO2 trace with the best-estimated delay was then used as the input function in the calculation of CVR.
To calculate BOLD CVR, the standard voxel-wise general linear model (GLM) analysis in SPM was applied to the pre-processed BOLD images [45], as outlined in equation [4]:
| [4] |
where SBOLD(t) is the BOLD time course at each voxel during the whole experiment,
PETCO2(t) is the shifted resampled end-tidal CO2 trace signal that is used as a reference function to predict the BOLD signal,
ε is the linear constant term which is modeled as white Gaussian noise in GLM fitting, and CVRBOLD is the coefficient that reflects the associated BOLD signal change.
Global CVR scaling was done for each subject to reduce inter-subject CVR variability [45]. In performing regional and global cortical CVR quantification, the CVR map was then co-registered to the T1 image space and warped to the standard MNI template. Similar ROI analysis as in CBF CVR analysis was applied to derive regional average BOLD CVR. The experimental paradigms for pCASL and BOLD sequences were dictated by differences in inherent SNR between the two sequences, which had to be taken into consideration for processing of the data as well. To compute ASL CVR, the data representing the two conditions were directly subtracted and PETCO2 scaling was applied. To obtain BOLD CVR, GLM regression analysis was performed with continuous PETCO2 signal serving as reference function. For ASL, the timing difference of each slice was calculated and corrected during the quantification of the CBF signal [46], while for the BOLD data an optimal time-matched PETCO2 between the BOLD time course averaged over center slices and PETCO2 trace was used as reference.
2.4.3 Correlations between CVR data measured with pCASL and BOLD
For voxel-wise correlation analysis, the CVR maps measured with pCASL and BOLD for each subject in the MNI space were concatenated, and correlation test of each voxel across subjects was performed using the Analysis of Functional NeuroImages (AFNI, http://afni.nimh.nih.gov/afni, version macosx 10.5) command 3dTcorrelate to derive the correlation coefficient Rho for each voxel. Both normal Pearson and non-linear (e.g. quadrant) correlations with 1st to 3rd polynomial trend removal parameters were tested. The Rho value was then transformed to a z-value using Fisher Z-transformation and finally converted to z-score statistics. Results were corrected at the cluster-level (minimum Z > 2; cluster level, P <0.05) to adjust for multiple comparisons on the basis of Gaussian random field theory by using FSL easythresh command.
The pCASL and BOLD CVR values in the whole brain and for each ROI were computed and compared with the template parcellations as previously described.
Additionally, brain volume was computed from the high-resolution T1 images for each subject using the ROBEX software [47], and segmented into GM, WM, and CSF. The normalized brain parenchymal ratio (BPR), an index of normalized brain tissue size, was computed as the ratio of the volume of parenchyma to the volume of the whole brain (parenchyma plus CSF). For regression analysis, the Pearson correlation coefficient was computed between age or global brain volume, and CVR derived from BOLD or pCASL in GM, lobar regions, and over the entire parenchyma.
3. Results
3.1 ASL CVR
The repeat examinations performed on the three subjects yielded an absolute CVR difference relative to the initial scans of 5%. Baseline CBF (room air) was weakly correlated with PETCO2 (r = 0.55, P = 0.027 for GM) (Figure 2A). The fractional change in CBF from baseline to hypercapnia correlated significantly with the change in PETCO2 (r = 0.7, P <0.005) (Figure 2B). Furthermore, there was a significant correlation between the parenchymal CBF measured with pCASL in room air and global CBF measured by phase-contrast MRI (r = 0.62, P = 0.007). The tagging efficiency calculated as ratio of parenchymal CBF scaled by the global CBF with phase-contrast MRA at baseline was 0.842 ± 0.021 across all subjects, consistent with the empirical value of pCASL sequence (0.85) used at 3T.
Figure 2.
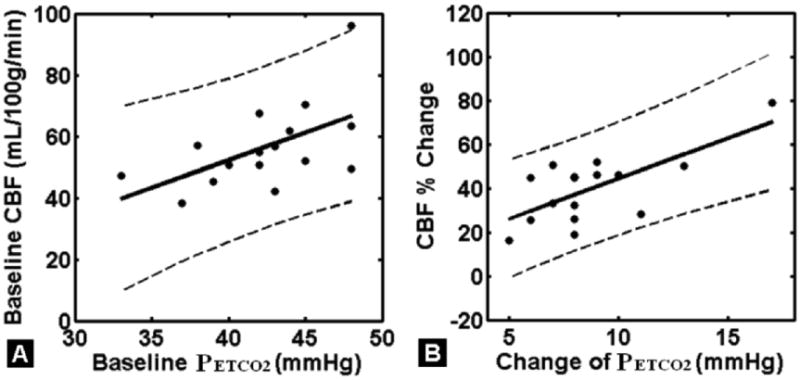
A: Significant correlation between average GM CBF and PETCO2 at baseline room air condition in control subjects (r=0.55, P=0.027). B: Significant correlation between average parenchymal CBF percentage change and change of PETCO2 (ΔPETCO2) at hypercapnia from room air (baseline) condition in healthy control subjects (r=0.70, P<0.005). The dashed lines represent 95% lower and upper confidence intervals of the linear correlation.
Group-wise average of CBF maps at baseline and during hypercapnia are shown in Figures 3A1 and A2, respectively. As expected, elevated CBF (i.e., positive absolute CBF differences subtracting baseline CBF from hypercapnia CBF) is observed in most cerebral regions under hypercapnia (Figure 3A3). Figure 3 B1 shows group-wise mean whole-brain CVR after correcting for change in end-tidal CO2 pressure (ΔPETCO2) for each subject. Large clusters from all cortical lobes and thalamic area showed consistent pattern of CVR after a strict family-wise error correction using 1-sample paired t-test, with T > 6.29 and cluster size K > 5 (Figure 3 B2).
Figure 3.
