Abstract
Background
Elucidation of the relative importance of commonly targeted biomechanical variables to poststroke long-distance walking function would facilitate optimal intervention design.
Objectives
To (1) determine the relative contribution of variables from three biomechanical constructs to poststroke long-distance walking and (2) identify the biomechanical changes underlying posttraining improvements in long-distance walking function.
Methods
Forty-four individuals > 6 months after stroke participated in this study. A subset of these subjects (n = 31) underwent 12 weeks of high-intensity locomotor training. Cross-sectional (pretraining) and longitudinal (posttraining change) regression quantified the relationships between poststroke long-distance walking function, as measured via the 6-Minute Walk Test (6MWT), and walking biomechanics. Biomechanical variables were organized into stance phase (paretic propulsion and trailing limb angle), swing phase (paretic ankle dorsiflexion and knee flexion), and symmetry (step length and swing time) constructs.
Results
Pretraining, all variables correlated with 6MWT distance (r’s = 0.39 to 0.75, p’s < 0.05); however, only propulsion (Prop) and trailing limb angle (TLA) independently predicted 6MWT distance (R2 = 0.655, F(6,36) = 11.38, p < .001). Interestingly, only ΔProp predicted Δ6MWT; however, pretraining Prop, pretraining TLA, and ΔTLA moderated this relationship (moderation model R2s = 0.383, 0.468, 0.289, respectively).
Conclusions
The paretic limb’s ability to generate propulsion during walking is a critical determinant of long-distance walking function after stroke. This finding supports the development of poststroke interventions that impact deficits in propulsion and trailing limb angle.
Keywords: Hemiparesis, Rehabilitation, Walking, Stroke, Biomechanics, Gait
INTRODUCTION
More than 5.5 million Americans are currently living with stroke – the leading cause of disability in the USA1. For the majority of stroke survivors, the restoration of walking is the ultimate goal of rehabilitation2. As such, a major focus of rehabilitation research has been on the development and testing of poststroke gait rehabilitation programs. However, activity and participation are often limited even after rehabilitation3–8. Indeed, persons poststroke walk less than 3500 steps per day; in contrast, even the most sedentary healthy adults walk more than 5000 steps per day9,10. Given the relationship between physical inactivity and diseases such as heart disease and diabetes1, a critical need exists for the development of interventions capable of increasing the physical activity of persons who have sustained a stroke.
The development of interventions directed toward improving poststroke walking function is confounded by the fact that improvements in walking function are achievable through a variety of recovery mechanisms – from improved neuromotor control to better compensation for lost neuromotor function11–14. Because compensatory strategies such as stiff-legged and circumduction gait are associated with a higher energy cost of walking, reduced endurance, and slower speeds5,15–17, recovery strategies that rely on gait compensations may limit the gains in long-distance walking function that are achievable through intervention. This is important as persons poststroke indicate that a major contributor to their lack of engagement in the community is a deficit in their ability to walk farther distances18. As such, training someone to walk faster may not be sufficient to improve their ability to walk farther if the strategy they use to walk faster is not economical and sustainable. Given the relationships between long-distance walking function19, community walking participation20, and the energy cost of walking, a better understanding of the biomechanical determinants of poststroke long-distance walking function is needed.
Improvements in poststroke long-distance walking function may be achieved through any number of biomechanical mechanisms. Indeed, previous investigations have shown relationships between various biomechanical variables and walking function after stroke21–32. For example, Sibley et al demonstrated that changes in spatiotemporal asymmetry were associated with less distance walked during the final two minutes of the 6-Minute Walk Test (6MWT) in those with the worst long-distance walking function21. Others have identified stance phase variables related to the propulsive force generating ability of the paretic limb as major contributors to poststroke walking function22–29. Still, others have shown that deficits in variables related to swing phase ground clearance correlate with poststroke walking function30–32. The primary purpose of this study was to determine the relative importance of variables from each of these biomechanical constructs – spatiotemporal symmetry, stance phase, or swing phase – to the long-distance walking function of persons in the chronic phase of stroke recovery. A secondary aim of this study was to identify the biomechanical changes underlying posttraining improvements in long-distance walking function. We hypothesized that the stance phase construct would be the best predictor of long-distance walking function and that improvement in stance phase mechanics would account for improvements in long-distance walking function. Moreover, we hypothesized that baseline stance phase function would moderate the relationship between stance phase improvements and improvements in long-distance walking function; specifically, that improvements in stance phase mechanics would be more meaningful in those most impaired at baseline.
METHODS
Subjects
Forty-five subjects with poststroke hemiparesis participated in this study. A subset of these subjects (n = 31) underwent 12 weeks of locomotor training as described below. Subjects were recruited over two years from Delaware, New Jersey, and Pennsylvania health care facilities, advertisements, and patient support groups. Subjects were at least 6 months post a single stroke, able to walk at a self-selected pace for six minutes without orthotic support but with observable gait deficits, were able to passively dorsiflex the ankle to a neutral position with the knee extended (tested in the prone position), and were able to passively extend the hip at least ten degrees (tested in a side lying position). Subjects were excluded if they had evidence of moderate to severe chronic white matter disease or cerebellar stroke on MRI, a history of lower extremity joint replacement due to arthritis, an inability to communicate with the investigators, neglect (tested via the star cancellation test33) or hemianopia, a score of >1 on question 1b and >0 on question 1c on the NIH Stroke Scale, or unexplained dizziness in the last 6 months. All subjects signed written informed consent forms approved by the Human Subjects Review Board of the University of Delaware, received written medical clearance from their physician, and completed a submaximal stress test to determine exercise safety prior to participation in the intervention protocol described below. Subjects completed clinical and biomechanical evaluations prior to (pretraining) and immediately following 12 weeks of training (posttraining).
Clinical Testing
Clinical evaluations were conducted by licensed physical therapists and included the 6-meter walk test34 and the 6-minute walk test (6MWT)35. Derived from the 6-meter walk test was each subject’s self-selected and maximum walking speeds (m/s), which are reported in Table 1 as an indication of baseline walking disability7. The distance walked during the 6MWT served as our a priori measure of long-distance walking function. The 6MWT is thought to be reflective of a person’s ability to maintain a moderate amount of exertion over a period of time similar to the activities of daily living, has been identified as an excellent measure of poststroke walking capacity and community ambulation36,37, and as indicative of community reintegration following stroke5,38. Subjects were allowed the use of their regular assistive device (e.g. cane) during testing, if necessary. Subjects who used an assistive device at their pretraining evaluation also used one during their posttraining evaluation.
