An understanding of protective immunity to mycobacterial infection is critical for the development of effective strategies to control tuberculosis (TB), a major public health problem worldwide. Mendelian susceptibility to mycobacterial disease (MSMD) is a rare condition characterized by clinical disease caused by weakly virulent mycobacteria, such as Mycobacterium bovis Bacille Calmette-Guérin (BCG) vaccines and nontuberculous, environmental mycobacteria (EM) (OMIM209950)1. Patients are also susceptible to M. tuberculosis. Nine genes have been found to be mutated in patients with MSMD (IFNGR1, IFNGR2, STAT1, IL12B, IL12RB1, IRF8, ISG15, NEMO, and CYBB). All these genes are involved in interferon (IFN)-γ immunity, which is therefore essential for defense against mycobacterial infections in humans 2. X-linked recessive (XR)- MSMD caused by mutations in CYBB, results in impaired nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity in monocyte-derived macrophages (MDMs) and EBV-transformed B (EBV-B) cell lines, but not in monocytes or granulocytes3. By contrast, germline mutations in CYBB that impair NADPH oxidase activity in all cell types, result in X-linked chronic granulomatous disease (CGD)4. An increasing number of case reports from various countries have shown that BCG disease and TB are important features of CGD, particularly in countries in which BCG vaccine is routinely administered, TB is endemic, or both4.
Macrophages are known to be the first line of defense against mycobacteria, generating the reactive oxygen species (ROS) and probably responsible for microbicidal activity 5. Phagocyte NADPH oxidase activity can be enhanced by treatment with IFN-γ and the corresponding genes can also be induced by IFN-γ 6. A contribution of NADPH oxidase deficiency to mycobacterial disease in patients with inborn errors of IFN-γ is however uncertain. The occurrence of BCG disease and TB in CGD patients and in patients with macrophage-tropic mutations of the NADPH oxidase complex suggests that impaired macrophage NADPH oxidase activity may contribute to both diseases in patients with IFN-γR deficiency 4.We therefore tested the hypothesis that the function of the NADPH oxidase complex might be partly dependent on IFN-γ, at least in human MDMs in vitro.
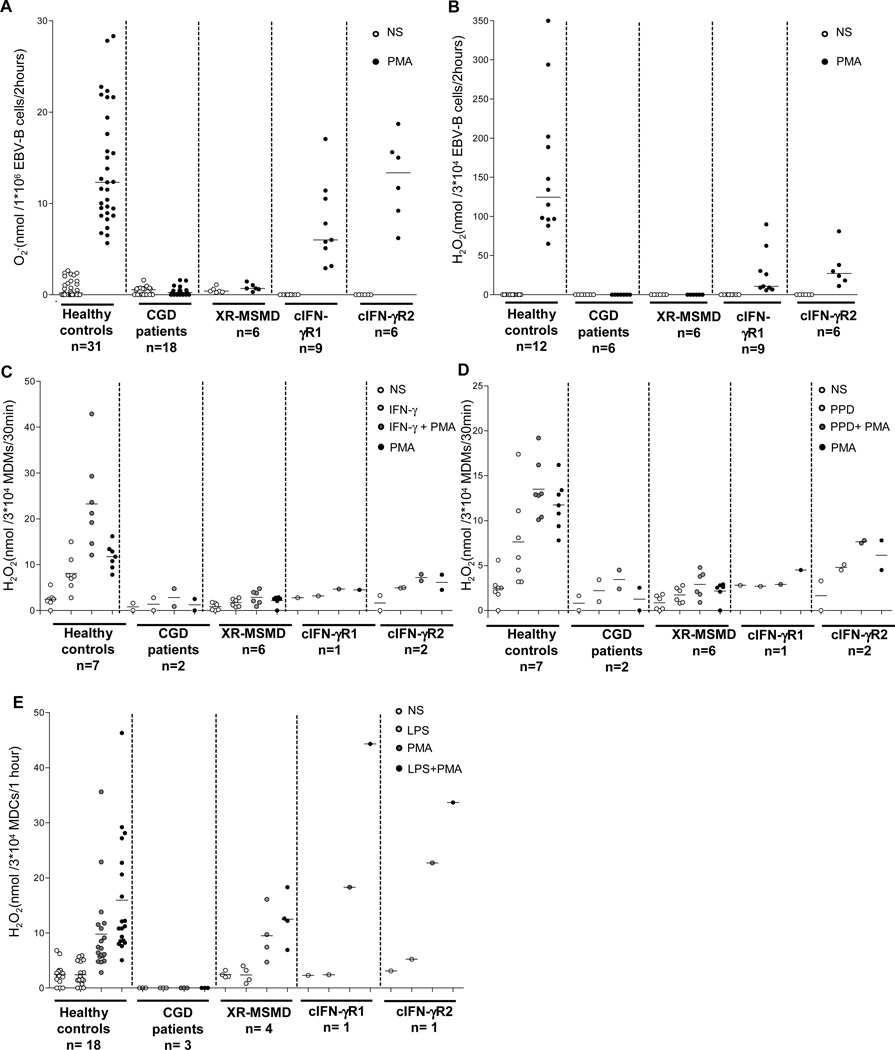
We first evaluated NADPH oxidase activity in EBV-B cells from MSMD patients without cellular responses to IFN-γIFN-γR1/IFN-γR2 [c] complete deficiencies), patients without NADPH oxidase activity (CGD), and healthy controls. We selected EBV-B cells from patients with cIFN-γR1 (n=9) or cIFN-γR2 (n=6) deficiencies, XR-MSMD (n=6), or CGD (n= 18), and healthy controls. We assessed the ability of EBV-B cells to produce superoxide (O2-) in the cytochrome-c reduction assay and to release hydrogen peroxide (H2O2) by reducing 10-acetyl-3,7 dihydroxyphenoxazine (Resurfin or Amplex Red®). We also investigated the NADPH oxidase activity by luminal and isoluminol assays (supplemental figure 1 A-B). As previously reported3, the EBV-B cells of the healthy controls produced and released ROS following stimulation with a phorbol ester, such as phorbol myristate acetate (PMA), for two hours. All EBV-B cells from patients with cIFN-γR1/cIFN-γR2 had levels of O2- production similar to those of healthy controls (Figure 1A and supplemental figure 1A), unlike the EBV-B cells of CGD and XR-MSMD patients. However, H2O2 release from the EBV-B cells of all patients with cIFN-γR1/cIFN-γR2 deficiencies was severely impaired (Figure 1B and supplemental figure 1B).