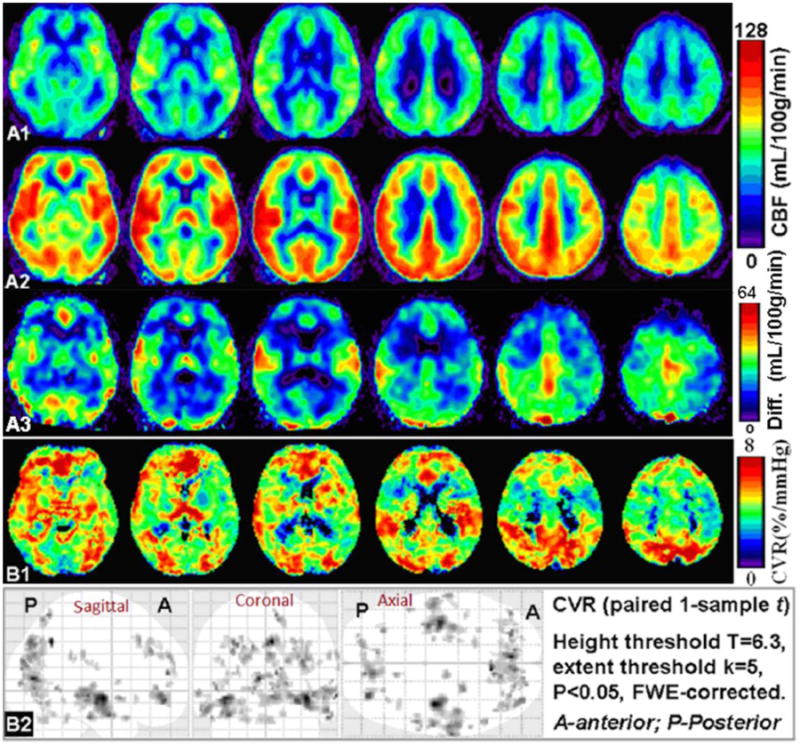
Group average of CBF in MNI space at baseline (room air, A1) and hypercapnia (A2). The absolute difference of average CBF map comparing hypercapnia to baseline was shown in A3. CBF was elevated significantly in most cerebral areas during hypercapnia than in room air (A3). B1: Group average of CBF derived CVR (in %/mmHg) calculated as fractional CBF change during hypercapnia relative to PETCO2 change. B2: Group-wise 1-sample paired t-test of pCASL CVRs showing consistent large clusters of significant CBF changes (i.e. CVR) across subject, in somatosensory, occipital, temporal, parietal and frontal regions after application of a comprehensive threshold with family-wise error (FWE) correction (T>6.29 and cluster size K>5).
Absolute mean CBF values were obtained by averaging over each tissue type with an empirical minimum threshold of 10% of maximum of the whole brain. The group means of global CBF from parenchyma, GM and fractional change due to hypercapnic challenge are shown in Figure 4A. The mean fractional change of CBF during hypercapnia was 42.5 ± 6.1 % in GM, with a mean parenchymal factional change of 39.9 ± 3.9 %. The mean ΔPETCO2 across subject was 8.75 ± 0.74 mmHg. The global mean CVR calculated based on global CBF in GM and ΔPETCO2 was 5.11 ± 0.87 %/mmHg and mean global parenchymal CVR was 4.64 ± 0.37 %/mmHg. The brain clusters showing consistent pCASL CVR patterns across group using a paired 1-sample t-test with ΔPETCO2 as a co-variant (family-wise error corrected P < 0.05, cluster size ≥ 100), including middle frontal, middle occipital, temporal and subcortical regions (e.g. thalamus and hippocampus) are listed in Table 1.
Figure 4.
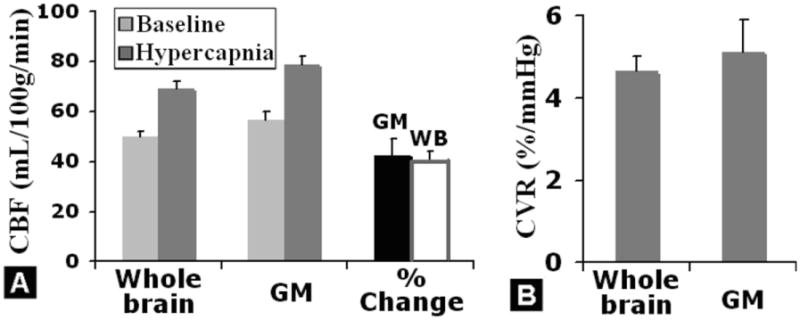
A: Quantitative pCASL-based average CBF in room air (baseline) and hypercapnia, for the whole brain (WB) parenchyma and gray matter (GM). CBF values were obtained by averaging over each tissue type with an empirical minimum threshold of 10% of maximum of the whole brain. During hypercapnia, the CBF in whole brain and gray matter was significantly higher than CBF at baseline, with an approximately 40% increase, as shown in the rightmost column in A. B: Average pCASL CVR in gray matter and whole brain parenchyma across subject.
Table 1.
Brain regions of significant pCASL CBF CVR changes comparing blood flow differences under CO2 hypercapnia to room air across group using paired 1-sample t-test with ΔPetCO2 as a co-variant (family-wise error corrected P<0.05, cluster size ≥ 100). Brain regions were identified based on the automated anatomical labeling template, and their corresponding cytoarchitecture-based Brodmann areas were also reported.
| Brain Region | BA | X,Y,Z, MNI mm | Cluster size | Max. Z Score |
|---|---|---|---|---|
| Right middle frontal /anterior cingulum | 11 | 8, 40, -2 | 1593 | 6.72 |
| Left middle occipital | 18 | -26, -86, 18 | 5811 | 6.66 |
| Right superior temporal /insular | 48 | 64, 2, 6 | 904 | 6.16 |
| Right middle temporal | 21 | 62, -14, -18 | 268 | 6.09 |
| Right thalamus | - | 4, -14, 6 | 267 | 5.95 |
| Right parahippocampus | 37 | 32, -30, -12 | 147 | 5.93 |
| Right medial-superior frontal | 9 | 16, 36, 46 | 119 | 5.84 |
| Right hippocampus | 20 | 38, -12, -10 | 223 | 5.75 |
Note: BA - Brodmann area, MNI – Montreal Neurological Institute convention.
3.2 BOLD CVR
The time course of BOLD CVR data is given in Figure 5A for a study subject. The average maximal correlation coefficient between PETCO2 and BOLD signal was about 0.9, indicating a strong association between physiologic and imaging parameters (Figure 5B). The mean latency between the BOLD signal averaged over three contiguous brain slices centered at the thalamus, and PETCO2 was about 6 seconds (Figure 5C), subtracting 5 seconds of gas sensor delay (mouth piece to the capnometer). The mean fractional BOLD signal percentage change during hypercapnia was 2.0% ± 0.3%. Whole-brain group averaged parametric images of BOLD CVR using optimal shifted resampled PETCO2 as a reference function for each individual is shown in Fig. 6 A&B, with quantitative group mean values shown in Fig. 6C (GM: 0.23 ± 0.04%/mmHg and whole-brain parenchyma: 0.22 ± 0.04%/mmHg).
Figure 5.