Table 1.
Subject (n = 44) Characteristics
| Variable | Median (SIQR) or Frequency (%) |
|---|---|
| Age, y | 60.08 (2.49) |
| Time Since Stroke, y | 1.72 (0.73) |
| Sex, Female | 39% |
| Side of Paresis, Left | 66% |
| Self-Selected Walking Speed, m/s | 0.74 (0.12) |
| Maximum Walking Speed, m/s | 1.03 (0.15) |
Motion Analysis
Previous work has described in detail the methods used during this investigation19,39,40. Briefly, kinetic and kinematic data were collected using an 8-camera motion analysis system (Motion Analysis 3D Eagle, Santa Rosa, CA, USA) as subjects walked for thirty seconds at the maximum walking speed they could maintain for four minutes. For baseline motion analysis testing, this maximum walking speed was determined during an acclimatization session conducted prior to the start of training. The speed used for posttraining motion analysis was determined during the final week of training. During motion analysis testing, subjects walked on a dual-belt treadmill instrumented with two independent 6-degree of freedom force platforms. Ground reaction force (GRF) data were collected at 2000Hz (Bertec Corporation, Worthington, OH). Kinematic data were sampled at a rate of 100 Hz and based on the motion of retro-reflective markers placed over the pelvis, and bilaterally over the thigh, shank, and foot segments, and on the medial and lateral malleoli, at the medial and lateral femoral condyles, the greater trochanters, and the iliac crests. All kinematic and kinetic variables were computed for each stride and averaged across the first 15 strides recorded during motion analysis testing using a custom-written LabVIEW program (National Instruments, Austin, TX, USA).
Six biomechanical variables, divided into three biomechanical constructs – 1) stance phase, 2) swing phase, and 3) spatiotemporal symmetry – quantified biomechanical function during walking. These constructs were selected due to their prevalent study13,21–32,41–44 and common consideration by clinicians during poststroke rehabilitation. Peak paretic propulsion and peak paretic trailing limb angle (measured during the paretic double support phase) comprised the stance phase construct, peak knee flexion and peak ankle dorsiflexion angles comprised the swing phase construct, and step length symmetry and swing time symmetry comprised the spatiotemporal symmetry construct. Peak propulsion was defined as the maximum anterior GRF recorded during the paretic double support phase, normalized to body weight. Peak trailing limb angle was defined as the maximum sagittal plane angle between the vertical axis of the lab and a vector joining the paretic limb’s lateral malleolus and greater trochanter. Peak ankle dorsiflexion was defined as the maximum ankle dorsiflexion angle during the paretic swing phase. Peak knee flexion was defined as the maximum knee flexion angle during the paretic swing phase.
Step length, stride duration, and swing time were calculated bilaterally per stride to allow calculation of the symmetry measures of interest. Step length was defined as the distance between heel markers at the leading limb’s initial contact. Stride duration was defined as the time from one initial contact to the subsequent ipsilateral initial contact. Swing time was defined as the time between toe off and initial contact. Swing time was normalized to stride duration. As per a previous study45, to calculate step length symmetry, the following equation was used: [larger step length / (larger step length + smaller step length)]. To calculate swing time symmetry, the following equation was used: [longer swing time / (longer swing time + shorter swing time)]. A value of 0.50 reflects perfect symmetry. For step length asymmetry, a value of 1.00 reflects a step-to gait pattern and values greater than 1.00 reflect a walking pattern where one limb does not pass the other.
Training Protocol
A subgroup of subjects (n = 31) completed 12 weeks of high intensity locomotor training. Subjects walked at their maximum walking speeds with (n = 15) or without (n = 16) the application of functional electrical stimulation to the paretic ankle dorsiflexors during swing phase and plantarflexors during late stance phase. The training protocol used has been previously described39,40. Regardless of whether subjects trained with or without FES, the training provided task-specific practice of thousands of steps per treatment session. Training occurred at a frequency of 3 sessions per week for 12 weeks. Approximately 36 minutes of total walking were completed during each session. Because the present manuscript is only concerned with a mechanistic investigation of the biomechanical changes underlying changes in long-distance walking function, change-score data from these treatment groups have been combined. A subsequent manuscript will test treatment efficacy by investigating group-specific effects as they relate to a control group.
Statistical Analyses
All statistical tests were performed in SPSS version 21. Sequential and moderated regression analyses46,47 of cross-sectional (ie, pretraining) and longitudinal (ie, posttraining change) data were performed. Centered variables were used in the analysis. Standardized regression coefficients (β) are reported, allowing us to infer the strongest predictor of long-distance walking function based on magnitude. Residuals for each of the regression models were screened for the presence of outliers. Alpha level of 0.05 was set as the threshold for statistical significance. One-tail tests were used for effects with an a priori directional hypothesis.
Sequential linear regression was used to test our hypothesis that the stance phase construct would be the strongest predictor of long-distance walking function. The order by which the swing phase and spatiotemporal symmetry constructs were added to the model was based on the magnitudes of the bivariate correlations, with strongest added first. With 44 subjects, and alpha set at 0.05, this study had power = 0.80 to detect an R2 increase between 0.20 (1-tail) and 0.24 (2-tail) when adding the swing phase and spatiotemporal symmetry constructs (ie, four variables) to the model containing the stance phase construct (ie, two variables).
Bivariate correlations of the longitudinal data were used to test our hypothesis that improvements in stance phase function would relate to improvements in long-distance walking function. Moderated regression was used to test our hypothesis that baseline stance phase function would moderate the relationship between changes in stance phase mechanics versus changes in long-distance walking function. Because only changes in paretic propulsion correlated to changes in long-distance walking function (see Results), only interactions with change in paretic propulsion were tested. The available sample size precluded us from examining all interactions in one model, so to avoid model over fit and to maintain adequate power, independent moderation models were generated to examine each interaction.
