To the Editor
The first few months of life represent a window of opportunity for neonatal oral tolerance induction. During this critical time, breast milk is a rich source of immunomodulatory factors, including TGF-β shown to influence the maturation of the mucosal immune system of the neonates.1 Reports on breast milk cytokines, chemokines and growth factors in cow’s milk allergy (CMA), including both IgE- and non-IgE-mediated mechanisms are largely lacking, although IL-5 and IL-13 were identified as risk factors for asthma and eotaxin for atopic dermatitis.2,3 Because food allergy represents a failure in development of mucosal tolerance to foods, immune factors in human milk may have a more direct impact on tolerance to foods compared to environmental allergens. In the present study, we sought to assess the immunologic milieu of human milk received by non-CMA infants and those who develop CMA.
We utilized stored frozen human milk samples from a prospective birth cohort of 145 mother-infant pairs, designed to assess immunologic factors in human milk and oversampled for newborns with high risk for food allergies.4 All available samples were assessed from 39 mothers of non-CMA infants and 35 mothers of CMA infants, verified by oral food challenges6 performed at median 5.5 months of age (IQR, 3.1–7.7 months). Clinical characteristics are shown in Table E1. 28 cytokines, chemokines and growth factors were measured in milk samples after processing4 by multiplex kits (Milliplex, Millipore, Billerica, MA) and read on a Luminex 200 Multiplex analyzer; TGF-β1 was measured by ELISA (Genzyme, Cambridge, Mass) from acid activated samples. As a control, we diluted samples or spiked samples with known amounts of analytes to assess whether levels could be accurately recovered. Those that were not, were excluded from further analysis including TNF-α and -β, CCL1, CCL17, IL-31, eotaxin 3 and CXCL9. Total protein was measured by Pierce Protein assay (Thermo Scientific, Rockford, IL). Please see the supplementary text in this article’s Online Repository at www.jacionline.org for details of statistical analysis.
Immune markers were detectable in the majority of samples, except for IL-5, GM-CSF and IL-12p70 detected in <10% of samples (Table EII). Because mothers of CMA infants had more atopic dermatitis (p=0.002), and a trend for longer duration of lactation at sampling (p=0.05, Table EI), we assessed whether maternal covariates (age, duration of breastfeeding, atopic manifestations) had an impact on immune marker levels. Maternal age positively correlated with IL-1α, and duration of lactation correlated positively with PDGF-BB, CCL27, and VEGF, and negatively with IL-6, TGF-β 1, TSLP, CCL11, CXCL10, and CXCL11 (Fig. E1). Atopic mothers had lowers levels of CCL22, TSLP, and total protein than non-atopic mothers (p=0.0002, p=0.001, and p=0.01, respectively, data not shown). Further analyses were adjusted for maternal atopy and duration of lactation as appropriate.
To assess whether individual human immune markers were associated with protection or risk of CMA, we performed univariate analysis. Milk received by non-CMA infants contained higher levels of IL-1β, IL-6, IL-10 and TSLP than that received by CMA infants, but only IL-1β and IL-6 reached statistical significance (p=0.01 and p=0.04, respectively, Figure 1A–D). The levels of these four cytokines combined were higher in milk received by non-CMA infants, even after adjusted for duration of lactation (p=0.049 muStat, Fig. 1E; p=0.047 after TGF-β1 was included in the analysis decreasing the sample size to 39). IL-1β and IL-6 positively correlated with milk macrophage numbers suggesting macrophages as their source (Fig. E1). Logistic univariate regression was then used to assess the influence of multiple immunologic factors in human milk on CMA onset, including milk immune markers, leukocytes, eosinophilic cationic protein (ECP), and the levels of β-lactoglobulin (BLG)- and casein-specific IgA, which have been previously reported in this cohort.4–6 After adjusting for maternal covariates, including atopic diseases, age and duration of lactation, the levels of IL-1β (p=0.001), IL-10 (p=0.04) and BLG-specific IgA (p=0.005) were lower in the milk of mothers with a CMA infant than in those with a non-CMA infant and there was a trend for lower IL-6 (p=0.07, Table EIII).