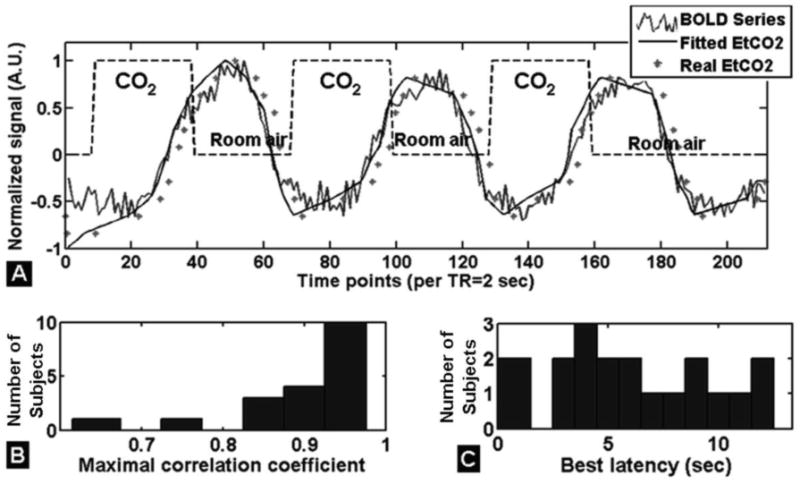
Time course of BOLD signal changes in response to a hypercapnic challenge interleaved with room air and averaged in the brain regions over three contiguous slices (slices centered at the thalamus) of a representative subject (A). The delay (i.e. latency) that best matched the BOLD signal to PETCO2 was obtained by shifting the PETCO2 signal to maximize the correlation between the two signals. B: Histogram of maximal correlation coefficient showing good match between BOLD and PETCO2 with an average of 0.9 for all subjects. C: Histogram of latency between BOLD and PETCO2 signal in all subjects, with an average delay of 6 seconds (including correction for the exhaled CO2 to reach the capnometer).
Figure 6.
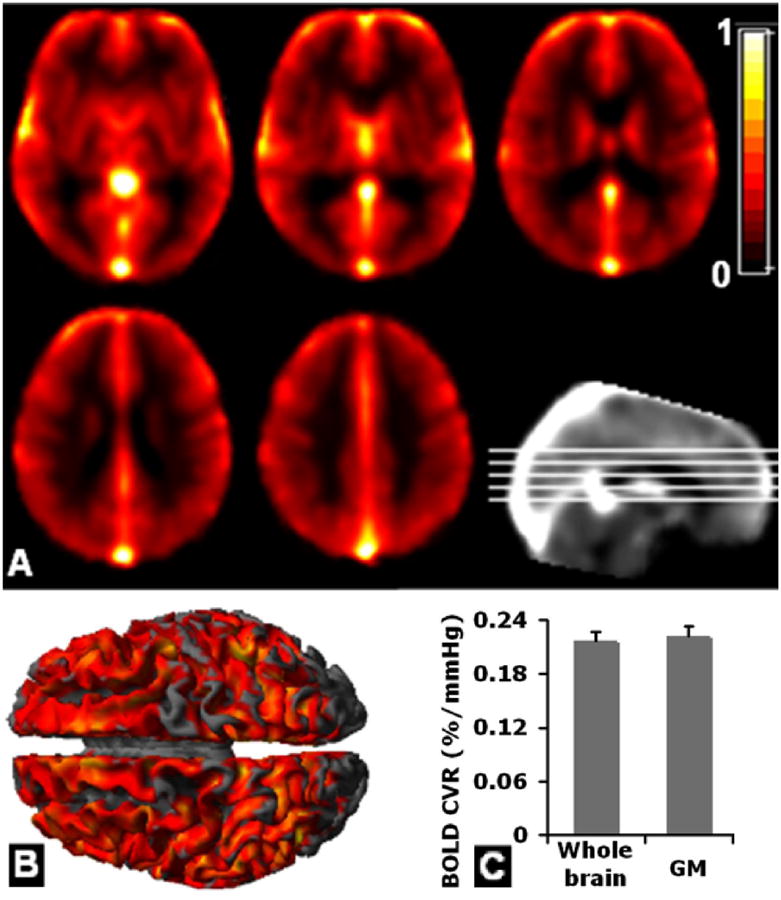
A: Group average of BOLD CVR (%/mmHg) parametric images obtained using shifted resampled PETCO2 as a reference function with general linear model. B: Smoothed 3D volume-rendered sagittal view (1 sample t-test, family wise error corrected P<0.05). C: Average whole-brain BOLD GM and parenchymal CVR.
Regional BOLD CVR obtained by comparing BOLD signal difference and using ΔPETCO2 as input function for regression analysis (family-wise error corrected P < 0.00001, cluster size ≥ 200), including superior temporal, cingulate and postcentral regions, are listed in Table 2. Only the left middle occipital region (highlighted in gray in Table 2) showed consistent CVR patterns for the two techniques after multiple comparisons were accounted for.
Table 2.
Brain regions of significant BOLD CVR changes comparing BOLD signal differences under CO2 hypercapnia to room air across group using ΔPetCO2 as input function for regression analysis (family-wise error corrected P<0.0001, cluster size ≥ 200). A more stringent threshold was used for BOLD signal in this table compared to pCASL signal in Table 1 due to less inter-subject variation and possibly more smoothing effects in BOLD signal. The cluster that survived in both techniques with cluster-wise threshold, namely left middle occipital region, is highlighted in Table 1 and Table 2.
| Brain Region | BA | X,Y,Z, MNI mm | Cluster size | Max. Z Score |
|---|---|---|---|---|
| Left superior temporal /insular | 48 | -32, -6, -10 | 5712 | 7.59 |
| Left postcentral / inferior parietal | 2 | -24, -40, 68 | 437 | 7.22 |
| Left superior temporal | 42 | -56, -40, 16 | 214 | 7.00 |
| Left middle occipital | 18 | -30, -88, 14 | 442 | 6.96 |
| Right precentral | 6 | 36, -10, 62 | 233 | 6.95 |
| Left posterior cingulum | 26 | -10, -38, 40 | 241 | 6.84 |
| Right middle cingulum | 23 | 6, -36, 36 | 217 | 6.77 |
| Right paracentral lobule | 4 | 4, -26, 70 | 443 | 6.75 |
| Right postcentral | 3 | 38, -28, 48 | 217 | 6.56 |
Note: BA - Brodmann area. MNI – Montreal Neurological Institute convention.