Specifically, the first model tested the interaction between pretraining propulsion and change in propulsion and the second tested the interaction between pretraining trailing limb angle and change in propulsion. Based on our finding of moderation by pretraining trailing limb angle (see Results), the third moderation model tested moderation by changes in trailing limb angle with an a priori hypothesis that changes in propulsion would have a stronger relationship to changes in long-distance walking function in those with concomitant changes in trailing limb angle. With 30 subjects, at an alpha level of 0.05, each moderated regression model was 80% powered to detect an R2 increase (1-tail) of 0.22.
RESULTS
Clinical data were available for all subjects (see Table 1 for subject characteristics); however, due to technical issues during data collection, pretraining biomechanical data were not available for 1 of the 45 subjects studied. Moreover, a single subject was found to be a statistical outlier and was removed prior to the analyses presented. These 2 subjects were also among the cohort (n = 31) who underwent training. As such, the cross-sectional analyses presented reflect the data collected for 43 subjects and the longitudinal analyses reflect the data collected for 29 subjects. Table 2 presents means, standard deviations, minimums, and maximums for the pretraining and change-score variables included in the regression analyses conducted.
Table 2.
| Pretraining and posttraining change-score mean (SD) values. | ||
|---|---|---|
| Pretraining (n = 43) | ||
| Variable | Mean (SD) | Min/Max |
| Paretic Propulsion, % body weight | 8.67 (5.00) | 0.00/20.12 |
| Paretic Trailing Limb Angle, degrees | 15.35 (8.15) | −3.98/29.61 |
| Paretic Knee Flexion, degrees | 46.04 (14.79) | 14.43/71.39 |
| Paretic Dorsiflexion, degrees | −1.65 (8.01) | −19.45/11.61 |
| Step Length Symmetry | 0.570 (0.147) | 0.501/1.250 |
| Swing Time Symmetry | 0.565 (0.057) | 0.504/0.738 |
| Six-Minute Walk Test Distance, m | 285 (134) | 44/546 |
| Change-Scores (n = 29) | ||
| Variable | Mean (SD) | Min/Max |
| Δ Paretic Propulsion, % body weight | 2.26 (3.78) | −4.26/14.74 |
| Δ Paretic Trailing Limb Angle, degrees | 2.76 (4.65) | −4.62/16.00 |
| Δ Paretic Knee Flexion, degrees | 2.62 (6.82) | −11.40/20.72 |
| Δ Paretic Dorsiflexion, degrees | 1.12 (6.29) | −10.94/18.23 |
| Δ Step Length Symmetry | 0.034 (0.141) | −0.055/0.746 |
| Δ Swing Time Symmetry | −0.011 (0.042) | −0.109/0.088 |
| Δ Six-Minute Walk Test Distance, m | 72 (61.68) | −37/207 |
| Sequential regression models predicting pretraining 6MWT distance (n = 43). | |||||||
|---|---|---|---|---|---|---|---|
| Model Statistics | Predictor Statistics | ||||||
| Block | Model R2 | Model F | Model p | Predictors | β | t | p |
| 1 | .615 | 31.92 | .000 | Paretic Propulsion | .363 | 2.15 | .019 |
| Trailing Limb Angle | .460 | 2.73 | .005 | ||||
| 1 + 2 | .621 | 15.54 | .000 | Paretic Propulsion | .331 | 1.86 | .035 |
| Trailing Limb Angle | .415 | 2.19 | .018 | ||||
| Ankle Dorsiflexion | .098 | 0.73 | .472 | ||||
| Knee Flexion | .017 | 0.14 | .887 | ||||
| 1 + 2 + 3 | .655 | 11.38 | .000 | Paretic Propulsion | .339 | 1.92 | .031 |
| Trailing Limb Angle | .564 | 2.75 | .005 | ||||
| Ankle Dorsiflexion | .166 | 1.20 | .237 | ||||
| Knee Flexion | .043 | 0.37 | .717 | ||||
| Step Length Symmetry | .156 | 1.19 | .243 | ||||
| Swing Time Symmetry | .184 | 1.40 | .170 | ||||
Cross-sectional Analyses
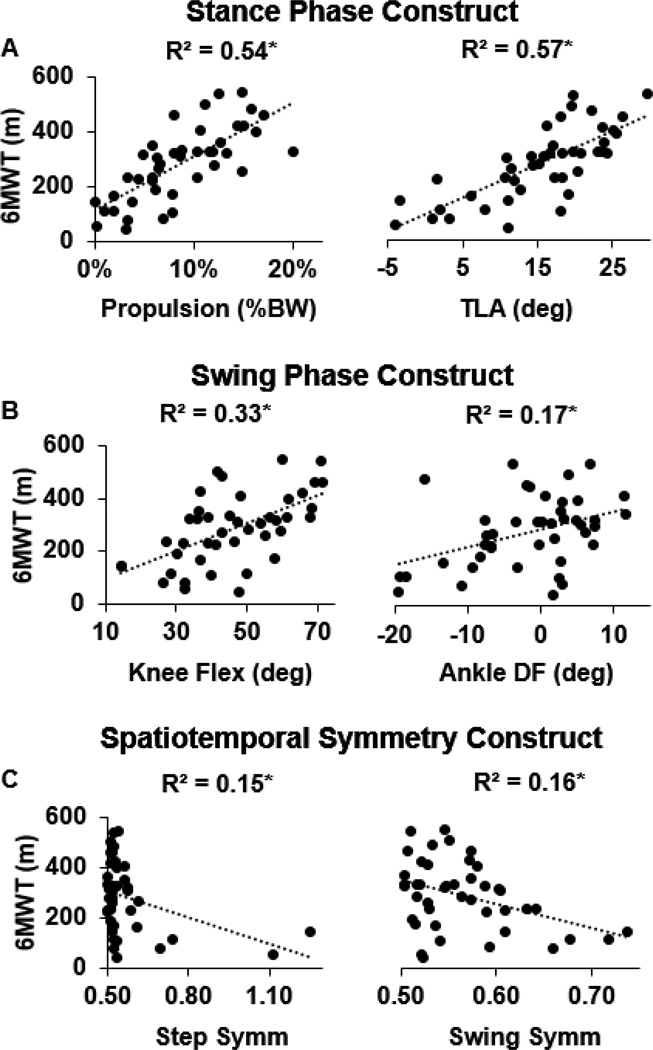
Despite variables from all three constructs correlating with performance on the 6MWT (Figure 1), only paretic propulsion (β = .339) and trailing limb angle (β = .564) independently predicted 6MWT distance (Table 2; R2 = .655, F(6,36) = 11.38, p < 0.001).