Figure 1.
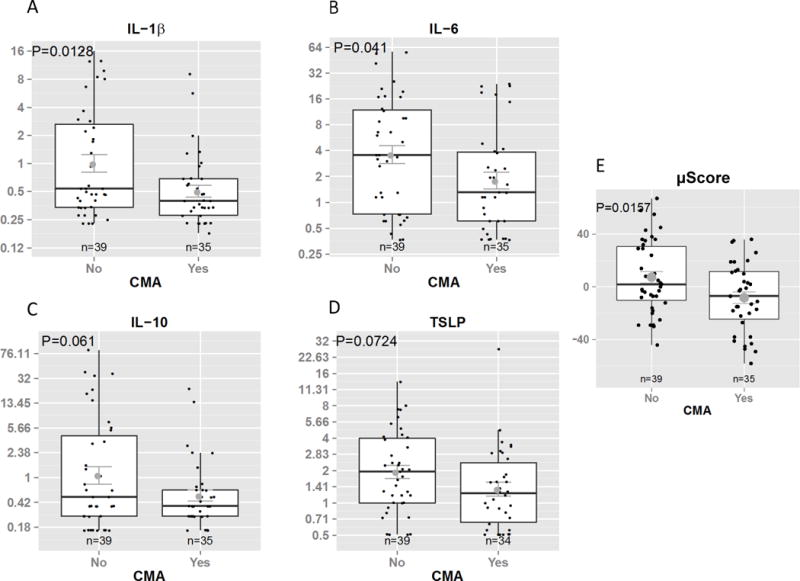
Human milk immune markers which were different between mothers with a CMAinfant and those with a non-CMA infant, A) IL-1β, B) IL-6, C) IL-10, and D) TSLP (t test, shown after logarithmic transformation. E) Multivariate score combining all 4 cytokines A–D (multivariate Ustatiscs). On the box plots horizontal line, median;box,25th to 75th percentile; whiskers,5th to 95th percentile; round symbol, geometric mean.
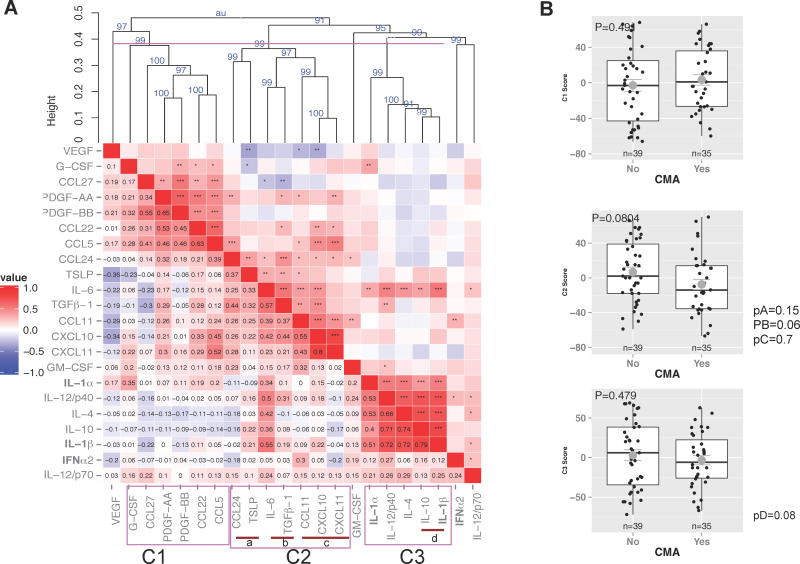
Due to many interactions seen between immune markers, Spearman correlation and hierarchical cluster analysis was carried out to see whether CMA or tolerance would better correlate with clusters of immune markers rather than individual factors. Three main clusters and 4 subclusters were identified (Fig. 2A). Although the p-values are only marginally significant (when considering the established 0.05 cut-off), they show a trend for networks of cytokines including IL-6 together with TGF-β1 and IL-10 together with IL-1β in protection against CMA (p=0.06 and p=0.08, respectively). Finally, we used classification and regression trees to classify CMA based on the immune markers, IgA, TGF- β1 and maternal demographics. The best decision tree fitting the data showed that high levels of IL-6, IL-1β and specific IgA and low levels of IL-12p40 and CXCL10 correctly classified 80% of the samples between CMA and non-CMA infants with sensitivity of 83% and specificity of 75% (Fig. E2).