3.3 Associations between ASL and BOLD CVR
Whole brain fractional CBF change correlated significantly with BOLD CVR (r = 0.61, P = 0.01, Figure 7A). After PETCO2 scaling, a trend of positive correlation was found between CBF and BOLD parenchymal CVRs (r=0.43, P=0.09) (Figure 7B). Based on voxel-wise analysis, significant positive correlations between the two CVRs are shown in Figure 8A (minimum Z > 2; cluster level, P < 0.05, corrected by the Gaussian random field theory after controlling for voxel-wise multiple comparisons). These correlations are based on the quadrant correlation with 3rd order polynomial detrending, which yielded the largest number of significantly correlated voxels between pCASL and BOLD CVR measures. The most significant correlations between BOLD and CBF-based CVRs were in GM structures, with greater vascular response in occipital cortex than in frontal and parietal lobes (6.8 %/mmHg versus 4.5 %/mmHg, 50% greater). Regional results from the major cortical lobes, subcortical regions, left and right hemisphere are shown in Figure 8B. The occipital lobe showed highest BOLD CVR (approximately 0.33 %/mmHg), followed by parietal lobe. The temporal lobe, especially the Wernicke's area, showed slightly higher pCASL CVR (approximately 5.1 %/mmHg) compared to other brain regions, followed by occipital lobe. There was no significant difference between left and right hemispheres for the measured CVR (P > 0.5), although pCASL CVR was slightly higher (4.4%) in the right hemisphere than the left hemisphere (Figure 8B). Note that although most brain regions showed consistent values between two CVRs after scaling (Figure 8A), the regional variation of CVRs still existed. For instance, the BOLD CVR was highest in the occipital lobe, with a higher degree than ASL CVR by comparing the ratio of occipital lobe BOLD CVR to BOLD CVRs of other brain regions.
Figure 7.
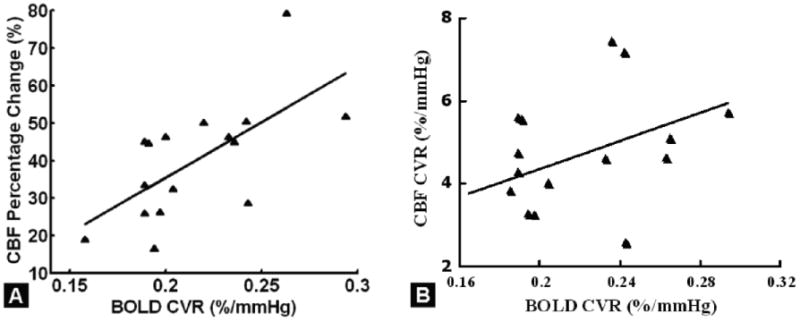
A: Significant correlation between fractional percentage change in CBF in response to hypercapnia and BOLD CVR (r=0.61, P=0.01) in parenchyma. B: A trend of positive correlation between two parenchymal CVRs after PETCO2 normalization (r=0.43, P=0.09).
Figure 8.

A: Voxel-wise correlation between pCASL and BOLD CVR (minimum Z > 2; cluster level, P <0.05, corrected by Gaussian random field theory) using non-linear (2nd order polynomial) correlation after high-order detrending. B: Regional data, displayed after scaling BOLD CVR by a factor of 22 to match average pCASL CVR. Note: bilateral lobar and sub-regional areas were defined with well-established parcellations by combining BA regions. Frontal cortex = BAs 4, 6, 8, 9, 10, 11, 44, 45; Motor region = BAs 4, 6 and 8; Prefrontal region = BAs 9, 10, 11; Broca's area = BAs 44, 45. Parietal cortex = BAs 1, 2, 3, 5, 7, 39, 40; Somatosensory area = BAs 1, 2, 3. Temporal cortex = BAs 21, 22, 41, 42, 37; Wernicke's area =BAs 21, 22, 42. Visual cortex = BAs 17, 18, 19. Subcortical area =caudate, thalamus, putamen, pallidum, hippocampus, amygdala, accumbens and brainstem. Left and right hemisphere contain combined lateral BA regions from four lobes specified.
Additional global significant correlations included negative correlations between the average baseline GM CBF and subject age (both r = -0.51, P = 0.05), but no association of CBF with age during hypercapnia. GM CBF decreased with age in room air with a rate of − 5.58 mL/100 g/min per decade. The resulted GM pCASL CVR correlated with subject age (r = 0.55, P = 0.044) (Figure 9), with a slight increment of 0.64 %/mmHg per decade for GM CVR. The BOLD CVR, on the hand, showed only a negative trend with aging (r = -0.27, P = 0.32). No other significant correlations were observed between regional pCASL or BOLD CVRs and age (P=0.21-0.98). A negative trend between the GM ASL CVR and GM volume across subjects (r = -0.4, P = 0.12) was also found.
Figure 9.
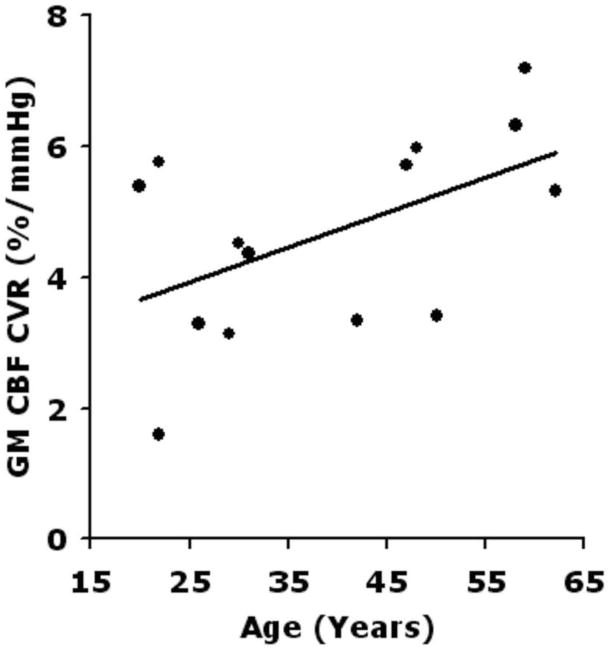
Association between average gray-matter (GM) pCASL CVR and subject age (r=0.55, P=0.044) after excluding two subjects with GM CVR values out of the range of one standard deviation from the group mean of GM CVR.
4. Discussion
4.1 Summary of major findings
Several findings suggest reliability of the independently obtained BOLD and pCASL measures. As expected, there was a significant increase in CBF under hypercapnia (43% increment in GM and 40% in parenchyma). The CBF and normalized CVR values based on pCASL (5.1 %/mmHg in GM and 4.6 %/mmHg in parenchyma) are consistent with values reported in current literature [23, 25, 30]. The tagging efficiency of pCASL with subject breathing room air (0.85) is also consistent with empirical value [48]. The BOLD signal change and CVR distribution maps, as well as regional CVR measurements, are in agreement with previously reported values, including observation of the highest BOLD CVR (not ASL CVR) in the occipital lobe [44, 49]. For instance, Kastrup et al. [33] and Yezhuvath et al. [45] have reported a trend of higher BOLD response in the occipital lobe than other brain regions. The measured left and right hemisphere CVR values were almost identical, further suggesting the robustness of the two techniques applied. The mean CVR delay from the PETCO2 was about 11 seconds, consistent with the reported values [50]. The slightly higher global pCASL CVR in the right hemisphere and significant regional pCASL CVR in most right-sided regions are consistent with reported values [30].