Figure 1.
Scatter plots present the relationships between stance phase (panel A), swing phase (panel B), and spatiotemporal symmetry (panel C) biomechanics versus long distance walking function. All variables considered were related to performance on the 6MWT; however, stance phase function exhibited the highest degree of correlation. * p < .05.
Longitudinal Analyses
Bivariate correlations of the longitudinal data revealed that only changes in paretic propulsion (r = 0.435, p = 0.009) correlated with changes in the 6MWT. Interestingly, changes in trailing limb angle and in the swing phase and the symmetry variables studied did not (r’s < 0.29 and p’s > 0.05).
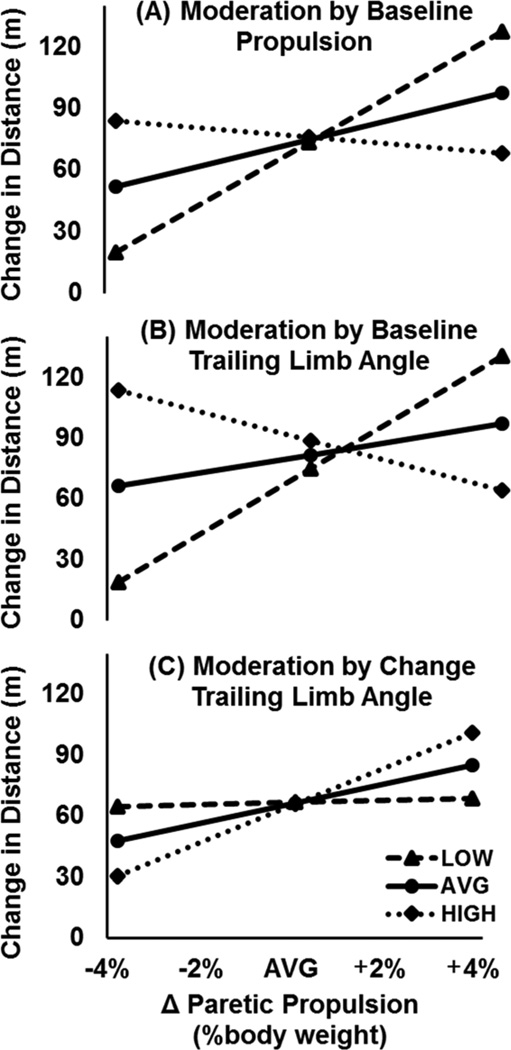
Moderated regression analyses revealed three independent moderators of the relationship between changes in paretic propulsion and changes in the 6MWT (see Table 3). The first was moderation by pretraining paretic propulsion (final model testing this interaction: F(3,25) = 5.17, p = 0.006, R2 = 0.383, ΔR2 = 0.193). Specifically, changes in propulsion were strongly positively related to changes in the 6MWT for those with low pretraining propulsion – that is, those with baseline propulsion lower than one standard deviation below the mean (ie, < 3.67% BW). For those with average propulsion (ie, 8.67% BW), changes in propulsion weakly positively related to changes in the 6MWT. For those with pretraining propulsion greater than one standard deviation above the mean (ie, > 13.67% BW), a weak negative relationship was observed (Figure 2, panel A).
Table 3.
Moderated regression analyses predicting change in 6MWT distance (n = 29).
| Model Statistics | Predictor Statistics | ||||||
|---|---|---|---|---|---|---|---|
| Model | Model R2 | Model F | Model p | Predictors | β | t | p |
| Moderation of Δ Prop by Pre-tx Prop |
.383 | 5.17 | .006 | Δ Prop | .340 | 2.11 | .023 |
| Pre-tx Prop | .098 | 0.62 | .539 | ||||
| Δ Prop * Pre-tx Prop | −.436 | −2.72 | .012 | ||||
| Moderation of Δ Prop by Pre-tx TLA |
.468 | 7.34 | .001 | Δ Prop | .227 | 1.43 | .082 |
| Pre-tx TLA | .216 | 1.43 | .082 | ||||
| Δ Prop * Pre-tx TLA | −.526 | −3.43 | .002 | ||||
| Moderation of Δ Prop by Δ TLA |
.289 | 3.39 | .034 | Δ Prop | .213 | 1.02 | .160 |
| Δ TLA | −.049 | −0.25 | .403 | ||||
| Δ Prop * Δ TLA | .408 | 1.82 | .041 | ||||
Figure 2.
Moderated regression plots present a visual representation of the relationship between changes in paretic propulsion (x-axis) versus changes in long-distance walking function (y-axis) as moderated by pretraining propulsion (panel A), pretraining trailing limb angle (panel B), and changes in trailing limb angle (panel C). This relationship was strongest in those with LOW (ie, 1 standard deviation below the mean) pretraining levels of propulsion (< 3.67% body weight, panel A) and trailing limb angle (< 7.2 degrees, panel B). Moreover, changes in paretic propulsion most strongly related to changes in long-distance walking function in those with the largest change in trailing limb angle (ie, 1 standard deviation above the mean: > 7.41 degrees) (panel C). Please note that panels A-C present simple slopes at each level of the moderator, not a grouping of subjects.
An even stronger effect was observed when testing moderation by pretraining trailing limb angle (Table 3; F(3,25) = 7.34, p = 0.001, R2 = 0.468, ΔR2 = 0.279). Similar to the effect of pretraining propulsion, the strongest relationship between changes in paretic propulsion and changes in 6MWT distance was observed in those with a pretraining trailing limb angle lower than one standard deviation below the mean (ie, < 7.2 degrees). A weaker positive relationship was observed in those with the average pretraining trailing limb angle (ie, 15.35 degrees) and a weak negative relationship was observed in those with the largest pretraining trailing limb angle (ie, > 23.5 degrees) (Figure 2, panel B).