Figure 2.
A) Hierarchical cluster analysis of human milk immune factors using Spearman correlation and average agglomeration algorithm. Correlation coefficient associated color coding shown, * p<0.05, ** p<0.01, *** p<0.001. P values (as %) in blue in the resultant dendogram indicate the degree of stability of each cluster. Height of ≤0.4 indicate clusters of significant correlation. Clusters C1, C2 and C3, and sub-clusters (a–d) were identified. B) Multivariate μ score combining all markers in the cluster (multivariate U-statiscs).
Here we showed that networks of pro-inflammatory and regulatory cytokines including IL-1β, IL-6, IL-10, and TGF-βl in human milk are associated with tolerance to cow’s milk. In early life, intragastric protein digestion is underdeveloped and high concentrations of antiproteases such as α1-antichymotrypsin, and α1-antitrypsin are present in human milk. With an exclusively breastfed infant ingesting about 500 ml of human milk a day in early life, daily ingestion of cytokines in infants totals micrograms daily. The effects of IL-1β on gut epithelial cells include stimulation of IL-6.7 IL-6, is involved in IgA synthesis, possibly induced by the follicular T helper cells in the Peyer’s patch germinal centers. Alternatively, these cytokines may provide stimulatory signals to mammary epithelium to increase secretion of milk IgA, shown to be protective against CMA.4 Lastly, IL-1, IL-6 and TGF-β are known to promote Thl7 differentiation, with some data suggesting a protective role for IL-17 in food allergy.8
We and others3,4 have found that human milk biomarker levels are largely, and surprisingly, not related to maternal atopy. Nevertheless, analyses were adjusted for maternal atopic diseases. Further strengths of this study include the prospective design and utilization of multiplex platform limiting variability. The limitations of the study include a relatively small sample size, and a high-risk population limiting applicability to the general population. TGF-β1 was not measured in all samples, compromising the statistical analysis.
In summary, breast milk is a complex substance that contains a variety of immune factors capable of influencing the infant microbiome,9 and cytokines such as IL-1β, IL-6 and IL-10, which may facilitate development of neonatal oral tolerance to foods. It remains to be seen whether these cytokines are directly protective by acting on the infant gastrointestinal tract or are a biomarker of another protective mechanism vertically transmitted through breast milk.
Supplementary Material
Figure E1. Spearman Rank correlation of human milk factors, including cytokines, chemokines, growth factors, specific IgA, leukocyte proportion, age of baby (i.e. duration of lactation), and age of mother. Shown is the correlation coefficient as well as color coding corresponding to values between −1.0 and 1.0 indicating negative and positive correlation, respectively. * p<0.05, ** p<0.01, ***p<0.001.
Figure E2. Classification and regression tree with input from human milk levels of cytokines, chemokines, growth factors, specific IgA, and maternal demographics. This model correctly classified CMA outcome in 80% of the samples. Numbers in blue indicate healthy and in red CMA infants. There are 15 misclassified patients: 9 healthy subjects were misclassified as CMA (7+2+0; see the blue numbers arriving to the red nodes) and 6 CMA patients misclassified as healthy (0+1+5; see red numbers arriving to the blue nodes).