4.2 BOLD vs. pCASL CVR
The two voxel-wise CVRs were correlated more prominently with a non-linear than linear association in GM structure. Interestingly, the BOLD CVR at whole brain level was more significantly associated with fractional CBF change than the CBF CVR with PETCO2 scaling, suggesting BOLD response to be significantly flow driven. ROI-based correlation tests and quantification showed BOLD and pCASL CVRs were associated more in the frontal and temporal regions, but differed in the parietal and occipital regions. The regional differences between BOLD and pCASL CVRs, which lead to decreased correlation between two CVRs, are thought to come from distinct physiology as well as different process strategies of two techniques. For example, the distinctive dense and rich micro-capillary vascular architecture in occipital cortex [33] might lead to significantly higher BOLD CVR in occipital regions with a less significant affect on ASL CVR, as found in our results. Other physiological parameters such as region-specific neurochemical modulation of neurovascular coupling effects (e.g. nitric oxide bioavailability and reactive oxygen species) are believed to contribute to the difference in coupling values in the results as well [51,52]. Finally, the correlations of BOLD and pCASL CVRs might increase if ASL and BOLD data were acquired simultaneously within the same protocol using interleaved or single shot designs [27].
4.3 CVR and Aging
In this study, baseline CBF deceased significantly with age without an accompanying change in CBF during the hypercapnic challenge. Consequently, the CBF CVR increased with age. The age related decline in CBF is consistent with the literature [53, 54]. The intact CBF reserve (as demonstrated by the lack of CBF decline with age in the hypercapnic condition) is not surprising, considering it has been long demonstrated that neither the extent nor pattern of CBF change during cognition is affected by age [55]. Nonetheless, our result of the extent of CBF decline with age is currently limited by small population size. The BOLD CVR, on the other hand, had been reported to reduce with aging recently [56, 57]. We observed similar negative trend of reduced BOLD CVR with aging.
4.4 Limitations and future work of pCASL CVR
CBF signal detection power could be improved through use of a GRASE readout acquisition together with multiple background suppression pulses for single-shot pCASL [60]. Additional investigation of whole brain vessel-wise hemodynamic parameters (e.g. OEF and CMRO2) with adaptive sampling echoes and temporal information [61] could provide extra neuro-metabolic information. Future application of a partial volume correction algorithm [53] to the pCASL data or improvement of spatial resolution might improve the CBF quantification accuracy [62]. A separate T1 map could be collected to correct for the T1 relaxation computation in CBF map. Relatively new ASL techniques have been proposed including velocity-selective (VS) ASL and acceleration-based ASL, that are able to generate the labeling contrast in the local brain regions [58, 59]. These techniques are less sensitive to the arterial transit time effect, and might overcome the SNR limitation in WM.
An intrinsic weakness of ASL lies in its ability to detect only the presence of flow, but not the source. This is potentially an issue if ASL were to be used in the clinical setting. In patients with vascular dysfunction (most chronic by nature), collateralization inevitably occurs, which can result in masking of the relative hypoperfusion. However, this weakness could be partially alleviated with addition of BOLD, as the deoxyhemoglobin to oxyhemoglobin ratio is altered in relative hypoperfusion, making ASL-BOLD coupling clinically desirable. Alternatively, adjustments in technique can also be employed, such as the use of vessel-encoded ASL [61, 63]. For example, in multiple sclerosis, neuronal degeneration causes a redistribution of vascular network that would affect flow rate and vessel dilation functions. The degree of this abnormality had been detected using both pCASL and BOLD CVR methods [64, 65], with improvement of classifying patients from controls by combining two CVRs together with some baseline measures [65].
4.5 Limitations and future work of BOLD CVR
The confounding effects in the large draining veins from the BOLD signal are well known limiting factors of BOLD [1, 8, 66]. The influence from many physiologic parameters also makes interpretation of BOLD somewhat complicated [27]. Furthermore, as BOLD does not directly measure CBF, but instead depends heavily on the oxygenation state, age related decline in CMRO2 could complicate the BOLD and CBF measurement [67].
Because BOLD is essentially based on T2* changes, sensitivity to susceptibility artifacts is another concern, particularly at the tissue interfaces. Many methods exist for improving the artifactual signal dropout, such as the z-shimming technique [68]. To account for other factors such as compartmental exchange that influence BOLD signal [69] and therefore the hemodynamic coupling between pCASL and BOLD CVRs, techniques that result in higher spatial resolution can be considered. A more general non-linearity modeling of BOLD signal in response to hypercapnia could be combined in the fitting at variable hypercapnic conditions and different magnetic strengths [70]. Due to the expected long hemodynamic lag that could last about 30 seconds, the relatively short challenge times of 60 second between the BOLD CO2 and room air switch was another limitation for BOLD CVR computation. Though the CVR values were found to be similar compared to a 4-min paradigm [45], prolonging the time of each condition slightly without inducing subject's discomfort could be considered in the future to increase the BOLD CVR SNR.
There existed several delays between the BOLD signal time course and the gas challenge paradigm including: a. The air delivery delay from the air bag to the mouth piece that was due to the physical distance between the air bag and the subject and was about 5-10 seconds in our study. b. The physiological lag from the BOLD to the PETCO2, due to the time it takes for the blood to travel from the pulmonary vascular system to the heart and then to the brain vessels which is typically around 15 seconds [45] after the CO2 valve was switched on. C. The hemodynamic latency which is around 5-10 seconds for the BOLD signal to reach the peak intensity. And finally the mechanical delay of gas sensor from the exhaled CO2 to the capnometer that showed the change of PETCO2, which was about 5 seconds in our study. All these delays induce transiting periods (i.e. long rising and falling periods from the challenge) in the BOLD time course. Even though this long latency would not affect much the fitting of the BOLD time-courses since BOLD signal follow the PETCO2 trace well and the transit periods of BOLD time course could be included in the general linear model to simplify the computation. However in a more complex and random-order BOLD CVR experiment, care must be taken to accurately use the time points to compute the CVR, such as excluding the transit time and including only the plateau periods [29].