The weakest moderator of the relationship between changes in propulsion and changes in the 6MWT was changes in trailing limb angle (Table 3; F(3,25) = 3.39, p = 0.034, R2 = 0.289, ΔR2 = 0.094). The relationship between changes in propulsion and changes in the 6MWT was strongest in those with the largest change in trailing limb angle (ie, > 7.41 degrees). Interestingly, for those with a change in trailing limb angle one standard deviation below the mean (ie, a decline of 1.89 degrees or greater), changes in propulsion were unrelated to changes in the 6MWT (Figure 2, panel C).
DISCUSSION
This report is the first to explore the relative importance of stance phase, swing phase, and spatiotemporal symmetry biomechanics to poststroke long-distance walking function. This investigation extends previous work that has studied the biomechanical determinants of short-distance walking function12,26,28,48–50. The present results reveal a relationship between the function of the paretic limb during stance phase – particularly the propulsive force generated during late stance – and long-distance walking function in persons in the chronic phase of stroke recovery. Moreover, the results of the moderated regression analyses indicate that this relationship is greatest in those persons presenting with large pretraining impairments in propulsion or trailing limb angle. Given that a majority of individuals in the chronic phase of stroke recovery identify deficits in their ability to walk farther as contributing to reduced engagement in the community18, by identifying key biomechanical determinants of poststroke long-distance walking function, this investigation facilitates the development of targeted interventions with the potential to increase community participation after stroke.
Previous investigators have posited that an assessment of post-intervention outcomes is lacking if limited to only gross clinical measures such as walking speed51. The present investigation’s elucidation of key biomechanical determinants of poststroke long-distance walking function therefore orients clinicians to important poststroke gait variables, ultimately informing clinical practice. Although task-specific practice forms a necessary basis for neurorehabilitation efforts16,52–54, the present findings support structuring practice in a manner that targets the specific impairments that may be limiting performance. For example, although walking practice is commonly prescribed as a therapeutic intervention, the present results suggest that training walking at a fast speed will produce improvements in long-distance walking function associated with the recovery of paretic limb biomechanical function – especially in those most impaired. Further development and testing of hypothesis-driven targeted locomotor interventions for persons poststroke is warranted.
Interestingly, despite not relating to changes in long-distance walking function, changes in trailing limb angle moderated the relationship between changes in propulsion and changes in long-distance walking function. Specifically, only in those with a large improvement in the paretic trailing limb angle did gains in propulsion relate to gains in long-distance walking (see Figure 2, panel C). One explanation for these apparently contradictory findings is that improvements in trailing limb angle are not meaningful – in terms of improving long-distance walking function – if they do not result in improvements in propulsion. Indeed, although increasing the paretic trailing limb angle yields a more effective biomechanical position for the generation of propulsive forces by the ankle musculature27, it is important to note that persons poststroke often use the hip flexors to advance the paretic limb during the stance to swing transition24,55,56 – which is a strategy known to negatively correlate with the propulsive forces generated15,27,57,58. That is, merely providing better resources (ie, a larger paretic trailing limb angle) may be insufficient to alter the strategy used to walk faster. The specific training of use of the ankle musculature may be necessary.
Multiple factors may influence performance on the 6MWT – our measure of long-distance walking ability. We have previously shown that changes in maximum walking speed account for greater than 50% of the variance in changes in 6MWT performance59. Other factors certainly contribute. One possible factor is changes in the energy cost of walking. Although the present report does not directly investigate the role that changes in walking energetics may play in modifying long-distance walking function, recent work from our laboratory demonstrates a meaningful relationship between posttraining changes in walking kinematics, specifically step length asymmetry, and changes in the energy cost of walking45. Surprisingly, the present investigation revealed that changes in walking kinematics were unrelated to changes in 6MWT performance, suggesting that deficits in walking kinematics were not limiting long-distance walking function as measured via the 6MWT. In contrast, it has been shown that compensatory kinematic strategies are energetically costly15–17,60 and previous work from our laboratory has shown that those with lower walking energy costs travel farther distances during the 6MWT19. Moreover, although not directly related to changes in the 6MWT in the present investigation, changes in the paretic trailing limb angle moderated the influence that changes in paretic propulsion had on changes in 6MWT performance. As such, further investigation of the interplay between walking kinematics, the energy cost of walking, and long-distance walking function is warranted.
Based on the findings of this investigation, a reasonable hypothesis would be that an intervention targeting deficits in paretic propulsion through specific effects on the paretic trailing limb angle could produce improvements in the functional status of persons in the chronic phase after stroke. Indeed, our laboratory recently published a preliminary study that supports this hypothesis by demonstrating improvements across the domains of the World Health Organization’s International Classification of Functioning, Health, and Disability following training targeting deficits in paretic propulsion through specific effects on the function of the paretic limb during late stance40. The findings from this preliminary study validate the present study’s emphasis on stance phase mechanics. However, future work is necessary to determine the efficacy of interventions targeting paretic propulsion across subgroups of patients stratified according to baseline biomechanical function.
An important point is that although this investigation considered the biomechanical constructs studied independently, for an individual, these variables are likely interrelated. That is, events during stance phase may have a direct impact on swing phase function, and changes in both stance phase and swing phase underlie changes in spatiotemporal symmetry. For example, increased propulsive force during late stance is one mechanism posited to increase knee flexion during swing61–63. Moreover, improvements in step length symmetry may result from a larger trailing limb angle – which would effectively increase the contralateral step length – or better propulsion – which may increase ipsilateral step length through its swing phase effects. Even so, by examining the relative importance of each of these constructs – particularly how changes in each relate to the changes in long-distance walking function observed after gait rehabilitation – this report reveals important information regarding the mechanisms that may be driving the recovery of poststroke walking function. Future work that examines how changes in other biomechanical measures, such as mechanical work or power, account for the variance in changes in long-distance walking function after poststroke locomotor intervention would further extend this work.