Figure 1. NADPH oxidase activity in human EBV-B cells, MDDCs and MDMs.
A. O2- generation of EBV-B cells from healthy controls (n=31), CGD patients (n=18), XR-MSMD (n=6) and IFN-γR1/IFN-γR2 complete (c) deficiencies (n=9 and 6 respectively), measured by cytochrome-c reduction test after 2 h PMA (400 ng/ml) activation. Each symbol represents an individual subject. B. Fluorimetric quatification of H2O2 release from EBV-B cells of healthy controls (n=12), CGD (n=6), XR-MSMD (n=6) and cIFN-γR1/cIFN-γR2 (n=9 and 6 respectively), measured by Amplex Red® assay after 2 h PMA (400 ng/ml) activation. C and D. Fluorimetric quatification of H2O2 release after 30 min from MDMs of healthy controls (n=7), CGD (n=2) and cIFN-γR1/cIFN-γR2 (n=1 and 2 respectively), measured by Amplex Red® assay then left untreated (NS) or treated for 18 h with IFN-γ (1×105 IU/ml), PPD (1 mg/ml), followed by no trigger or by treatment with PMA (400 ng/ml) activation. E. Release of H2O2 from MDDCs obtained from healthy controls (n=18), CGD (n=3), cIFN-γR1/cIFN-γR2 (n=1 and 1 respectively) and X-MSMD (n=4) deficiency, then left untreated (NS) or treated with LPS, followed by no trigger or by treatment with PMA (400 ng/ml). Each symbol represents an individual subject. Data are representative of two experiments. (A, B; mean of duplicates) and mean of duplicates (C, D, E).
We further explored MDMs from patients with cIFN-γR1 (n=1) or cIFN-γR2 (n=2) deficiency. MDMs from these patients produced very small amounts of H2O2 after stimulation with IFN-γ or PPD, suggesting an impairment of NADPH oxidase activity (Figure 1 C, D). This is consistent with the findings for EBV-B cells (Figure 1B). We also assessed NADPH oxidase activity in monocyte-derived dendritic cells (MDDCs) from all patients. MDDCs from XR-MSMD patients have not been tested previously 3. We derived MDDCs in vitro by treatment with GM-CSF plus IL-13 7 and the respiratory burst was evaluated with Amplex Red®. MDDCs from the healthy controls released H2O2 and, as expected, MDDCs from CGD patients did not (Figure 1E). Notably, MDDCs from XR-MSMD patients (n=4), and from patients with cIFN-γR1 (n=1) or cIFN-γR2 (n=1) deficiency produced similar amounts of H2O2 than those obtained from healthy controls (Figure 1E). The impairment of NADPH oxidase activity was, therefore, cell-specific and restricted to MDMs in these patients. Conversely, the normal respiratory burst activity of granulocytes and monocytes from MSMD patients with cIFN-γR1 or cIFN-γR2 deficiency (data not shown), like that herein documented in MDDCs, may account for their protection against fungi and bacteria other than mycobacteria, whereas CGD patients are typically susceptible to these microbes.
In conclusion, these results support a role for the IFN-γ pathway in the up-regulation of NADPH oxidase activity in MDMs and EBV-B cells, and suggest that impairment of the phagocyte respiratory burst contributes to BCG disease and TB in patients with inborn errors of IFN-γ immunity. IFN-γ can regulate NAPDH oxidase activity in different cell types 8. O2- is rapidly converted to H2O2 by spontaneous and enzymatic dismutation. Differences between the amounts of H2O2 and O2- produced in response to PMA were detected in the same cells. There are several possible reasons for this, including the role of endogenous superoxide dismutase in catalyzing the dismutation of O2- to H2O2, differences in kinetics and the techniques used to measure O2- and H2O2 levels, and variability due to differences in the pH of assay buffers. It was striking that some mycobacteria, e.g. M. tuberculosis, produced the enzyme such KatG, a catalase-peroxidase, that protect from killing by H2O2 but not O2- in mice 9. Probably NADPH oxidase activity contributes to cytokine production, granuloma genesis or autophagy more than killing of mycobacteria 5. The mechanisms affecting this activity in MDMs and EBV-B cells from MSMD patients remain unclear and require further investigation.