Conclusion
The regional and global CVR similarity of pCASL and BOLD suggests that the two techniques can provide consistent information and be suitable for studying disease processes with vascular dysfunction. The two CVRs were found to be consistent with reported values measured separately. Regionally, we found the highest CVRs in occipital lobe, with higher CVR for BOLD than pCASL. Globally, the two CVRs correlated more significantly without PETCO2 normalization. Furthermore, voxel-wise analysis revealed non-linear coupling between pCASL and BOLD CVRs in cortical GM.
Acknowledgments
The data for this paper were acquired at the Bernard and Irene Schwartz Center for Biomedical Imaging in the Department of Radiology at NYU School of Medicine. The authors thank NIH grants 5R01 NS076588 and 2R01 NS039135 that supported the data acquisition for this research. The authors also appreciate language editing from Ms. Erin E. Englund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fülesdi B, Limburg M, Bereczki D. Cerebrovascular reactivity and reserve capacity in type II diabetes mellitus. J Diabetes Complications. 1999;13:191–199. doi: 10.1016/s1056-8727(99)00044-6. [DOI] [PubMed] [Google Scholar]
- 2.Spano VR, Mandell DM, Poublanc J, Sam K, Battisti-Charbonney A, Pucci O, et al. CO2 blood oxygen level-dependent MR mapping of cerebrovascular reserve in a clinical population: safety, tolerability, and technical feasibility. Radiology. 2013 Feb;266:592–8. doi: 10.1148/radiol.12112795. [DOI] [PubMed] [Google Scholar]
- 3.Yonas H, Smith HA, Durham SR, Pentheny SL, Johnson DW. Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg. 1993;79:483–489. doi: 10.3171/jns.1993.79.4.0483. [DOI] [PubMed] [Google Scholar]
- 4.Rostrup E, Larsson HB, Toft PB, Garde K, Thomsen C, Ring P, et al. Functional MRI of CO2 induced increase in cerebral perfusion. NMR Biomed. 1994;7:29–34. doi: 10.1002/nbm.1940070106. [DOI] [PubMed] [Google Scholar]
- 5.Rostrup E, Law I, Blinkenberg M, Larsson HBW, Born AP, Hom S, et al. Regional differences in the CBF and BOLD responses to hypercapnia: A combined PET and fMRI study. Neuroimage. 2000;11:87–97. doi: 10.1006/nimg.1999.0526. [DOI] [PubMed] [Google Scholar]
- 6.Naqvi TZ, Hyuhn HK. Cerebrovascular mental stress reactivity is impaired in hypertension. Cardiovascular Ultrasound. 2009;7:32–45. doi: 10.1186/1476-7120-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandell DM, Han JS, Poublanc J, Crawley AP, Stainsby JA, Fisher JA, et al. Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in patients with arterial steno-occlusive disease: comparison with arterial spin labeling MRI. Stroke. 2008;39:2021–2028. doi: 10.1161/STROKEAHA.107.506709. [DOI] [PubMed] [Google Scholar]
- 8.Sicard KM, Duong TQ. Effects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus-evoked BOLD, CBF, and CMRO2 in spontaneously breathing animals. Neuroimage. 2005;25:850–858. doi: 10.1016/j.neuroimage.2004.12.010. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wey HY, Wang DJ, Duong TQ. Baseline CBF, and BOLD, CBF, and CMRO2 fMRI of visual and vibrotactile stimulations in baboons. J Cereb Blood Flow Metab. 2009;31:715–24. doi: 10.1038/jcbfm.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saxena I, Kumar G, Kumar M, Kumar J. The stress-induced cardiovascular reactivity in the fasting and fed states of healthy young indian males. J Clinical and Diagnostic Research. 2013;7:635–637. doi: 10.7860/JCDR/2013/5430.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pillai JJ, Zaca D. Clinical utility of cerebrovascular reactivity mapping in patients with low grade gliomas. World Journal of Clinical Oncology. 2011 Dec;2:397–403. doi: 10.5306/wjco.v2.i12.397. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao JH, Miller I, Lai S, Xiong J, Fox PT. Quantitative assessment of blood inflow effects in functional MRI signals. Magn Reson Med. 1996 Aug;36:314–9. doi: 10.1002/mrm.1910360219. [DOI] [PubMed] [Google Scholar]
- 13.Bright MG, Bulte DP, Jezzard P, Duyn JH. Characterization of regional hetergeneity in cerebrovascular reactivity dynamics using novel hypercapnia task and BOLD fMRI. Neuroimage. 2009;48:166–175. doi: 10.1016/j.neuroimage.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rusinek H, Glodzik L, Brys M, Haas F, Mcgorty KA, Chen Q, et al. Hippocampal blood flow and vascular reactivity in normal aging. Proceedings of the 18th scientific meeting ISMRM. 2010 [Google Scholar]
- 15.Rodgers ZB, Jain V, Englund EK, Langham MC, Wehrli FW. High temporal resolution MRI quantification of global cerebral metabolic rate of oxygen consumption in response to apneic challenge. J Cereb Blood Flow Metab. 2013;33(10):1514–22. doi: 10.1038/jcbfm.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leoni RF, Mazzetto-Betti KC, Silva AC, Dos Santos AC, de Araujo DB, Leite JP, et al. Assessing cerebrovascular reactivity in carotid steno-occlusive disease using MRI BOLD and ASL techniques. Radiol Res Pract. 2012;2012:1–28. doi: 10.1155/2012/268483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain V, Langham MC, Floyd TF, Jain G, Magland JF, Wehrli FW. Rapid magnetic resonance measurement of global cerebral metabolic rate of oxygen consumption in humans during rest and hypercapnia. J Cereb Blood Flow Metab. 2011 Jul;31:1504–12. doi: 10.1038/jcbfm.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu F, Uh J, Brier MR, Hart J, Jr, Yezhuvath US, Gu H, et al. The influence of carbon dioxide on brain activity and metabolism in conscious humans. J Cereb Blood Flow Metab. 2011 Jan;31:58–67. doi: 10.1038/jcbfm.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birn RM, Cox RW, Bandettini PA. Experimental designs and processing strategies for fMRI studies involving overt verbal responses. Neuroimage. 2004 Nov;23:1046–58. doi: 10.1016/j.neuroimage.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 20.Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci U S A. 1998 Feb 17;95:1834–9. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic-Resonance-Imaging of Perfusion Using Spin Inversion of Arterial Water. Proceedings of the National Academy of Sciences of the United States of America. 