Limitations
A potential limitation of this study is that biomechanical testing occurred on a treadmill. Conceptually, relating changes in overground gait mechanics to changes in overground long-distance walking function would have been preferable; however, it should be noted that treadmill biomechanical assessment has several advantages over overground testing. These include the averaging of consecutive strides, the ability to control speed – a major determinant of gait mechanics, increased patient safety, and a marked reduction in efforts by both patient and researcher to generate data for a large number of strides. Previous work has also shown that treadmill biomechanical data provides relevant information for understanding overground walking64,65.
A second potential limitation of this study is that some subjects utilized a handrail during testing. Specifically, subjects who typically used an assistive device or those who felt unsafe walking on the treadmill were allowed to use a handrail. The use of a handrail during testing may promote a forward trunk lean that could influence our measurement of trailing limb angle if the pelvis/trunk are not aligned with the vertical axis of the laboratory. However, it should be noted that subjects were only allowed to use a handrail located at the side of the treadmill. This mimicked walking with an assistive device and placed minimal constraint on the anterior/posterior displacement of the body during walking. It should also be noted that subjects were instructed to use the minimal amount of handrail support possible.
Conclusions
Because a rapid achievement of walking independence, not necessarily the reduction of impairment, is the goal of current neurorehabilitation practice66, the high prevalence of inefficient walking strategies among persons in the chronic phase of stroke recovery is not surprising15,16. Maximizing posttraining outcomes for persons in the chronic phase of stroke recovery may therefore necessitate the learning of new walking strategies. The findings of this investigation support the development of poststroke locomotor interventions that include the targeting of paretic limb stance phase deficits during walking – specifically propulsion and trailing limb angle.
Acknowledgements
We would like to thank Christopher Cutsail, BS and Jacqueline Palmer, PT, DPT for assistance with data collection and processing.
Financial Support: This study was supported by the following National Institutes of Health grants: R01NR010786, K01HD050582, U54GM104941, P30GM103333, and T32HD007490.
Footnotes
Disclosures: Authors have no conflicts of interest to disclose.
REFERENCES
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Bohannon RW, Horton MG, Wikholm JB. Importance of four variables of walking to patients with stroke. [Accessed October 29, 2012];Int J Rehabil Res. 1991 14(3):246–250. doi: 10.1097/00004356-199109000-00010. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1938039. [DOI] [PubMed] [Google Scholar]
- 3.Grimby G, Andrén E, Daving Y, Wright B. Dependence and perceived difficulty in daily activities in community-living stroke survivors 2 years after stroke: a study of instrumental structures. Stroke A J Cereb Circ. 1998;29:1843–1849. doi: 10.1161/01.str.29.9.1843. [DOI] [PubMed] [Google Scholar]
- 4.Lord SE, McPherson K, McNaughton HK, Rochester L, Weatherall M. Community ambulation after stroke: how important and obtainable is it and what measures appear predictive? [Accessed August 15, 2013];Arch Phys Med Rehabil. 2004 85(2):234–239. doi: 10.1016/j.apmr.2003.05.002. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14966707. [DOI] [PubMed] [Google Scholar]
- 5.Mayo NE, Wood-Dauphinee S, Ahmed S, et al. Disablement following stroke. [Accessed August 15, 2013];Disabil Rehabil. 1999 21(5–6):258–268. doi: 10.1080/096382899297684. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10381238. [DOI] [PubMed] [Google Scholar]
- 6.Lamb SE, Ferrucci L, Volapto S, Fried LP, Guralnik JM. Risk factors for falling in home-dwelling older women with stroke: the Women’s Health and Aging Study. [Accessed August 15, 2013];Stroke. 2003 34(2):494–501. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12574566. [PubMed] [Google Scholar]
- 7.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. [August 15, 2013];Stroke. 1995 26(6):982–989. doi: 10.1161/01.str.26.6.982. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7762050. [DOI] [PubMed] [Google Scholar]
- 8.Reisman DS, Rudolph KS, Farquhar WB. Influence of speed on walking economy poststroke. Neurorehabil Neural Repair. 2009;23(6):529–534. doi: 10.1177/1545968308328732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil. 2005;86(8):1552–1556. doi: 10.1016/j.apmr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Michael K, Macko RF. Ambulatory activity intensity profiles, fitness, and fatigue in chronic stroke. Top Stroke Rehabil. 14(2):5–12. doi: 10.1310/tsr1402-5. [DOI] [PubMed] [Google Scholar]
- 11.Combs SA, Dugan EL, Ozimek EN, Curtis AB. Effects of body-weight supported treadmill training on kinetic symmetry in persons with chronic stroke. Clin Biomech (Bristol, Avon) 2012;27(9):887–892. doi: 10.1016/j.clinbiomech.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Hall AL, Bowden MG, Kautz SA, Neptune RR. Biomechanical variables related to walking performance 6-months following post-stroke rehabilitation. Clin Biomech (Bristol, Avon) 2012;27(10):1017–1022. doi: 10.1016/j.clinbiomech.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88:43–49. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Bowden MG, Behrman AL, Neptune RR, Gregory CM, Kautz SA. Locomotor rehabilitation of individuals with chronic stroke: difference between responders and nonresponders. Arch Phys Med Rehabil. 2013;94(5):856–862. doi: 10.1016/j.apmr.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 15.Olney SJ, Richards C. Hemiparetic gait following stroke. Part I: characteristics. Gait Posture. 1996;4:136–148. [Google Scholar]
- 16.Richards CL, Malouin F, Dean C. Gait in stroke: assessment and rehabilitation. [January 21, 2014];Clin Geriatr Med. 1999 15(4):833–855. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10499938. [PubMed] [Google Scholar]