Supplementary Material
Acknowledgments
We would like to thank the patients and their families, whose cooperation was essential for collection of the data used in this study. We thank all members of the Laboratory of Human Genetics of Infectious Diseases for helpful discussions; Martine Courat, Lahouari Amar and Yelena Nemirovskaya for secretarial assistance; and Mélanie Migaud for technical assistance. We would like to thank the patients and their families, whose cooperation was essential for collection of the data used in this study.
Funding: The Laboratory of Human Genetics of Infectious Diseases is supported by institutional grants to INSERM and The Rockefeller University, and grants from the French National Research Agency (ANR) (IFNGPHOX-ANR13-ISV3-0001-01), the “Investments for the Future” program (grant number ANR-10-IAHU-01°), Laboratoire d’Excellence Integrative Biology of Emerging Infectious Diseases (ANR-10-LABX-62-IBEID), the National Center for Research Resources and the National Center for Advancing Sciences (NCATS), National Institutes of Health (8UL1TR000043), the National Institute of Allergy and Infectious Diseases (R37AI095983) and the St. Giles Foundation. Antonio Condino-Neto are supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP grants 2012/11757-2, 2010/51814-0, 2012/51094-2 and 2013/50303-0) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ grant 306902/2013). Francesca Conti was supported by the Department of Public Health and Cellular Biology, University of Rome Tor Vergata.
Abbreviations used
- CGD
chronic granulomatous disease
- H2O2
hydrogen peroxide
- MSMD
Mendelian susceptibility to mycobacterial disease
- NADPH
nicotinamide dinucleotide phosphate
- O2-
superoxide
- TB
tuberculosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have indicated they have no financial relationships relevant to this article to disclose.
Short summary: The role of NADPH oxidase activity was evaluated in MDMs, MDDCs and EBV-B cells in patients with inborn errors of IFN-γ immunity.
Contributions: JB. and A.C.-N. designed the study, contributed to the recruitment and follow-up of the patients, provided CGD controls and contributed intellectually to the experimental process. Experimental studies were performed by F.C., W.C.A.F., C.D. and C.P. under the supervision of. J.B. and A.C.-N. M.H. and P.N. provided important experimental advice concerning cell cultures and activation. F.C. J.-L.C, A.C.-N. and J.B. wrote the paper. All authors commented on and discussed the paper. F.C. and W.C.A.F. contributed equally to this work. J.B. and A.C.-N. contributed equally to this work.
References
- 1.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 2.Bustamante J, Boisson-Dupuis S, Abel L, Casanova J-L. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-g immunity. Semin Immunol. 2014 doi: 10.1016/j.smim.2014.09.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bustamante J, Arias AA, Vogt G, Picard C, Galicia LB, Prando C, et al. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat Immunol. 2011;12:213–221. doi: 10.1038/ni.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bustamante J, Aksu G, Vogt G, de Beaucoudrey L, Genel F, Chapgier A, et al. BCG-osis and tuberculosis in a child with chronic granulomatous disease. J Allergy Clin Immunol. 2007;120:32–38. doi: 10.1016/j.jaci.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 5.Deffert C, Cachat J, Krause KH. Phagocyte NADPH oxidase, chronic granulomatous disease and mycobacterial infections. Cell Microbiol. 2014 doi: 10.1111/cmi.12322. [DOI] [PubMed] [Google Scholar]
- 6.Condino-Neto A, Newburger PE. Interferon-gamma improves splicing efficiency of CYBB gene transcripts in an interferon-responsive variant of chronic granulomatous disease due to a splice site consensus region mutation. Blood. 2000;95:3548–3554. [PubMed] [Google Scholar]
- 7.Vulcano M, Dusi S, Lissandrini D, Badolato R, Mazzi P, Riboldi E, et al. Toll receptor-mediated regulation of NADPH oxidase in human dendritic cells. J Immunol. 2004;173:5749–5756. doi: 10.4049/jimmunol.173.9.5749. [DOI] [PubMed] [Google Scholar]
- 8.Casbon AJ, Long ME, Dunn KW, Allen LA, Dinauer MC. Effects of IFN-gamma on intracellular trafficking and activity of macrophage NADPH oxidase flavocytochrome b558. J Leukoc Biol. 2012;92:869–882. doi: 10.1189/jlb.0512244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trivedi A, Singh N, Bhat SA, Gupta P, Kumar A. Redox biology of tuberculosis pathogenesis. Adv Microb Physiol. 2012;60:263–324. doi: 10.1016/B978-0-12-398264-3.00004-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.