1992 Jan 1;89:212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SP, Duong TQ, Yang G, Iadecola C, Kim SG. Relative changes of cerebral arterial and venous blood volumes during increased cerebral blood flow: implications for BOLD fMRI. Magn Reson Med. 2001;45:791–800. doi: 10.1002/mrm.1107. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Wang DJ, Detre JA. Test-retest reliability of arterial spin labeling with common labeling strategies. J Magn Reson Imaging. 2011 Apr;33:940–9. doi: 10.1002/jmri.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tancredi FB, Gauthier CJ, Madjar C, Bolar DS, Fisher JA, Wang DJ, et al. Comparison of pulsed and pseudocontinuous arterial spin-labeling for measuring CO2 -induced cerebrovascular reactivity. J Magn Reson Imaging. 2012 Aug;36:312–21. doi: 10.1002/jmri.23658. [DOI] [PubMed] [Google Scholar]
- 25.Bulte DP, Kelly M, Germuska M, Xie J, Chappell MA, Okell TW, et al. Quantitative measurement of cerebral physiology using respiratory-calibrated MRI. Neuroimage. 2012 Mar;60:582–591. doi: 10.1016/j.neuroimage.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heijtel DF, Mutsaerts HJ, Bakker E, Schober P, Stevens MF, Petersen ET, et al. Accuracy and precision of pseudo-continuous arterial spin labeling perfusion during baseline and hypercapnia: a head-to-head comparison with (1)(5)O H(2)O positron emission tomography. Neuroimage. 2014 May 15;92:182–92. doi: 10.1016/j.neuroimage.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Blockley NP, Griffeth VE, Simon AB, Buxton RB. A review of calibrated blood oxygenation level-dependent (BOLD) methods for the measurement of task-induced changes in brain oxygen metabolism. NMR Biomed. 2013 Aug;26:987–1003. doi: 10.1002/nbm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leontiev O, Dubowitz DJ, Buxton RB. CBF/CMRO2 coupling measured with calibrated BOLD fMRI: sources of bias. Neuroimage. 2007 Jul 15;36:1110–22. doi: 10.1016/j.neuroimage.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faraco CC, Strother MK, Dethrage LM, Jordan L, Singer R, Clemmons PF, et al. Dual echo vessel-encoded ASL for simultaneous BOLD and CBF reactivity assessment in patients with ischemic cerebrovascular disease. Magn Reson Med. 2014 doi: 10.1002/mrm.25268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hare HV, Germuska M, Kelly ME, Bulte DP. Comparison of CO2 in air versus carbogen for the measurement of cerebrovascular reactivity with magnetic resonance imaging. J Cereb Blood Flow Metab. 2013 Nov;33:1799–805. doi: 10.1038/jcbfm.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mark CI, Slessarev M, Ito S, Han J, Fisher JA, Pike GB. Precise control of end-tidal carbon dioxide and oxygen improves BOLD and ASL cerebrovascular reactivity measures. Magn Reson Med. 2010 Sep;64:749–56. doi: 10.1002/mrm.22405. [DOI] [PubMed] [Google Scholar]
- 32.Donahue MJ, Ayad M, Moore R, van Osch M, Singer R, Clemmons P, et al. Relationships between hypercarbic reactivity, cerebral blood flow, and arterial circulation times in patients with moyamoya disease. J Magn Reson Imaging. 2013 Nov;38:1129–39. doi: 10.1002/jmri.24070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kastrup A, Kruger G, Glover GH. Regional variability of cerebral blood oxygenation response to hypercapnia. Neuroimage. 1999;10:675–681. doi: 10.1006/nimg.1999.0505. [DOI] [PubMed] [Google Scholar]
- 34.Shen Q, Ren H, Duong TQ. CBF, BOLD, CBV, and CMRO2 fMRI Signal Temporal Dynamics at 500-msec Resolution. J Magn Reson Imaging. 2008;27:599–606. doi: 10.1002/jmri.21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tancredi FB, Hoge RD. Comparison of cerebral vascular reactivity measures obtained using breath-holding and CO2 inhalation. J Cereb Blood Flow Metab. 2013 Jul;33:1066–74. doi: 10.1038/jcbfm.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alsop DC. Arterial spin labeling: its time is now. MAGMA. 2012 Apr;25:75–7. doi: 10.1007/s10334-012-0309-8. [DOI] [PubMed] [Google Scholar]
- 37.Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. Journal of Cerebral Blood Flow and Metabolism. 1996;16:1236–1249. doi: 10.1097/00004647-199611000-00019. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Aguirre GK, Rao H, Wang J, Fernandez-Seara MA, Childress AR, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008 Feb;26:261–9. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998 Sep;40:383–96. doi: 10.1002/mrm.1910400308. [DOI] [PubMed] [Google Scholar]
- 40.Wu WC, Fernandez-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med. 2007 Nov;58:1020–7. doi: 10.1002/mrm.21403. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence KS, Ye FQ, Lewis BK, Weinberger DR, Frank JA, McLaughlin AC. Effects of Indomethacin on Cerebral Blood Flow at Rest and During Hypercapnia: An Arterial Spin Tagging Study in Humans. J Magn Reson Imaging. 2002;15:628–635. doi: 10.1002/jmri.10111. [DOI] [PubMed] [Google Scholar]
- 42.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcriox N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Y, Yu F, Duong T. Multiparametric MRI characterization and prediction in autism spectrum disorder using graph theory and machine learning. PLoS One. 2014;12;9(6):e90405. doi: 10.1371/journal.pone.0090405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh M, Sungkarat W, Jeong JW, Zhou YX. Extraction of temporal information in functional MRI. Ieee Transactions on Nuclear Science. 2002 Oct;49:2284–2290. [Google Scholar]
- 45.Yezhuvath US, Lewis-Amezcua K, Varghese R, Xiao G, Lu H. On the assessment of cerebrovascular reactivity using hypercapnia BOLD MRI. NMR Biomed. 2009 Aug;22:779–86. doi: 10.1002/nbm.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magn Reson Med. 1998 May;39:702–8. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- 47.Iglesias JE, Liu CY, Thompson PM, Tu ZW. Robust Brain Extraction Across Datasets and Comparison With Publicly Available Methods. Ieee Transactions on Medical Imaging. 2011 Sep;30:1617–1634. doi: 10.1109/TMI.2011.2138152. [DOI] [PubMed] [Google Scholar]