- 17.Cruz TH, Lewek MD, Dhaher YY. Biomechanical impairments and gait adaptations post-stroke: multi-factorial associations. J Biomech. 2009;42(11):1673–1677. doi: 10.1016/j.jbiomech.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Combs SA, Van Puymbroeck M, Altenburger PA, Miller KK, Dierks TA, Schmid AA. Is walking faster or walking farther more important to persons with chronic stroke? Disabil Rehabil. 2013;35(10):860–867. doi: 10.3109/09638288.2012.717575. [DOI] [PubMed] [Google Scholar]
- 19.Reisman DS, Binder-MacLeod S, Farquhar WB. Changes in metabolic cost of transport following locomotor training poststroke. Top Stroke Rehabil. 20(2):161–170. doi: 10.1310/tsr2002-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franceschini M, Rampello A, Agosti M, Massucci M, Bovolenta F, Sale P. Walking performance: correlation between energy cost of walking and walking participation. new statistical approach concerning outcome measurement. PLoS One. 2013;8(2):e56669. doi: 10.1371/journal.pone.0056669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sibley KM, Tang A, Patterson KK, Brooks D, McIlroy WE. Changes in spatiotemporal gait variables over time during a test of functional capacity after stroke. J Neuroeng Rehabil. 2009;6:27. doi: 10.1186/1743-0003-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowden MG, Balasubramanian CK, Neptune RR, Kautz SA. Anterior-posterior ground reaction forces as a measure of paretic leg contribution in hemiparetic walking. Stroke A J Cereb Circ. 2006;37:872–876. doi: 10.1161/01.STR.0000204063.75779.8d. [DOI] [PubMed] [Google Scholar]
- 23.Chen G, Patten C, Kothari DH, Zajac FE. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture. 2005;22(1):51–56. doi: 10.1016/j.gaitpost.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. [August 15, 2013];Clin Biomech (Bristol, Avon) 1999 14(2):125–135. doi: 10.1016/s0268-0033(98)00062-x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10619100. [DOI] [PubMed] [Google Scholar]
- 25.Mulroy SJ, Klassen T, Gronley JK, Eberly VJ, Brown DA, Sullivan KJ. Gait parameters associated with responsiveness to treadmill training with body-weight support after stroke: an exploratory study. Phys Ther. 2010;90(2):209–223. doi: 10.2522/ptj.20090141. [DOI] [PubMed] [Google Scholar]
- 26.Hall AL, Peterson CL, Kautz SA, Neptune RR. Relationships between muscle contributions to walking subtasks and functional walking status in persons with post-stroke hemiparesis. Clin Biomech (Bristol, Avon) 2011;26(5):509–515. doi: 10.1016/j.clinbiomech.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson CL, Cheng J, Kautz SA, Neptune RR. Leg extension is an important predictor of paretic leg propulsion in hemiparetic walking. Gait Posture. 2010;32(4):451–456. doi: 10.1016/j.gaitpost.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. [August 15, 2013];J Biomech. 2001 34(11):1387–1398. doi: 10.1016/s0021-9290(01)00105-1. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11672713. [DOI] [PubMed] [Google Scholar]
- 29.Peterson CL, Hall AL, Kautz SA, Neptune RR. Pre-swing deficits in forward propulsion, swing initiation and power generation by individual muscles during hemiparetic walking. J Biomech. 2010;43(12):2348–2355. doi: 10.1016/j.jbiomech.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng SS, Hui-Chan CW. Contribution of ankle dorsiflexor strength to walking endurance in people with spastic hemiplegia after stroke. Arch Phys Med Rehabil. 2012;93(6):1046–1051. doi: 10.1016/j.apmr.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Dorsch S, Ada L, Canning CG, Al-Zharani M, Dean C. The strength of the ankle dorsiflexors has a significant contribution to walking speed in people who can walk independently after stroke: an observational study. Arch Phys Med Rehabil. 2012;93(6):1072–1076. doi: 10.1016/j.apmr.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Lin P-Y, Yang Y-R, Cheng S-J, Wang R-Y. The relation between ankle impairments and gait velocity and symmetry in people with stroke. Arch Phys Med Rehabil. 2006;87(4):562–568. doi: 10.1016/j.apmr.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 33.Azouvi P, Samuel C, Louis-Dreyfus A, et al. Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. [August 15, 2013];J Neurol Neurosurg Psychiatry. 2002 73(2):160–166. doi: 10.1136/jnnp.73.2.160. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1737990&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plummer P, Behrman AL, Duncan PW, et al. Effects of stroke severity and training duration on locomotor recovery after stroke: a pilot study. Neurorehabil Neural Repair. 21(2):137–151. doi: 10.1177/1545968306295559. [DOI] [PubMed] [Google Scholar]
- 35.Pohl PS, Duncan PW, Perera S, et al. Influence of stroke-related impairments on performance in 6-minute walk test. [August 15, 2013];J Rehabil Res Dev. 2002 39(4):439–444. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17638141. [PubMed] [Google Scholar]
- 36.Donovan K, Lord SE, McNaughton HK, Weatherall M. Mobility beyond the clinic: the effect of environment on gait and its measurement in community-ambulant stroke survivors. Clin Rehabil. 2008;22(6):556–563. doi: 10.1177/0269215507085378. [DOI] [PubMed] [Google Scholar]
- 37.Fulk GD, Reynolds C, Mondal S, Deutsch JE. Predicting home and community walking activity in people with stroke. Arch Phys Med Rehabil. 2010;91(10):1582–1586. doi: 10.1016/j.apmr.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Pang MYC, Eng JJ, Miller WC. Determinants of satisfaction with community reintegration in older adults with chronic stroke: role of balance self-efficacy. Phys Ther. 2007;87(3):282–291. doi: 10.2522/ptj.20060142. [DOI] [PubMed] [Google Scholar]
- 39.Reisman DS, Kesar TM, Perumal R, et al. Time course of functional and biomechanical improvements during a gait training intervention in persons with chronic stroke. J Neurol Phys Ther. 2013 doi: 10.1097/NPT.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Awad LN, Reisman DS, Kesar TM, Binder-Macleod SA. Targeting Paretic Propulsion To Improve Post-Stroke Walking Function: A Preliminary Study. Arch Phys Med Rehabil. 2013 doi: 10.1016/j.apmr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allen JL, Kautz SA, Neptune RR. Step length asymmetry is representative of compensatory mechanisms used in post-stroke hemiparetic walking. Gait Posture. 2011;33(4):538–543. doi: 10.1016/j.gaitpost.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patterson KK, Mansfield A, Biasin L, Brunton K, Inness EL, McIlroy WE. Longitudinal Changes in Poststroke Spatiotemporal Gait Asymmetry Over Inpatient Rehabilitation. Neurorehabil Neural Repair. 2014 doi: 10.1177/1545968314533614. [DOI] [PubMed] [Google Scholar]