Acknowledgments
Funding: The project described was supported by Grant Number K08 AI091655 (K.M. Järvinen) from the National Institute of Allergy and Infectious Diseases. H.A. Sampson is supported in part by grants from the NIH, RR026134, AI44236 and AI066738. Cecilia Berin is supported in part by NIH grant AI093577. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
We thank the Human Immune Monitoring Center at Mount Sinai for their technical expertise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Verhasselt V, Milcent V, Cazareth J, Kanda A, Fleury S, Dombrowicz D, Glaichenhaus N, Julia V. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med. 2008;14:170–5. doi: 10.1038/nm1718. [DOI] [PubMed] [Google Scholar]
- 2.Soto-Ramirez N, Karmaus W, Yousefi M, Zhang H, Liu J, Gangur V. Maternal immune markers in serum during gestation and in breast milk and the risk of asthma-like symptoms at ages 6 and 12 months: a longitudinal study. Allergy Asthma Clin Immunol. 2012;8 doi: 10.1186/1710-1492-8-11. 11,1492-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ochiai S, Shimojo N, Morita Y, Tomiita M, Arima T, Inoue Y, Nakaya M, Uehara N, Sato Y, Mori C, Suzuki Y, Kohno Y. Cytokine biomarker candidates in breast milk associated with the development of atopic dermatitis in 6-month-old infants. Int Arch Allergy Immunol. 2013;160:401–8. doi: 10.1159/000342995. [DOI] [PubMed] [Google Scholar]
- 4.Jarvinen KM, Westfall JE, Seppo MS, James AK, Tsuang AJ, Feustel PJ, Sampson HA, Berin C. Role of maternal elimination diets and human milk IgA in the development of cow’s milk allergy in the infants. Clin Exp Allergy. 2014;44:69–78. doi: 10.1111/cea.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Järvinen K-M, Suomalainen H. Leucocytes in human milk and leucocyte subsets in cow’s milk-allergic infants. Pediatr Allergy Immunol. 2002;13:243–54. doi: 10.1034/j.1399-3038.2002.00087.x. [DOI] [PubMed] [Google Scholar]
- 6.Österlund P, Smedberg T, Hakulinen A, Heikkilä H, Järvinen K-M. Eosinophil cationic protein in human milk is associated with development of food allergies and atopic eczema in breastfed infants. Pediatr Res. 2004;55:296–301. doi: 10.1203/01.PDR.0000106315.00474.6F. [DOI] [PubMed] [Google Scholar]
- 7.Moon MR, Parikh AA, Pritts TA, Kane C, Fischer JE, Salzman AL, Hasselgren PO. Interleukin-1beta induces complement component C3 and IL-6 production at the basolateral and apical membranes in a human intestinal epithelial cell line. Shock. 2000;13:374–8. doi: 10.1097/00024382-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Ganeshan K, Bryce PJ. Regulatory T cells enhance mast cell production of IL-6 via surface-bound TGF-beta. J Immunol. 2012;188:594–603. doi: 10.4049/jimmunol.1102389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogier EW, Frantz AL, Bruno ME, Wedlund L, Cohen DA, Stromberg AJ, Kaetzel CS. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci U S A. 2014;111(8):3074–9. doi: 10.1073/pnas.1315792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure E1. Spearman Rank correlation of human milk factors, including cytokines, chemokines, growth factors, specific IgA, leukocyte proportion, age of baby (i.e. duration of lactation), and age of mother. Shown is the correlation coefficient as well as color coding corresponding to values between −1.0 and 1.0 indicating negative and positive correlation, respectively. * p<0.05, ** p<0.01, ***p<0.001.
Figure E2. Classification and regression tree with input from human milk levels of cytokines, chemokines, growth factors, specific IgA, and maternal demographics. This model correctly classified CMA outcome in 80% of the samples. Numbers in blue indicate healthy and in red CMA infants. There are 15 misclassified patients: 9 healthy subjects were misclassified as CMA (7+2+0; see the blue numbers arriving to the red nodes) and 6 CMA patients misclassified as healthy (0+1+5; see red numbers arriving to the blue nodes).