- 48.Duhamel G, Callot V, Tachrount M, Alsop DC, Cozzone PJ. Pseudo-continuous arterial spin labeling at very high magnetic field (11.75 T) for high-resolution mouse brain perfusion imaging. Magn Reson Med. 2012 May;67:1225–36. doi: 10.1002/mrm.23096. [DOI] [PubMed] [Google Scholar]
- 49.Kassner A, Winter JD, Poublanc J, Mikulis DJ, Crawley AP. Blood-Oxygen Level Dependent MRI Measures of Cerebrovascular Reactivity Using a Controlled Respiratory Challenge: Reproducibility and Gender Differences. J Magn Reson Imaging. 2010;31:298–304. doi: 10.1002/jmri.22044. 2010. [DOI] [PubMed] [Google Scholar]
- 50.Blockley NP, Driver ID, Francis ST, Fisher JA, Gowland PA. An Improved Method for Acquiring Cerebrovascular Reactivity Maps. Magn Reson Med. 2011;65:1278–1286. doi: 10.1002/mrm.22719. [DOI] [PubMed] [Google Scholar]
- 51.Cooke JP, Dzau VJ. Nitric oxide synthase: role in the genesis of vascular disease. Annu Rev Med. 1997;48:489–509. doi: 10.1146/annurev.med.48.1.489. [DOI] [PubMed] [Google Scholar]
- 52.Barua RS, Ambrose JA, Srivastava S, DeVoe MC, Eales-Reynolds LJ. Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: an in vitro demonstration in human coronary artery endothelial cells. Circulation. 2003;107(18):2342–7. doi: 10.1161/01.CIR.0000066691.52789.BE. [DOI] [PubMed] [Google Scholar]
- 53.Buijs PC, Krabbe-Hartkamp MJ, Bakker CJ, de Lange EE, Ramos LM, Breteler MM, et al. Effect of age on cerebral blood flow: measurement with ungated two-dimensional phase-contrast MR angiography in 250 adults. Radiology. 1998;209:667–674. doi: 10.1148/radiology.209.3.9844657. [DOI] [PubMed] [Google Scholar]
- 54.Bangen KJ, Restom K, Lui TT, Jak AJ, Wierenga CE, Salmon DP, et al. Differential age effects on cerebral blood flow and BOLD response to encoding: Associations with cognition and stroke risk. Neurobiology of Aging. 2009;30:1276–1287. doi: 10.1016/j.neurobiolaging.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gur RC, Gur RE, Obrist WD, Skolnick BE, Reivich M. Age and Regional Cerebral Blood Flow at Rest and During Cognitive Activity. Arch Gen Psychiatry. 1987;44:617–621. doi: 10.1001/archpsyc.1987.01800190037006. [DOI] [PubMed] [Google Scholar]
- 56.Liu P, Hebrank AC, Rodrigue KM, Kennedy KM, Section J, Park DC, et al. Age-related differences in memory-encoding fMRI responses after accounting for decline in vascular reactivity. Neuroimage. 2013 Sep;78:415–25. doi: 10.1016/j.neuroimage.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas BP, Liu P, Park DC, van Osch MJ, Lu H. Cerebrovascular reactivity in the brain white matter: magnitude, temporal characteristics, and age effects. J Cereb Blood Flow Metab. 2014 Feb;34:242–7. doi: 10.1038/jcbfm.2013.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duhamel G, de Bazelaire C, Alsop DC. Evaluation of systematic quantification errors in velocity-selective arterial spin labeling of the brain. Magn Reson Med. 2003;50:145–153. doi: 10.1002/mrm.10510. [DOI] [PubMed] [Google Scholar]
- 59.Schmid S, Ghariq E, Teeuwisse WM, Webb A, van Osch MJP. Acceleration-Selective Arterial Spin Labeling. Magn Reson Med. 2014;71:191–199. doi: 10.1002/mrm.24650. [DOI] [PubMed] [Google Scholar]
- 60.Fernandez-Seara MA, Wang Z, Wang J, Rao H, Guenther M, Feinberg DA, et al. Continuous arterial spin labeling perfusion measurements using single shot 3D GRASE at 3T. Magnetic resonance in medicine. 2005;54:1241–1247. doi: 10.1002/mrm.20674. [DOI] [PubMed] [Google Scholar]
- 61.Wong EC. Vessel-encoded arterial spin-labeling using pseudocontinuous tagging. Magn Reson Med. 2007 Dec;58:1086–91. doi: 10.1002/mrm.21293. [DOI] [PubMed] [Google Scholar]
- 62.Ye FQ, Berman KF, Ellmore T, Esposito G, van Horn JD, Yang Y, et al. H215O PET validation of steady-state arterial spin tagging cerebral blood flow measurements in humans. Magn Reson Med. 2000;44:450–456. doi: 10.1002/1522-2594(200009)44:3<450::aid-mrm16>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 63.Okell TW, Chappell MA, Kelly ME, Jezzard P. Cerebral blood flow quantification using vessel-encoded arterial spin labeling. J Cereb Blood Flow Metab. 2013;33(11):1716–24. doi: 10.1038/jcbfm.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ge Y, Zhou Y, Lu H, Xu F, Kister I, Jaggi H, et al. Impaired cerebrovascular reactivity in multiple sclerosis measured with hypercapnia perfusion magnetic resonance imaging. Multiple Sclerosis Journal International Conference. 2012:56. [Google Scholar]
- 65.Zhou Y, Lu H, Xu F, Kenul D, Jaggi H, Herbert J, et al. Impaired Cerebrovascular Reactivity (CVR) in MS Measured with Hypercapnia Perfusion MRI. Neuroimage. 2013 Aug 15;62:774–81. [Google Scholar]
- 66.Pattinson KT, Rogers R, Mayhew SD, Tracey I, Wise RG. Pharmacological FMRI: measuring opioid effects on the BOLD response to hypercapnia. J Cereb Blood Flow Metab. 2007 Feb;27:414–23. doi: 10.1038/sj.jcbfm.9600347. [DOI] [PubMed] [Google Scholar]
- 67.Mohtasib RS, Lumley G, Goodwin JA, Emsley HCA, Slumin V. Calibrated fMRI during a cognitive Stroop task reveals reduced metabolic response with increasing age. NeuroImage. 2012;12:1143–1151. doi: 10.1016/j.neuroimage.2011.07.092. [DOI] [PubMed] [Google Scholar]
- 68.Song AW. Single-shot EPI with signal recovery from the susceptibility-induced losses. Magn Reson Med. 2001;46:407–411. doi: 10.1002/mrm.1205. [DOI] [PubMed] [Google Scholar]
- 69.Griffeth VE, Buxton RB. A theoretical framework for estimating cerebral oxygen metabolism changes using the calibrated-BOLD method: modeling the effects of blood volume distribution, hematocrit,oxygen extraction fraction, and tissue signal properties on the BOLD signal. Neuroimage. 2011 Sep;58:198–212. doi: 10.1016/j.neuroimage.2011.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bhogal AA, Siero JC, Fisher JA, Froeling M, Luijten P, Philippens M, et al. Investigating the non-linearity of the BOLD cerebrovascular reactivity response to targeted hypo/hypercapnia at 7T. Neuroimage. 2014 Sep;98:296–305. doi: 10.1016/j.neuroimage.2014.05.006. [DOI] [PubMed] [Google Scholar]