- 43.Lewek MD, Bradley CE, Wutzke CJ, Zinder SM. The relationship between spatiotemporal gait asymmetry and balance in individuals with chronic stroke. J Appl Biomech. 2014;30(1):31–36. doi: 10.1123/jab.2012-0208. [DOI] [PubMed] [Google Scholar]
- 44.Combs SA, Dugan EL, Ozimek EN, Curtis AB. Bilateral coordination and gait symmetry after body-weight supported treadmill training for persons with chronic stroke. Clin Biomech (Bristol, Avon) 2013;28(4):448–453. doi: 10.1016/j.clinbiomech.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Awad LN, Palmer JA, Pohlig RT, Binder-Macleod SA, Reisman DS. Walking speed and step length asymmetry modify the energy cost of walking after stroke. Neurorehabil Neural Repair. 2014 doi: 10.1177/1545968314552528. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd Edition. Routledge; 2003. [Google Scholar]
- 47.Aiken LS, West SG. Multiple Regression: Testing and interpreting interactions. SAGE; 1991. [Google Scholar]
- 48.Routson RL, Clark DJ, Bowden MG, Kautz SA, Neptune RR. The influence of locomotor rehabilitation on module quality and post-stroke hemiparetic walking performance. Gait Posture. 2013;38(3):511–517. doi: 10.1016/j.gaitpost.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teixeira-salmela LF, Nadeau S, Mcbride I, Olney SJ. EFFECTS OF MUSCLE STRENGTHENING AND PHYSICAL CONDITIONING TRAINING ON TEMPORAL , KINEMATIC AND KINETIC VARIABLES DURING GAIT IN CHRONIC STROKE SURVIVORS. J Rehabil Med. 2001;(33):53–60. doi: 10.1080/165019701750098867. [DOI] [PubMed] [Google Scholar]
- 50.Parvataneni K, Olney SJ, Brouwer B. Changes in muscle group work associated with changes in gait speed of persons with stroke. [November 11, 2013];Clin Biomech. 2007 22(7):813–820. doi: 10.1016/j.clinbiomech.2007.03.006. Available at: http://www.sciencedirect.com/science/article/pii/S0268003307000630. [DOI] [PubMed] [Google Scholar]
- 51.Bowden MG, Behrman AL, Woodbury M, Gregory CM, Velozo CA, Kautz SA. Advancing measurement of locomotor rehabilitation outcomes to optimize interventions and differentiate between recovery versus compensation. J Neurol Phys Ther. 2012;36(1):38–44. doi: 10.1097/NPT.0b013e3182472cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1):S225–S239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 53.Poltawski L, Abraham C, Forster A, et al. Synthesising practice guidelines for the development of community-based exercise programmes after stroke. Implement Sci. 2013;8:115. doi: 10.1186/1748-5908-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duncan PW, Zorowitz R, Bates B, et al. Management of Adult Stroke Rehabilitation Care: a clinical practice guideline. Stroke. 2005;36(9):e100–e143. doi: 10.1161/01.STR.0000180861.54180.FF. [DOI] [PubMed] [Google Scholar]
- 55.Olney SJ, Griffin MP, McBride ID. Temporal, kinematic, and kinetic variables related to gait speed in subjects with hemiplegia: a regression approach. Phys Ther. 1994;74:872–885. doi: 10.1093/ptj/74.9.872. [DOI] [PubMed] [Google Scholar]
- 56.Jonsdottir J, Recalcati M, Rabuffetti M, Casiraghi A, Boccardi S, Ferrarin M. Functional resources to increase gait speed in people with stroke: strategies adopted compared to healthy controls. Gait Posture. 2009;29(3):355–359. doi: 10.1016/j.gaitpost.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Turns LJ, Neptune RR, Kautz SA. Relationships between muscle activity and anteroposterior ground reaction forces in hemiparetic walking. Arch Phys Med Rehabil. 2007;88(9):1127–1135. doi: 10.1016/j.apmr.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olney SJ, Griffin MP, Monga TN, McBride ID. Work and power in gait of stroke patients. [November 18, 2013];Arch Phys Med Rehabil. 1991 72(5):309–314. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2009047. [PubMed] [Google Scholar]
- 59.Awad LN, Reisman DS, Wright TW, Roos MA, Binder-MacLeod S. The Importance of Maximum Walking Speed In Determining Walking Function After Stroke. Top Stroke Rehabil. 2014 doi: 10.1310/tsr2106-502. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stoquart G, Detrembleur C, Lejeune TM. The reasons why stroke patients expend so much energy to walk slowly. Gait Posture. 2012;36(3):409–413. doi: 10.1016/j.gaitpost.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 61.Neptune RR, Sasaki K, Kautz SA. The effect of walking speed on muscle function and mechanical energetics. Gait Posture. 2008;28(1):135–143. doi: 10.1016/j.gaitpost.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knarr BA, Kesar TM, Reisman DS, Binder-Macleod SA, Higginson JS. Changes in the activation and function of the ankle plantar flexor muscles due to gait retraining in chronic stroke survivors. J Neuroeng Rehabil. 2013;10:12. doi: 10.1186/1743-0003-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen G, Patten C. Joint moment work during the stance-to-swing transition in hemiparetic subjects. J Biomech. 2008;41(4):877–883. doi: 10.1016/j.jbiomech.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 64.Goldberg E, Kautz SA, Neptune RR. Can treadmill walking be used to assess propulsion generation? [August 27, 2014];J Biomech. 2008 41(8):1805–1808. doi: 10.1016/j.jbiomech.2008.03.009. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18436229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kautz SA, Bowden MG, Clark DJ, Neptune RR. Comparison of motor control deficits during treadmill and overground walking poststroke. [August 27, 2014];Neurorehabil Neural Repair. 2011 25(8):756–765. doi: 10.1177/1545968311407515. Available at: http://www.ncbi.nlm.nih.gov.proxy.nss.udel.edu/pubmed/21636831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kitago T, Krakauer JW. Motor learning principles for neurorehabilitation. Handb Clin Neurol. 2013;110:93–103. doi: 10.1016/B978-0-444-52901-5.00008-3. [DOI] [PubMed] [Google Scholar]